Abstract
Musculoskeletal reconstruction is an ongoing challenge for surgeons as it is required for one out of five patients undergoing surgery. In the past three decades, through the close collaboration between clinicians and basic scientists, several regenerative strategies have been proposed. These have emerged from interdisciplinary approaches that bridge tissue engineering with material science, physiology, and cell biology. The paradigm behind tissue engineering is to achieve regeneration and functional recovery using stem cells, bioactive molecules, or supporting materials. Although plenty of preclinical solutions for bone and cartilage have been presented, only a few platforms have been able to move from the bench to the bedside. In this review, we highlight the limitations of musculoskeletal regeneration and summarize the most relevant acellular tissue engineering approaches. We focus on the strategies that could be most effectively translate in clinical practice and reflect on contemporary and cutting‐edge regenerative strategies in surgery. Stem Cells Translational Medicine 2017;6:2186–2196
Keywords: Mesenchymal stem cells, Stem cell‐microenvironment interactions, Tissue regeneration, Tissue engineering
Significance Statement.
Musculoskeletal tissue engineering has been proposed as an innovative therapeutic approach aiming at replacing lost or severely damaged bone, cartilage, ligaments, and tendons. Although 30 years have passed since the field was born, the clinical solutions for musculoskeletal regeneration are still not sufficient to fulfill the demands of the many patients. In fact, only few tissue engineering platforms have been successfully translated to humans. This review gives a brief overview of the current needs of musculoskeletal tissue engineering in order to speed up the transition from “bench to bedside” emphasizing the essential need for a close collaboration between clinicians and tissue engineers.
Introduction
Amphibians, the most regenerative vertebrates, are capable of limb regeneration after amputation through the formation of a structure called a blastema. The blastema forms through the degradation of the extracellular matrix (ECM) and the subsequent re‐organization of the regenerative tissues driven by dedifferentiated cells, along with satellite cells released from skeletal muscle 1. The blastema grows rapidly by mitosis and its cells re‐differentiate into the original tissue pattern 2. Although mammals do not seem to be able to activate this process in tissue regeneration 3, an endogenous regenerative potential still exists. In all tissues there is a resident population of stem and progenitor cells programmed to respond to certain stimuli to produce replacement cells 4. These stem cells have been exploited therapeutically with different approaches, especially to boost the repair of bone and cartilage 5.
Stem cells, however, are not the only essential components to restoring mammalian tissues. The ECM is the other integral player that not only offers physical support to the cells, but also serves as a plastic and dynamic structure that controls and directs tissue homeostasis 6. Furthermore, the ECM is part of a dynamic environment called a niche that provides biochemical and biomechanical cues that allow stem cells' survival and define their identity 7. Depending on external stimuli, the niche finely regulates state of quiescence, self‐renewal or active differentiation of stem cells. Moreover, the niche also has an important “shield role” as it protects the residing stem cells from gene mutations, preventing their transformation into cancer cells.
Given these main roles, an ECM that properly responds to the environment could improve tissue restoration. For decades, tissue engineers have tried to develop a stem cell niche replacement by designing and fabricating the “perfect ECM” to improve tissue regeneration.
This review discusses recent developments in biomaterial designs that aim to stimulate the endogenous healing potential of an organism in order to achieve tissue restoration. We will explore the tissue‐specific regenerative limitation of musculoskeletal system and highlight different regenerative strategies focusing on cell‐free approaches. We will focus our attention particularly on biomimetic tissue engineering strategies aimed toward guiding the endogenous regenerative niche of musculoskeletal tissues 8.
Endogenous Regenerative Potential of Bone
Bone innately regenerates following trauma, typically without scarring. This mainly happens due to a powerful stem cell niche present within the bone 9. As we have previously anticipated, stem cell niches are not only defined by the cells that inhabit them, but also by the ECM, as the latter plays an important role in the cellular commitment. The protein and proteoglycan composition of a stem cell niche ECM is tissue‐specific and differentially modulates the stem cells behavior. It does this through interaction with growth factors (GFs) and bioactive molecules that ultimately determine the fate of the cells 10. Mesenchymal stem cells (MSCs) are the main players of musculoskeletal tissue building, from bone formation in the embryo to fracture repair and remodeling in an adult 11. MSCs differentiate toward bone, cartilage, tendon, ligament, and muscle 12, 13. MSCs can be recruited from multiple tissues surrounding the site of injury including the bone marrow or from other stem cells niches found in compact bone 14, 15, muscle 16 tendons' cuffs 17, and periosteum 18. However, MSCs alone are not sufficient to repair injured bone, the healing is a regulated process in which resident stromal, progenitor, and inflammatory cells orchestrate a complex signaling cascade that leads to repair and remodeling 19, 20. If bone can regenerate itself, what are the limitations in bone healing and why do we need tissue engineering strategies to improve bone repair? While we cannot isolate a unique effector for bone healing, we can exploit different strategies that rely either on stem or on inflammatory cells to improve bone regeneration 21.
Bone Regeneration Limitations
Although bone remains one of the most‐regenerative tissue, there are still many different clinical conditions that require therapeutic support to improve bone repair 9. The size of the fracture could be so large (i.e., critical size defects) that it results in the physical loss of stem cell niche sites. This type of injury is so severe that even autologous or heterologous grafts would not be sufficient to repair it 22. The ability of bone to regenerate can be also compromised when the complex repair mechanisms become insufficient either at the fracture site or, more frequently, at the systemic level. An essential aspect to bone regeneration is the inflammatory response and, as a consequence, organisms with compromised immune systems have impaired bone regeneration 21. Diabetic, immunocompromised, or elderly patients represent a high percentage of the Western countries' populations and are categories that could experience bone regeneration impairment 21.
Biomimetic Approach in Bone Repair
Bone tissue engineering (BTE) platforms have been proposed as a valid alternative to bone grafts. BTE offers several advantages: reduced disease transmission, lower risk of infection or immunogenicity, as well as implant personalization and limitless availability. Although there have been almost three decades of investigations, only few products have been translated to clinical practice 23. One of the reasons is the complexity of the devices and scaffolds proposed. They serve as a temporary ECM for bone growth and provide specific environment and architectures according to the tissues to be repaired. In addition, they can be combined with drugs such as GFs, bioactive molecules, or antibiotics. The design iterations of a BTE platform have to take into account several variables: (a) the type of materials, (b) the source of chosen materials, (c) the materials' functionalization, and (d) the eventual combination with bioactive molecules/drugs. Moreover, another variable is the possibility to combine the BTE with cells from different sources. All of these iterations are very exciting from an engineering perspective, but they can easily become detrimental when it comes to the regulatory process toward clinical approval.
To speed up the translation process, one strategy could be used to simplify the synthesis and composition of the BTE platform, therefore limiting the associated risks. However, this task is quite difficult to perform. Reducing the variables in the manufacturing and makeup of the device could help, but is it possible to enhance bone regeneration without including cells and osteoinductive factors? In order to achieve efficient bone deposition, the scaffold should be able to support the recruitment, proliferation, and differentiation of infiltrating cells. It should also allow for the formation of new blood vessels throughout the defect, facilitate the deposition on new bone, allow integration with surrounding native tissues, and promote long‐term remodeling of the injured site. This list of requirements is extensive and very complex, but an effective solution could be found in nature. Nature often gives the best and simple solutions to complex problems. Biomimicry (the imitation of nature) has significantly contributed to recent advances in biomedical research. The field of tissue engineering has been populated by biomimetic solutions aiming to restore the shape, composition, and finally function of damaged or lost tissue 24.
Biomimetic approaches in BTE have taken cues from the native bone structure to develop bioactive scaffolds 25. Biomimicry in BTE could be extended to different aspects of bone structure/function including composition, architecture, mechanical properties, and bioactivity.
Mimicking Bone Compositions Through Composite Materials
Human bone is composed of 10%–20% collagen, 60%–70% minerals, 9%–20% water, and small quantities of other proteins, lipids, and polysaccharides 26. Overall, bone ECM is a complex inorganic–organic nanocomposite material, in which hydroxyapatite [HA, Ca10(PO4)6(OH)2] nanocrystallites and a collagen network are organized in a hierarchical structure 27, 28. For this reason many BTE biomaterials are mainly fabricated using this structure as template (Fig. 1). However, the most used strategy that became a design principle in biomimetic BTE is the combination between scaffolds with different GFs 28. Bone morphogenetic proteins, for example, are the most important GFs in bone restoration and have been clinically used to enhance bone healing for over a decade. Nevertheless, the combination of GFs on the scaffold results in a longer regulatory pathway due to the use of molecules with a biological effect. Moreover, the use of GFs sometimes resulted in some drawbacks due to the uncontrolled release kinetics 29, 30.
Figure 1.
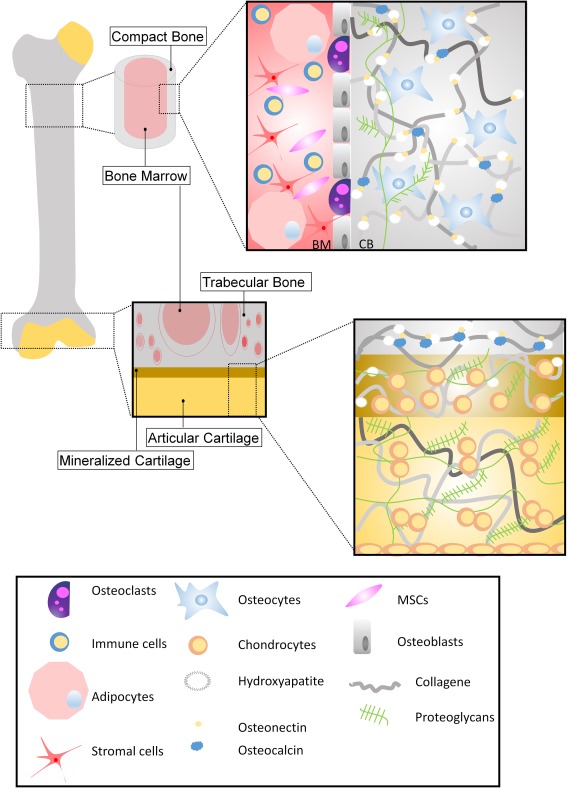
Bone and cartilage structure. The schematic summarizes the architecture of the extracellular matrix (ECM) of bone and cartilage of a long bone. Bone tissue consists mostly of ECM. ECM is composed of osteoid, which represents the organic matrix composed of type I collagen, proteoglycans, and hydroxyapatite, a calcium salt crystal. Cellular components are basically three types of cells: osteoblasts, osteocytes, and osteoclasts. Osteoblasts synthesize matrix and are responsible for its mineralization. They are directly derived from MSCs. Osteocytes are inactive osteoblasts trapped within bone ECM. Osteoclasts are derived from monocytes and they activate bone resorbtion in the continuous remodeling process. Bone marrow is a spongy tissue present in the hollow spaces of bones and consist mainly of hematopoietic, stem, immune, and adipose cells. Articular cartilage is composed of a dense ECM with a bare distribution of chondrocytes. The ECM is principally composed of collagen (type II mostly), and proteoglycans. Articular cartilage is progressively mineralized—like bone matrix—at the junction between cartilage and bone. Abbreviation: MSCs, mesenchymal stem cells.
As previously anticipated, several mineral phase platforms, with similar compositions to native bone, have been shown to be highly bioactive in vivo without the use of GFs 31, 32, 33. More efficient strategies aim toward reproducing both the organic and mineral components of bone ECM 34. Both organic and mineral phases have been chosen over a variety of combinations. For instance, the organic phase could be either proteins (collagen/gelatin/chitosan/silk) or small peptides (collagen‐mimetic), while other approaches utilize synthetic polymers (polylactide‐co‐glycolide (PLGA), polycaprolactone (PCL)). At the same time the mineral phase, represented by hydroxyapatite (HA), could vary depending on ion substitutions. Several BTE strategies induced in vivo bone formation with neither GFs nor stem cells in ectopic sites mostly due to the presence of different HA forms. HAs are often, calcium‐deficient apatites with many di‐ and tri‐valent ion substitutions (e.g., Zn2+, Sr2+, Mg2+, Mn2+, and ions) 35. HA—and its functionalization—was shown to be highly osteoinductive in different animal models, even if the exact mechanisms of osteoinduction are still relatively unknown 36, 37, 38. Zinc substituted HA (Zn2+) has been used in combination with collagen scaffolds to induce bone formation both in vitro and in vivo 39, together with its antibacterial properties seems to be a good candidate for BTE. Strontium (Sr2+) is another common HA substituent with osteogenic potential. Similar to Zn2+, showed very promising results when used in BTE 40, 41. However, magnesium (Mg2+) is one of the most important ion substitutions in an HA lattice, and physiologically present in the early stages of osteogenesis. Several BTE strategies used magnesium substituted HA and showed tremendous osteogenic potential in ectopic site implantations 42, 43 as well as in clinical studies 44.
Other nanostructured biocomposites have been reported to induce bone formation even in the absence of HA. For instance, the group of Gaharwar recently showed that nanostructured hydrogels functionalized with nano‐silicate could be osteogenic in vitro. The presence of nano‐silicate enhances the mechanical properties of the hydrogel together with bone induction 45. Nanostructured materials, in general, have proven to be bone biomimetic. The surface roughness of natural bone is around 32 nm, thus, modification of implant topography introducing nano‐features has demonstrated enchanced bone formation mimicking the cellular environment compared to micro‐features 46. In addition, the mimicking of the hierarchical structure of bone ECM proved to be a potent osteogenic strategy. The composite HA collagen fibrils show a banding period of 67 nm due to the mineral nanocrystals randomly nucleated on collagen gap zones. Several proposed attempts have been successful, including the pioneering work of Stupp, where in 2001 he produced self‐assembling mineralized peptides mimicking bone structure 47. A more recent and refined study by Wang et al., showed nucleation to be completely directed by the collagen template as it happens in the natural process of biomineralization 48. The list of nanomaterials in BTE is very long, and includes nanofibers, nanotubes, nanoparticles, or nanostructured hydrogels, all of which have been proposed as a promising candidate to efficiently replace bone defects 49. However, the nanostructure could also be combined with the macrostructure in order to achieve a better regeneration.
Mimicking Bone Structure
Cell‐conductive porosity and pore interconnectivity are both necessary to support early angiogenesis and tissue infiltration within the scaffold. However, a new dogma in the regeneration of critical size bone defects predicts that the close mimicking of the chemical‐physical and morphological‐structural features of the intact tissue is instrumental in achieving proper bone healing 50. The complex, hierarchically organized structure of bone from the nano‐ to the macro‐scale accounts for its outstanding mechanical performances (e.g., resistance and flexibility associated with lightness) and is necessary for the transfer of the appropriate mechanical stimuli to the bone cells 51. Mimicking bone architecture typically results in mimicking the mechanical features of the bone itself, which is a crucial parameter for in vivo osteoinductivity 52. Furthermore, bone cells and their progenitors are very sensitive to the biomechanical environment, so this aspect is crucial to this type of mimicry 52. As the complete structure of bone is very complex, there are only few strategies that aim to mimic the overall architecture. Some studies exploit three‐dimensional (3D) printing methods to fabricate biomaterials with precisely controlled structures 53. This technology is relatively new in bone repair and it is still far from having the resolution necessary to mimic bone ultrastructure 54. So far, the biomimetic architecture achieved by a 3D printer is too basic in comparison with that of natural bone. Moreover, only synthetic polymers 55 have been proved to be reliable with such technology while no natural materials have been successfully used thus far. However, some attempts have been done using 3D printer BTE constructs based on synthetic polymers (hydroxyapatite and either PCL or PLGA), mimicking the structure of the native bone with excellent results 56. Nonetheless, as there is a huge hope in 3D printing right now, we believe there will be further implementations in the technique that will allow for the use of natural polymers (ECM‐based materials) in order to achieve a more osteoinductive BTE biomaterial 57. To reach this goal, an interesting approach has been developed by Tampieri et al., in which wood was used as a natural template and its chemical composition transformed in mineral phase by a sequence of reactions while maintaining the original micro‐structure of wood 58.
Cartilage Endogenous Healing Potential
Unlike bone, articular cartilage (AC) has a very low endogenous healing capacity and is not able to properly regenerate the lesions due to injury or disease 59. Cartilage tissue, found at the epiphysis of long bones, functions mainly to bear and distribute the loads away from the joints and to reduce the friction during movement 60. AC unique composition and organization allows for compression and deformation, moreover, it confers the resistance to the loads and shear stresses originated within the movements of the musculoskeletal system in motion 61. This tissue is avascular, aneural, and alymphatic, it also has a low cell‐to‐matrix ratio and high water content 62. The minimally abundant chondrocytes produce a rich an ECM formed mainly by collagen type II fibrils, with lesser quantities of types IX and XI, and proteoglycans, mainly aggrecan, which is bound to hyaluronic acid through linker proteins 63.
Mature healthy cartilage ECM is very strictly organized and divided into four zones: superficial zone, middle zone, deep zone, and calcified cartilage. These are all clearly differentiated by their cellular organization, ECM components, and orientation (Fig. 2). The ECM composition can also be divided into three categories depending on its proximity to the cellular chondrocyte: the pericellular matrix, the territorial matrix, and the interterritorial matrix. The particular structure and components of these three territories differs again in collagens and proteoglycans content 63. Softer and proximal to the cells, the pericellular matrix provides cushion to the cells and simultaneously aids their communication with the ECM. Distally to the cells, the matrix composition becomes gradually stiffer with higher collagen content to support the body weight forces 64.
Figure 2.
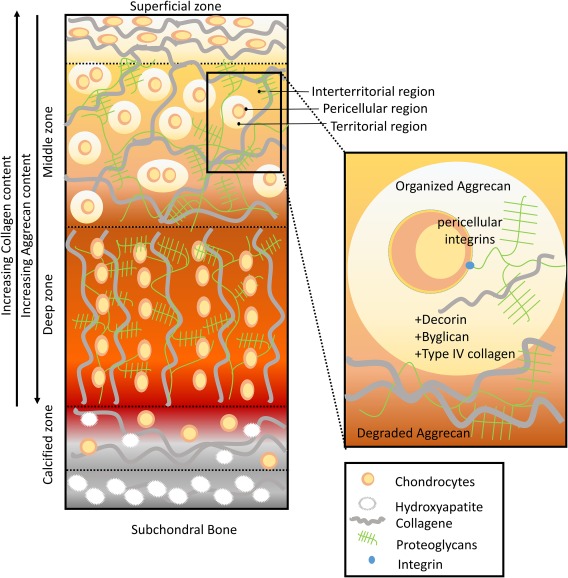
Human articular cartilage. Articular cartilage is divided into four different zones: superficial, middle, deep, and calcified zone. The four zones differ in their collagen, cell orientation, and proteoglycan density. In the superficial zone, collagen fibers are thin and organize themselves parallel to the plane of the articular surface. In the middle zone, the collagen fibers appear more randomly oriented. In the deep zone collagen fibers become thicker and align orthogonally to the superficial zone. At the same time aggrecan density increases from the superficial zone toward the deep zone. Also chondrocyte morphology and density changes depending on the zone. In the superficial zone, chondrocytes are disc‐shaped, aligned parallel to the articular surface as well as the collagen fibers. The middle zone is characterized by randomly oriented spherical cells that could be either isolated or in small clusters. In the deep zone chondrocytes are ellipsoid and aligned in columns. Cartilage's extracellular matrix (ECM) is differentially organized around the chondrocytes in pericellular, territorial, and interterritorial region. These different ECM organizations serve to protect the cells and transmit mechanical signals. The pericellular zone is full of proteoglycans (byglicans, aggrecans). The territorial zone also contains thin collagen fibrils. Instead, the interterritorial matrix constitutes 90% of cartilage volume and contains the largest collagen fibrils.
Cartilage's Stem Cell Niche
Cartilage is a tissue of mesenchymal origin and possesses a stem cell niche first described by Dowthwaite et al. 65. Cartilage chondroprogenitor stem cells (CPSCs) possess trilineage differentiation, self‐renewal, colony formation capacity, and express markers common to MSCs 66. CPSCs have been shown to reside at the surface of the AC and migrate to the full depth of the tissue during development and to site of injury responding to inflammatory signals in conditions such as osteoarthritis 67. The appropriate microenvironmental, physical, and biochemical cues are responsible for the differentiation or maintenance of the stemness phenotype of CPSCs, features exploited for the novel tissue engineering technologies 68.
In order to tune stem cells, it is important to understand the nature of the cartilage stem cell niche under healthy and diseased conditions. The avascular nature of the cartilage tissue requires that the oxygen, nutrients, and regulatory molecules diffuse primarily from the synovial fluid, aided by the load motion that creates extracellular fluid flow in and out of the tissue 69. This feature makes the environment become hypoxic and nutrient‐deprived. Energy is therefore produced mainly through glycolysis, which acidifies the niche with lactic acid accumulation 67. This particular niche changes due to age, injury, and early or late stages of osteoarthritis and other inflammatory conditions. It has been shown how CPSCs migrate and differentiate toward these areas of damaged matrix, responding to the inflammatory signals through pGR4 secretion and proliferation 70.
Limitation in Regeneration
The presence of CPSCs at different repair sites account for tissue regenerative capabilities. Unfortunately, the regeneration is normally inadequate as it lacks the fundamental requirements of successful cartilage healing: ECM composition and organization and bioactivity 71. The anatomist William Hunter first said in 1743: “… ulcerated cartilage… when destroyed, it is never recovered” 72. This dogma has remained true until now because nothing has achieved perfect regeneration, which has rendered prosthetics as the gold standard for treatment of critical size defects, with all their associated complications. The interesting attempts of autograft or allograft have the limitations of creating new defects and producing immunogenicity, increasing the need for research strategies ready to be translated into medical clinical trials 73.
There are three main limitations of cartilage regeneration in comparison to other tissues with much better natural regeneration capacity. First, the cartilage tissue has a low cellular number together with a characteristic faint metabolic activity, exemplified with a low ECM turnover 67. Second, the access to nutrients is limited and molecules only diffuse through synovial fluid. Third, cartilage exists in a biomechanically harsh environment with simultaneous high compression, tensile forces, and friction 74.
Many efforts are needed to overcome these obstacles since we are far from a real solution to ameliorate cartilage repair and its post‐traumatic degenerations 75. Regeneration strategies are currently focused on simultaneously optimizing scaffolds, cells, bioactive molecules, and environmental forces 61, 69, 76, 77. Hence, tissue‐engineering strategies are aimed to develop biomimetic tissues that recapitulate the biological, structural, and functional features of native cartilage, increasing its ability to withstand and adapt to the highly loaded environment of the joint.
Cell Free Biomaterials for Endogenous Potential
Despite the many attempts to solve the challenge of cartilage regeneration, only a scarce amount of studies have advanced to the clinical stage 73. Here, we highlight only the relevant acellular strategies, provided that they offer a greater advantage in comparison to the cellular approaches in terms of handling, time, cost, and regulation 77.
Within this field, aside from overcoming the traditional hurdles of mechanics, integration, and biodegradation, new research studies are trying, with some success, to recruit progenitor cells to repair, direct differentiation, reorganize the ECM, and modulate inflammation 78, 79. To do so, biomimicry of the native cartilage tissue seems to be the appropriate approach 80.
There is a wide variety of synthetic polymeric, polyesters, polyurethanes, and natural biological materials (e.g., collagen, chitosan, fibroins, and hyaluronan) 81 that provide different advantages and disadvantages in terms of use, structure, and functionalization 61. These materials are currently being investigated in the form of hydrogels, meshes, or foam‐like structures (9), alone or in combination 64. They can be physically modified to change their properties with compression, UV treatments, or fiber orientation, and chemically modified with the addition of molecules like proteins, GFs, peptides, or glycosaminoglycans 80, 82, 83, 84, 85, 86. 3D printing is also showing some progress toward aiding the fabrication of scaffolds 87.
Differently, part of the scientific community has been focused on research toward bioinspired and biomimetic cell free nanotherapeutics, used as nanotechnology for drug delivery. This includes technologies such as virosomes, liposomes, and exosomes among others 88. Exosomes, first discovered in 1981 89, are extracellular membrane‐bound vesicles secreted by most cell types. They originate from the formation of multivesicular bodies and are released upon exocytosis 90. Their expression of surface ligands and receptors confers their biological activity 91. Exosomes derived from MSCs have been shown to possess cardioprotective, neuroprotective, and osteoregenerative effects 91, 92, 93. In a rat osteochondral defect model, MSC exosomes promote osteochondral regeneration 94. Moreover, Liu et al. showed promising cartilage regeneration in vivo by fabricating a gelatin hyaluronic acid hydrogel combined with encapsulated stem cell exosome vesicles 95. Despite of these significant results, there still lacks standardized methods to produce, isolate, and purify exosomes in sufficient amounts 90.
Currently, the trend is to use combinatorial technologies to improve the characteristics of a single material. Kim et al. combined hyaluronic acid fibers and poly (ɛ‐caprolactone) fibers with transforming growth factor‐β3 for an increased histological score with higher collagen type II production in a porcine model 96. Immunomodulation has also been achieved using a collagen scaffold combined with resveratrol, an anti‐inflammatory and immunomodulatory drug. Authors demonstrated how this anti‐inflammatory scaffold, once implanted in a rabbit osteochondral region, revealed remarkable anti‐inflammatory and regenerative properties in comparison to an untreated control 97. The group of Elisseeff showed successful prospective in cartilage repair with the use of scaffolds integrated with anti‐inflammatory drugs 98. They implanted 3,4,6‐O‐Bu3GlcNAc‐loaded poly(lactic‐co‐glycolic acid) microfiber scaffolds into rats in order to assess its ability to modulate the inflammatory reaction through b cells. Although the work has not been applied to an osteoarthritic model, there are high hopes that this device could be useful to decrease the inflammatory environment of osteoarthritis and ameliorate cartilage regeneration.
If we widen our scope to find more relevant clinical studies, biphasic osteochondral scaffolds allow for the fabrication of bigger scaffolds, facilitating the fixation of the material, thus having success at the implantation in vivo 99. However, the ultimate goal is to achieve early repair of the cartilage tissue to stop the development of osteoarthritis and any damage that also occurs to the subchondral bone 71.
Clinical Applications of Emerging Cell‐Free Technologies
In this section, we will discuss the present state of clinical utilization of cell‐free biomaterials to address bone and cartilage defects in symptomatic patients. There is a clinical need to regenerate bone to improve and accelerate fracture healing. This technology has implications for routine fractures, non‐unions, and critical‐sized bone defects, any of which may occur following trauma or infection. It is also the foundational component for osteochondral defects where there is loss of both subchondral bone and the overlying AC (i.e., degenerative lesions, such as in osteoarthritis). Defects on the AC are common clinical observation. They have been shown to be present in 63% of symptomatic patients undergoing knee arthroscopy 100. As AC lesions have limited spontaneous healing and have a propensity for progressing to osteoarthritis, addressing lesions of the AC can be particularly challenging.
Clinical Impact of Cell‐Free Approach for Treatment of Bone Defects
The treatment strategies for osseous defects include osseous autograft transplantation, allograft transplantation or augmentation, and cell‐free scaffold implantation. Limited by donor‐site morbidity, autograft transplantation has significant clinical utility for small defects secondary to trauma, degenerative disease (e.g., osteoarthritis), iatrogenic (e.g., total joint arthroplasty revision), or infection. The iliac crest is the most common source for osseous cortical, corticocancellous, and cancellous grafts with high MSC concentration. Local autografts may also be obtained for a variety of fractures (e.g., distal radius for scaphoid non‐union, calcaneus for fifth metatarsal non‐union), arthroplasty (e.g., proximal femoral or acetabular/pelvic bone loss in total hip arthroplasty revision, distal femoral and/or proximal tibial bone loss in total knee arthroplasty revision), degenerative joint disease (e.g., full thickness AC defect with subchondral bone loss requiring restoration of articular surface contour), or infectious problems (e.g., osteomyelitis, septic arthritis) in the extremities. In the U.S., widespread availability of allograft allows osseous transplant or augment for joint‐, whole bone‐, and whole limb‐specific purposes. Disease transmission, cost, immune response, and compromised healing are factors associated with limitations in allograft bone transplant.
There are clear disadvantages to both autograft and allograft cell‐based osseous transplantation for bone defects. This creates a significant clinical need for cell‐free scaffold implantation areas of bone loss. The “holy grail” of bone regeneration and remodeling for any size, location, or chronicity of defects has many general requirements. The product should be an off‐the‐shelf tool, possible inexpensive and moldable. Moreover, the bone scaffold should (a) support early cells' infiltration, (b) avoid the host reaction, and (c) have ability to be completely regenerated by normal host bone (with subsequent remodeling to the correct shape and corticocancellous architecture without over‐ or under‐growth). Unfortunately, no publications exists that have successfully utilized a cell‐free osseous scaffold in human trials with at least a short‐term follow‐up.
Clinical Impact of Cell‐Free Approach for Treatment of Cartilage Defects
The treatment strategies for chondral and osteochondral defects include prosthetics, allograft reconstructions, biodegradable scaffolds, and tissue‐forming cell therapies, or some combination of these. Each has its own advantages and limitations. Despite advances in the field of cartilage repair, orthopedic surgeons report significant challenges to overcome in order to consistently achieve good, long‐term clinical outcomes in patients 101. Prosthetics, such as total knee arthroplasty, can provide significant symptomatic relief, but they also wear over time and are not appropriate in early stages or in younger patients. In countries with advanced tissue banking, such as the U.S., fresh allografts can be used to reconstruct damaged articular surfaces 102. However, they are expensive and have limited global availability, in additions to concerns regarding the potential for immunologic or infectious sequelae 103. Attempts to use allograft tissues in a decellularized manner have resulted in unacceptable failure rates 104.
The “perfect” chondrogenic material should be implantable in a single‐stage procedure that can lead to regeneration of the complex multi‐layered architecture of the osteochondral unit. Moreover, this material should allow for a good incorporation with the native bone and surrounding cartilage, be readily available (“off‐the‐shelf”), and be sufficiently strong to allow for normal mechanical function within the joint (allows for early weight‐bearing) during the process of regeneration. Biomimetic scaffolds can potentially provide a cost‐effective, off‐the‐shelf treatment option that can be manufactured to exact specifications. 3D printed scaffolds may be useful to match the size, depth, and location in a joint in which they are to be used 105. Controversy still exists as to whether cartilage treatments with or without cells is likely to yield better clinical results and there is no study that directly compares the same scaffold with and without the addition of cells 106. Cell‐free treatments have the significant advantage of avoiding cell manipulation and the regulatory hurdles that come with it and rely on the presence of endogenous cells of the native tissues. Some scaffolds are designed to use microfracture as a source for influx of cells. The clinical challenge is how to attract, activate, and direct their differentiation into the desired tissue. The scaffold needs to support or direct their production of matrix and lead to mature tissue that integrates with both the subchondral bone and the surrounding cartilage 107. A single‐stage scaffold implantation that does not require cell‐expansion is ideal from both a regulatory approval process and from a patient acceptance standpoint 108.
There are four primary materials currently used in cartilage scaffolds: protein polymers, carbohydrate polymers, synthetic or artificial polymers, and composites 109, 110. The composites are often constructed in bi‐ or tri‐layered constructs that are tuned to direct cells to form AC on the surface and bone on the deep surface 111 (Table 1). In addition, hydrogels have emerged as a promising scaffold due to their highly tunable mechanical properties and ability to entrap cells or other materials. They also have the potential to be injected into sites of injury 101, 112, 113. The ability to recruit cells and direct their differentiation can be modulated using molecules, such as GFs, either bound to the polymer or released in a controlled fashion 114.
Table 1.
Available scaffolds for articular cartilage repair.
| Biologic scaffolds |
| Protein‐based matrices |
| 1. Collagen |
| 2. Fibrin |
| 3. Gelatin |
| Carbohydrate‐based matrices |
| 1. Hyaluronic Acid |
| 2. Chitosan |
| 3. Agarose and alginate |
| Combinations: Synthetic scaffolds |
| 1. Polylactic Acid |
| 2. Polyglycolic Acid |
| 3. Polylactide‐co‐glycolide |
| 4. Polycaprolactone |
While there are several studies showing clinical success with expanded cells cocultured in a scaffold matrix (e.g., “MACI” Vericel, Cambridge, MA) 109, 115, there are only a few cell‐free scaffolds presently available for clinical or investigational use in humans (Table 2). While initial reports with scaffolds such as the “TrueFit” (Smith & Nephew, Andover, MA) plug showed questionable integration and clinical results 116, others such as “MaioRegen” (FinCeramica, Faenza, Italy) have advanced to a multi‐center clinical study after initial published success 117. Many barriers including cost, regulatory, insurance, and logistical issues still exist in attempting to bring such treatments to clinical practice 118. Table 2 is a summary of cell‐free scaffolds involved in human trials that can be accessed via ClinicalTrials.gov.
Table 2.
Summary of acellular scaffolds listed in ClinicalTrials.gov for treatment of chondral or osteochondral defects in the knee.
| Product name | Company | Composition | Identifier |
|---|---|---|---|
| TruFit | Smith & Nephew, (Andover, MA) | Biphasic poly[d,l‐lactide]/glycolide and calcium sulfate polymer | NCT01246635 |
| Chondromimetic | Orthomimetics (Cambridge, U.K.) | Biphasic scaffold composed of collagen, calcium phosphate, and glycosaminoglycans | NCT01209390 |
| MaioRegen | FinCeramica Faenza S.p.A., (Faenza, Italy) | Tri‐layered scaffold. Type I collagen in the chondral layer, and differing concentrations of collagen and HA in the middle and deep layers | NCT01282034 |
| “BiCRI” BiPhasic Cartilage Repair Implant | Exactech Taiwan Ltd. (Gainsville, FL) | Bi‐phasic scaffold. Unknown composition | NCT01477008 |
| HYTOP | TRB Chemedica AG (Germany) | Bi‐layer bioresorbable matrix. Upper layer purified porcine splint‐skin, and lower layer of collagen fleece containing hyaluronan (HA) | NCT01791062 |
| BioMatrix CRD | Arthrex, (Naples, FL) | Bi‐layer scaffold with a top layer of type I collagen and a subchondral layer composed of β‐Tricalciumphosphate with PLA at the ratio of 80%–20% | NCT02309957 |
| BST‐CarGel | BioSyntech, (Quebec, Canada) | Chitosan‐glycerol phosphate‐based hydrogel scaffold whose active component is a polyglucosamine thrombogenic polysaccharide | NCT02981355 |
| GelrinC | Regentis, (Haifa, Israel) | Hydrogel composed of polyethelyne glycolated fibrinogen which polymerizes upon contact with ultraviolet light | |
| Chondro‐Gide | Geistlich Pharma AG (Wolhusen, Switzerland) | Bi‐layer type I/III collagen membrane | NCT02993510 |
| Agili‐C | CartiHeal Ltd. (Kfar Saba, Israel) | Bi‐phasic implant. The bone phase is composed of calcium carbonate in an aragonite crystalline form, and the cartilage phase is composed of modified aragonite and HA | NCT01471236 |
| CartiFill | Sewon Cellontech, (Seoul, Korea) | Atelocollagen, highly purified porcine derived type I collagen modified by removal of telopeptide | NCT02685917 |
Abbreviations: CRD, Cartilage Repair Device; HyA, hyaluronic acid; PLA, polylactic acid.
Conclusion
Physicians have an immediate clinical need for biomaterials that will help treat patients with AC defects in their weight bearing joints as well as for critical size defects in bone. It is unclear whether acellular or cell‐seeded scaffolds are most likely to be successful. In the authors' opinion, the key in either scenario is to activate those pathways that lead to regeneration, and to do so while blocking the inflammatory and reparative pathways that presently lead to scar formation, failure of integration, and failure to generate native tissue. To that end, it may not be necessary to populate biomaterials with a finite number of specific cells, but rather to provide the surrounding tissues with the appropriate signals to recruit those cells. Either way, it is not just the presence of cells but the ability to direct them into a specific pathway that is going to be required for true regeneration. This makes understanding of these activation pathways one of the critical directions for research. The mechanical properties of the scaffold alone can induce some of these responses. Moreover, multi‐layered composite scaffolds with varied materials, pore sizes, and inductive GFs seem to hold significant promise. Making these scaffolds with sufficient initial stability, strength, and integrity to function under high loads in a weight bearing joint while retaining the ability to bioabsorb over an appropriate time course remains a challenge. The use of 3D printing allows for more complex designs to be developed reproducibly, which will be critical for clinical applicability. There are still many open challenges in musculoskeletal repair; however, tissue engineering aligns well with conventional orthopedic practice in order to improve the final healing. Although the transition of tissue engineering platforms from bench to bedside could be very draining, the multidisciplinary approach involving material scientists, biologists, engineers, and clinicians seems to be a winning strategy in order to speed up the translational process.
Author Contributions
F.T., G.B., P.M., J.H., and E.T.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
F.T. has compensated employment. P.M. has stock options in Orthobullets.com. J.H. is a consultant Smith and Nephew, Ossur, NIA Magellan, Arthroscopy Journal and has research funding from Smith and Nephew. The other authors indicated no potential conflicts of interest.
Acknowledgments
We acknowledge Kelly Hartman and Dr. Chris Tsao for their help in editing this publication. We gratefully acknowledge funding support from the following sources: the Hearst Foundation (Project ID, 18130017), the Cullen Trust for Health Care Foundation (Project ID, 18130014), and the DoD USAMRMC (Project ID, W81XWH‐15‐1‐0718).
References
- 1. Morrison JI, Lööf S, He P et al. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol 2006;172:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santosh N, Windsor LJ, Mahmoudi BS et al. Matrix metalloproteinase expression during blastema formation in regeneration‐competent versus regeneration‐deficient amphibian limbs. Dev Dyn 2011;240:1127–1141. [DOI] [PubMed] [Google Scholar]
- 3. Odelberg SJ. Inducing cellular dedifferentiation: A potential method for enhancing endogenous regeneration in mammals Semin Cell Dev Biol 2002;13:335–343. [DOI] [PubMed] [Google Scholar]
- 4. Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell 2012;10:362–369. [DOI] [PubMed] [Google Scholar]
- 5. Bianco P, Cao X, Frenette PS et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim Biophys Acta 2014;1840:2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nie H, Lee CH, Tan J et al. Musculoskeletal tissue engineering by endogenous stem/progenitor cells. Cell Tissue Res 2012;347:665–676. [DOI] [PubMed] [Google Scholar]
- 9. Grayson WL, Bunnell BA, Martin E et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol 2015;11:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bi Y, Stuelten CH, Kilts T et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem 2005;280:30481–30489. [DOI] [PubMed] [Google Scholar]
- 11. Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J Cell Biochem 1994;56:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krampera M, Pizzolo G, Aprili G et al. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone 2006;39:678–683. [DOI] [PubMed] [Google Scholar]
- 13. Chamberlain G, Fox J, Ashton B et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez‐Moure JS, Corradetti B, Chan P et al. Enhanced osteogenic potential of mesenchymal stem cells from cortical bone: A comparative analysis. Stem Cell Res Ther 2015;6:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corradetti B, Taraballi F, Powell S et al. Osteoprogenitor cells from bone marrow and cortical bone: Understanding how the environment affects their fate. Stem Cells Dev 2015;24:1112–1123. [DOI] [PubMed] [Google Scholar]
- 16. Gates CB, Karthikeyan T, Fu F et al. Regenerative medicine for the musculoskeletal system based on muscle‐derived stem cells. J Am Acad Orthop Surg 2008;16:68–76. [DOI] [PubMed] [Google Scholar]
- 17. Bi Y, Ehirchiou D, Kilts TM et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 2007;13:1219–1227. [DOI] [PubMed] [Google Scholar]
- 18. Hankenson K, Zimmerman G, Marcucio R. Biological perspectives of delayed fracture healing. Injury 2014;45:S8–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998;355S:S7–S21. [DOI] [PubMed] [Google Scholar]
- 20. Einhorn TA, Gerstenfeld LC. Fracture healing: Mechanisms and interventions. Nat Rev Rheumatol 2015;11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loi F, Córdova LA, Pajarinen J et al. Inflammation, fracture and bone repair. Bone 2016;86:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gómez‐Barrena E, Rosset P, Lozano D et al. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015;70:93–101. [DOI] [PubMed] [Google Scholar]
- 23. Hollister SJ, Murphy WL. Scaffold translation: Barriers between concept and clinic. Tissue Eng Part B Rev 2011;17:459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang G. Biomimicry in biomedical research. Organogenesis 2012;8:101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reed EJ, Klumb L, Koobatian M et al. Biomimicry as a route to new materials: What kinds of lessons are useful? Philos Trans R Soc Lond A Math Phys Eng Sci 2009;367:1571–1585. [DOI] [PubMed] [Google Scholar]
- 26. Shekaran A, García AJ. Extracellular matrix‐mimetic adhesive biomaterials for bone repair. J Biomed Mater Res Part A 2011;96A:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou J, Xu C, Wu G et al. In vitro generation of osteochondral differentiation of human marrow mesenchymal stem cells in novel collagen–hydroxyapatite layered scaffolds. Acta Biomater 2011;7:3999–4006. [DOI] [PubMed] [Google Scholar]
- 28. Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 2012;30:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shields LBE, Raque GH, Glassman SD et al. Adverse effects associated with high‐dose recombinant human bone morphogenetic protein‐2 use in anterior cervical spine fusion. Spine 2006;31:542–547. [DOI] [PubMed] [Google Scholar]
- 30. Crawford CH, Carreon LY, McGinnis MD III et al. Perioperative complications of recombinant human bone morphogenetic protein‐2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine 2009;34:1390–1394. [DOI] [PubMed] [Google Scholar]
- 31. Denry I, Kuhn LT. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater 2016;32:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu YX, Thomopoulos S, Birman V et al. Bi‐material attachment through a compliant interfacial system at the tendon‐to‐bone insertion site. Mech Mater 2012;44:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antoniac IV, Albu MG, Antoniac A et al. Collagen–bioceramic smart composites In: Antoniac IV, ed. Handbook of Bioceramics and Biocomposites. Basel, Switzerland: Springer, 2016:301–324. [Google Scholar]
- 34. Pina S, Oliveira JM, Reis RL. Natural‐based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv Mater 2015;27:1143–1169. [DOI] [PubMed] [Google Scholar]
- 35. Farzadi A, Bakhshi F, Solati‐Hashjin M et al. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceram Int 2014;40:6021–6029. [Google Scholar]
- 36. Habibovic P, de Groot K. Osteoinductive biomaterials—properties and relevance in bone repair. J Tissue Eng Regen Med 2007;1:25–32. [DOI] [PubMed] [Google Scholar]
- 37. Minardi S, Corradetti B, Taraballi F et al. Evaluation of the osteoinductive potential of a bio‐inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015;62:128–137. [DOI] [PubMed] [Google Scholar]
- 38. Meagher MJ, Weiss‐Bilka HE, Best ME et al. Acellular hydroxyapatite‐collagen scaffolds support angiogenesis and osteogenic gene expression in an ectopic murine model: Effects of hydroxyapatite volume fraction. J Biomed Mater Res Part A 2016;104:2178–2188. [DOI] [PubMed] [Google Scholar]
- 39. Begam H, Nandi SK, Chanda A et al. Effect of bone morphogenetic protein on Zn‐HAp and Zn‐HAp/collagen composite: A systematic in vivo study. Res Vet Sci 2017;115:1–9. [DOI] [PubMed] [Google Scholar]
- 40. Wong CT, Lu WW, Chan WK et al. In vivo cancellous bone remodeling on a strontium‐containing hydroxyapatite (sr‐HA) bioactive cement. J Biomed Mater Res Part A 2004;68A:513–521. [DOI] [PubMed] [Google Scholar]
- 41. Lei Y, Xu Z, Ke Q et al. Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Mater Sci Eng C 2017;72:134–142. [DOI] [PubMed] [Google Scholar]
- 42. Sartori M, Giavaresi G, Tschon M et al. Long‐term in vivo experimental investigations on magnesium doped hydroxyapatite bone substitutes. J Mater Sci Mater Med 2014;25:1495–1504. [DOI] [PubMed] [Google Scholar]
- 43. Bròdano GB, Giavaresi G, Lolli F et al. Hydroxyapatite‐based biomaterials versus autologous bone graft in spinal fusion: An in vivo animal study. Spine 2014;39:E661–E668. [DOI] [PubMed] [Google Scholar]
- 44. Grigolato R, Pizzi N, Brotto MC et al. Magnesium‐enriched hydroxyapatite as bone filler in an ameloblastoma mandibular defect. Int J Clin Exp Med 2015;8:281. [PMC free article] [PubMed] [Google Scholar]
- 45. Xavier JR, Thakur T, Desai P et al. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth‐factor‐free approach. ACS Nano 2015;9:3109–3118. [DOI] [PubMed] [Google Scholar]
- 46. Chen Z, Bachhuka A, Han S et al. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano 2017;11:4494–4506. [DOI] [PubMed] [Google Scholar]
- 47. Hartgerink JD, Beniash E, Stupp SI. Self‐assembly and mineralization of peptide‐amphiphile nanofibers. Science 2001;294:1684–1688. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Azaïs T, Robin M et al. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat Mater 2012;11:724. [DOI] [PubMed] [Google Scholar]
- 49. Gong T, Xie J, Liao J et al. Nanomaterials and bone regeneration. Bone Res 2015;3:15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Basu J, Ludlow JW. Platform technologies for tubular organ regeneration. Trends Biotechnol 2010;28:526–533. [DOI] [PubMed] [Google Scholar]
- 51. Sprio S, Ruffini A, Valentini F et al. Biomimesis and biomorphic transformations: New concepts applied to bone regeneration. J Biotechnol 2011;156:347–355. [DOI] [PubMed] [Google Scholar]
- 52. Rath B, Nam J, Knobloch TJ et al. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech 2008;41:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inzana JA, Olvera D, Fuller SM et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014;35:4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lantada AD. Microsystems for Enhanced Control of Cell Behavior. Basel, Switzerland: Springer, 2016. [Google Scholar]
- 55. Saijo H, Igawa K, Kanno Y et al. Maxillofacial reconstruction using custom‐made artificial bones fabricated by inkjet printing technology. J Artif Organs 2009;12:200–205. [DOI] [PubMed] [Google Scholar]
- 56. Jakus AE, Rutz AL, Jordan SW et al. Hyperelastic “bone”: A highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med 2016;8:358ra127–358ra127. [DOI] [PubMed] [Google Scholar]
- 57. Cox SC, Thornby JA, Gibbons GJ et al. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater Sci Eng C 2015;47:237–247. [DOI] [PubMed] [Google Scholar]
- 58. Tampieri A, Sprio S, Ruffini A et al. From wood to bone: Multi‐step process to convert wood hierarchical structures into biomimetic hydroxyapatite scaffolds for bone tissue engineering. J Mater Chem 2009;19:4973–4980. [Google Scholar]
- 59. Tan AR, Hung CT. Concise review: Mesenchymal stem cells for functional cartilage tissue engineering: Taking cues from chondrocyte‐based constructs. Stem Cells Translational Medicine 2017;6:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hunziker EB. The elusive path to cartilage regeneration. Adv Mater 2009;21:3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cao Z, Dou C, Dong S. Scaffolding biomaterials for cartilage regeneration. J Nanomater 2014;2014:4. [Google Scholar]
- 62. Embree MC, Chen M, Pylawka S et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat Commun 2016;7:13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Correa D, Lietman SA. Articular cartilage repair: Current needs, methods and research directions Semin Cell Dev Biol 2017;62:67–77. [DOI] [PubMed] [Google Scholar]
- 64. Camarero‐Espinosa S, Rothen‐Rutishauser B, Foster EJ et al. Articular cartilage: From formation to tissue engineering. Biomater Sci 2016;4:734–767. [DOI] [PubMed] [Google Scholar]
- 65. Dowthwaite GP, Bishop JC, Redman SN et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci 2004;117:889–897. [DOI] [PubMed] [Google Scholar]
- 66. Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol 2015;11:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta 2014;1840:3181–3189. [DOI] [PubMed] [Google Scholar]
- 68. Headland SE, Jones HR, Norling LV et al. Neutrophil‐derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med 2015;7:315ra190–315ra190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang Y, Cai Y, Zhang W et al. Human cartilage‐derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Translational Medicine 2016;5:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koelling S, Kruegel J, Irmer M et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009;4:324–335. [DOI] [PubMed] [Google Scholar]
- 71. Makris EA, Gomoll AH, Malizos KN et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 2014;11:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hunter W. Of the structure and diseases of articulating cartilages, by William Hunter, surgeon. Philos Trans 1742;42:514–521. [Google Scholar]
- 73. Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 2015;11:213–222. [DOI] [PubMed] [Google Scholar]
- 74. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science 2012;338:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang Y, Lin H, Tuan RS. Overview: State of the art and future prospectives for cartilage repair In: Grässel S, Aszódi A, eds. Cartilage. Cham, Switzerland: Springer, 2017:1–34. [Google Scholar]
- 76. Gibson JD, O'sullivan MB, Alaee F et al. Regeneration of articular cartilage by human ESC‐derived mesenchymal progenitors treated sequentially with BMP‐2 and Wnt5a. Stem Cells Translational Medicine 2017;6:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen J, Yuan Z, Liu Y et al. Improvement of in vitro three‐dimensional cartilage regeneration by a novel hydrostatic pressure bioreactor. Stem Cells Translational Medicine 2017;6:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burdick JA, Mauck RL, Gorman JH et al. Acellular biomaterials: An evolving alternative to cell‐based therapies. Sci Transl Med 2013;5:176ps4–176ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taraballi F, Corradetti B, Minardi S et al. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J Tissue Eng 2016;7:2041731415624667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Corradetti B, Taraballi F, Minardi S et al. Chondroitin sulfate immobilized on a biomimetic scaffold modulates inflammation while driving chondrogenesis. Stem Cells Translational Medicine 2016;5:670–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Puppi D, Chiellini F, Piras AM et al. Polymeric materials for bone and cartilage repair. Prog Polym Sci 2010;35:403–440. [Google Scholar]
- 82. Russo L, Gautieri A, Raspanti M et al. Carbohydrate‐functionalized collagen matrices: Design and characterization of a novel neoglycosylated biomaterial. Carbohydr Res 2014;389:12–17. [DOI] [PubMed] [Google Scholar]
- 83. Russo L, Battocchio C, Secchi V et al. Thiol–ene mediated neoglycosylation of collagen patches: A preliminary study. Langmuir 2014;30:1336–1342. [DOI] [PubMed] [Google Scholar]
- 84. Cipolla L, Russo L, Taraballi F et al. Smart biomaterials: The contribution of glycoscience. Spec Period Rep SPR Carbohydr Chem 2012;38:416–445. [Google Scholar]
- 85. Taraballi F, Russo L, Battocchio C et al. A model study for tethering of (bio) active molecules to biomaterial surfaces through arginine. Org Biomol Chem 2014;12:4089–4092. [DOI] [PubMed] [Google Scholar]
- 86. Russo L, Gloria A, Russo T et al. Glucosamine grafting on poly (ɛ‐caprolactone): A novel glycated polyester as a substrate for tissue engineering. RSC Adv 2013;3:6286–6289. [Google Scholar]
- 87. Reed S, Lau G, Delattre B et al. Macro‐and micro‐designed chitosan‐alginate scaffold architecture by three‐dimensional printing and directional freezing. Biofabrication 2016;8:015003. [DOI] [PubMed] [Google Scholar]
- 88. Hafner A, Lovrić J, Lakoš GP et al. Nanotherapeutics in the EU: An overview on current state and future directions. Int J Nanomedicine 2014;9:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trams EG, Lauter CJ, Norman Salem J et al. Exfoliation of membrane ecto‐enzymes in the form of micro‐vesicles. Biochim Biophys Acta 1981;645:63–70. [DOI] [PubMed] [Google Scholar]
- 90. Kooijmans SAA, Vader P, van Dommelen SM et al. Exosome mimetics: A novel class of drug delivery systems. Int J Nanomedicine 2012;7:1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Johnsen KB, Gudbergsson JM, Skov MN et al. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 2014;1846:75–87. [DOI] [PubMed] [Google Scholar]
- 92. Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res 2014;114:333–344. [DOI] [PubMed] [Google Scholar]
- 93. Zhang J, Liu X, Li H et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang S, Chu WC, Lai RC et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage 2016;24:2135–2140. [DOI] [PubMed] [Google Scholar]
- 95. Liu X, Yang Y, Li Y et al. Integration of stem cell‐derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017;9:4430–4438. [DOI] [PubMed] [Google Scholar]
- 96. Kim IL, Pfeifer CG, Fisher MB et al. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng Part A 2015;21:2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang W, Sun L, Zhang P et al. An anti‐inflammatory cell‐free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater 2014;10:4983–4995. [DOI] [PubMed] [Google Scholar]
- 98. Kim C, Shores L, Guo Q et al. Electrospun microfiber scaffolds with anti‐inflammatory tributanoylated N‐acetyl‐d‐glucosamine promote cartilage regeneration. Tissue Eng Part A 2016;22:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kon E, Filardo G, Shani J et al. Osteochondral regeneration with a novel aragonite‐hyaluronate biphasic scaffold: Up to 12‐month follow‐up study in a goat model. J Orthop Surg Res 2015;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Curl WW, Krome J, Gordon ES et al. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456–460. [DOI] [PubMed] [Google Scholar]
- 101. Kessler MW, Ackerman G, Dines JS et al. Emerging technologies and fourth generation issues in cartilage repair. Sports Med Arthrosc Rev 2008;16:246–254. [DOI] [PubMed] [Google Scholar]
- 102. McCulloch PC, Kang RW, Sobhy MH et al. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle minimum 2‐year follow‐up. Am J Sports Med 2007;35:411–420. [DOI] [PubMed] [Google Scholar]
- 103. Frank RM, Lee S, Levy D et al. Osteochondral allograft transplantation of the knee: Analysis of failures at 5 years. Am J Sports Med 2017;45:864–874. [DOI] [PubMed] [Google Scholar]
- 104. Farr J, Gracitelli GC, Shah N et al. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med 2016;44:2015–2022. [DOI] [PubMed] [Google Scholar]
- 105. Zhang Z‐Z, Wang SJ, Zhang JY et al. 3D‐printed poly (ɛ‐caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution: A 12‐and 24‐week animal study in a rabbit model. Am J Sports Med 2017;45:1497–1511. [DOI] [PubMed] [Google Scholar]
- 106. Deng Z, Jin J, Zhao J et al. Cartilage defect treatments: With or without cells? Mesenchymal stem cells or chondrocytes? Traditional or matrix‐assisted? A systematic review and meta‐analyses. Stem Cells Int 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stammen JA, Williams S, Ku DN et al. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001;22:799–806. [DOI] [PubMed] [Google Scholar]
- 108. Ko IK, Lee SJ, Atala A et al. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med 2013;45:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Safran MR, Kim H, Zaffagnini S. The use of scaffolds in the management of articular cartilage injury. J Am Acad Orthop Surg 2008;16:306–311. [DOI] [PubMed] [Google Scholar]
- 110. LaPrade RF, Dragoo JL, Koh JL et al. AAOS research symposium updates and consensus: Biologic treatment of orthopaedic injuries. J Am Acad Orthop Surg 2016;24:e62–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Levingstone TJ, Ramesh A, Brady RT et al. Cell‐free multi‐layered collagen‐based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 2016;87:69–81. [DOI] [PubMed] [Google Scholar]
- 112. Vega S, Kwon M, Burdick J. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater 2017;33:59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 2011;17:281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nguyen LH, Kudva AK, Saxena NS et al. Engineering articular cartilage with spatially‐varying matrix composition and mechanical properties from a single stem cell population using a multi‐layered hydrogel. Biomaterials 2011;32:6946–6952. [DOI] [PubMed] [Google Scholar]
- 115. Saris D, Price A, Widuchowski W et al. Matrix‐applied characterized autologous cultured chondrocytes versus microfracture: Two‐year follow‐up of a prospective randomized trial. Am J Sports Med 2014;42:1384–1394. [DOI] [PubMed] [Google Scholar]
- 116. Verhaegen J, Clockaerts S, Van Osch GJVM et al. TruFit plug for repair of osteochondral defects—where is the evidence? Systematic Review of Literature. Cartilage 2015;6:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Di Martino A, Kon E, Perdisa F et al. Surgical treatment of early knee osteoarthritis with a cell‐free osteochondral scaffold: Results at 24 months of follow‐up. Injury 2015;46:S33–S38. [DOI] [PubMed] [Google Scholar]
- 118. Farr J, Gomoll AH. 2016 barriers to cartilage restoration. J Clin Orthop Trauma 2016;7:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]


