Abstract
Purpose
To evaluate dry eye manifestations following photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK) and determine the incidence and predictive factors of chronic dry eye using a set of dry eye criteria.
Setting
Walter Reed Army Medical Center, Washington, DC, USA
Methods
This is a prospective non-randomized clinical study of 143 active duty U.S. Army personnel aged 29.9±5.2 years with myopia or myopic astigmatism (manifest spherical equivalent −3.83±1.96 diopters) undergoing either PRK or LASIK. Dry eye evaluation was performed pre- and postoperatively. Main outcome measures included dry eye manifestations, incidence, and predictive factors of chronic dry eye.
Results
Schirmer scores, corneal sensitivity, ocular surface staining, surface regularity index (SRI), and responses to dry eye questionnaire significantly changed over time after PRK. After LASIK, significant changes were observed in tear breakup time, corneal sensitivity, ocular surface staining, and responses to questionnaire. At twelve months postoperatively, 5.0% of PRK and 0.8% of LASIK participants developed chronic dry eye. Regression analysis showed preoperatively lower Schirmer score will significantly influence development of chronic dry eye after PRK whereas preoperatively lower Schirmer score or higher ocular surface staining score will significantly influence the occurrence of chronic dry eye after LASIK.
Conclusions
Chronic dry eye is uncommon after PRK and LASIK. Ocular surface and tear film characteristics during preoperative examination may help predict chronic dry eye development in PRK and LASIK.
Keywords: dry eye, LASIK, PRK
INTRODUCTION
Corneal refractive surgery is a recognized risk factor for developing dry eye. Dysfunction in the ocular surface-lacrimal gland functional unit contributes to the occurrence of dry eye following photorefractive keratectomy (PRK) and laser in-situ keratomileusis (LASIK). Damage to the corneal afferent nerves during PRK and LASIK disrupts sensory input into the ocular surface lacrimal gland feedback system.1 In PRK, the neural damage occurs at the nerve endings that terminate in the anterior stroma and epithelium. In LASIK, however, nerve damage occurs deeper. When the corneal flap is created in LASIK, the nerves are cut at their trunks where they enter the eye in the peripheral, mid-stromal cornea with additional damage occurring during photoablation.
Available epidemiologic data on refractive surgery-induced dry eye is limited. According to the 2007 International Dry Eye Workshop (DEWS),2 the prevalence of LASIK-induced dry eye in patients without prior dry eye history ranged between 0.25% up to 48%. While a transient ocular surface disease is generally expected in most individuals in the early postoperative period, it is unclear how many individuals without apparent dry eye history develop chronic post-refractive surgery dry eye. The epidemiology subcommittee of the 2007 International DEWS also recognized the need to further investigate risks factors for dry eye after refractive surgery.2
In this study, we evaluated dry eye signs and symptoms following PRK and LASIK with the goal of identifying individuals who develop transient ocular surface disease versus those who progress to chronic dry eye after PRK and LASIK. We also explored if there were any significant preoperative tear and ocular surface findings that could be associated with developing chronic dry eye after refractive surgery.
METHODS
The Institutional Review Board (IRB) at Walter Reed Army Medical Center Department of Clinical Investigation granted approval prior to the initiation of the study. Each participant gave informed consent and all research adhered to the tenets of the Helsinki Declaration. This trial was registered at www.clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00411827). HIPAA compliance was maintained throughout the study.
Active duty U.S. Army participants (n=143), with mean age 29.9±5.2 years at the time surgery, were enrolled in the study. Each participant met the eligibility criteria prior to enrollment. Inclusion criteria included: male or female; of any race; between 21 and 40 years old at the time of preoperative examination; manifest refractive spherical equivalent (MSE) of up to 10.00 diopters (D) at the spectacle plane with refractive cylinder up to 3.00 D; corrected distance visual acuity (CDVA) of 20/20 or better in both eyes; and refractive stability, defined as a change in the spherical or cylindrical component of their refractive error of no more than 0.50D during the twelve-month period preceding the baseline examination. Patients were excluded if they had history or examination findings of preoperative dry eye: Schirmer test (with anesthesia) of 0, subjective complaints or symptoms of dry eye, or findings during the slit lamp exam consistent with dry eye (e.g. superficial punctate keratitis); previous surgery or trauma to the eye; medical condition, concurrent topical or systemic medication that may impair healing; active ophthalmic disease; neovascularization of the cornea within 1mm of the intended ablation zone; clinically significant lens opacity; evidence of glaucoma, keratoconus, corneal irregularity or abnormal videokeratography in either eye; history of recurrent erosions or epithelial basement dystrophy; hypersensitivity or inappropriate response to any of the postoperative medications; and for female participants, positive pregnancy test or intention of getting pregnant during the study period.
Prior to surgery participants underwent a complete ocular history and examination including uncorrected and corrected distance visual acuity (UDVA and CDVA), manifest and cycloplegic refractions, slit lamp biomicroscopy, dilated posterior segment examination, intraocular pressure measurement, computerized corneal topography, corneal pachymetry and dry eye testing, as described in the subsequent section. Before preoperative examinations, soft contact lens wearers were asked to discontinue using their lenses for at least two weeks and hard contact lens wearers at least four weeks.
Two surgeons (K.S.B and R.D.S) performed PRK and LASIK for the study. The PRK technique included epithelial debridement using a rotary brush (Amoils Epithelial Scrubber, Innovative Excimer Solutions, Toronto, Canada), photoabalation of the stroma and prophylactic mitomycin C application (0.1 mg/mL for 30 seconds) to the stromal bed for all cases with ablation depth equal to or greater than 70 microns. The LASIK technique used a superior-hinged, 120 micron thick, 9.0 mm diameter corneal flap created using the Intralase femtosecond laser (Abbott Medical Optics, Sta. Ana, CA). All treatments were wavefront optimized ablations using the WaveLight Allegretto Wave Eye-Q 400 Hz excimer laser system (Alcon Surgical, Ft. Worth, TX).
The postoperative regimen for PRK treated eyes was topical moxifloxacin four times daily for one week; a ten-week course of topical fluorometholone 0.1% ophthalmic solution (four times daily for four weeks, three times daily for two weeks, twice daily for two weeks, then once daily for two weeks); topical ketorolac up to four times daily during the first 48 hours as needed for pain; frequent lubrication with preservative-free artificial tears; and oral vitamin C 500 mg twice a day for three months. A therapeutic contact lens omafilcon A (Proclear, CooperVision, Fairport, NY) was used postoperatively in all eyes after PRK until complete re-epithelialization, typically between four and seven days.
The postoperative medication regimen for LASIK treated eyes was: moxifloxacin hydrochloride 0.5% ophthalmic solution (Vigamox, Alcon Inc, Ft. Worth, TX) four times a day for one week; prednisolone acetate 1% ophthalmic suspension every two hours for 24 hours and then four times a day for one week; topical ketorolac tromethamine 0.4% ophthalmic solution (Acular-LS, Allergan Inc, Irvine, CA) up to four times daily during the first 48 hours as needed for pain; and frequent preservative free artificial tear supplements as needed.
PRK participants followed up on postoperative day one and again on postoperative day four or five to remove the bandage contact lens. If the epithelium was not completely healed, the bandage lens was replaced and the patient was seen every one to two days until complete re-epithelialization. LASIK participants followed up on day one and seven postoperatively. Subsequent visits for both PRK and LASIK were at one, three, six, and twelve months postoperatively. Postoperative exams from these regularly scheduled visits included UDVA, manifest refraction, CDVA and identifying complications, including but not limited to corneal haze, dry eye, corneal infiltrate and steroid response ocular hypertension or glaucoma.
Diagnostic tests for dry eye were performed at baseline preoperatively, and repeated at one, three, six, and twelve months postoperatively.
Psychometric Questionnaire
Participants completed the McMonnies dry eye questionnaire, a previously-validated questionnaire that correlates subjective symptoms of dry eye with clinically evident dry eye disease.3
Computed videokeratoscopy
Corneal topography was performed using the Tomey TMS4 (Nagoya, Japan). The Surface Regularity Index (SRI) was calculated to assess the ocular surface and to objectively evaluate dry eye.4–6
Tear film break up time
The tear film break up time test (TBUT) was used to evaluate the stability of the tear film. A fluorescein strip moistened with balanced salt solution was lightly touched against the inferior tarsal conjunctiva. After blinking several times to distribute the dye throughout the tear film, the participant stared straight ahead without blinking while the cornea was observed under the slit lamp using a cobalt blue filter. The time between a complete blink and the appearance of the first defect in the fluorescein was measured in seconds. The test was repeated five times using a digital chronometer and the average was used. A tear film break up time of 10 seconds or longer was considered normal.
Rose bengal staining
Rose bengal staining was used to assess the damage to the ocular surface epithelia. One drop of 1 % rose bengal was placed in the lower cul-de-sac. Staining of the cornea and conjunctiva was scored on a scale of 0 to 3 (0 = no staining, 1 = mild staining, 2 = moderate staining, 3 = severe staining) for the nasal conjunctiva, temporal conjunctiva, and cornea. A total possible score for one eye was 9. The cut-off value for dry eye was a score of 3 or higher.
Esthesiometry
The Cochet-Bonnet esthesiometer measured the corneal sensitivity. The corneal touch threshold was defined as the mean length of the filament that produced a positive response from a minimum of three stimulus applications. The test was performed with the participant on upright position. Mean filament length was converted to applied pressure (g/mm2) using a conversion table provided by the manufacturer.
Schirmer test
The Schirmer test determined the rate of tear secretion. It measured reflex tearing when performed without anesthesia and basal tear secretion when performed with topical anesthesia (proparacaine 0.5%). Schirmer test strips were inserted in the inferior cul-de-sac for five minutes. Diagnostic cut-off values were wetting of less than 10 mm and 5 mm per five minutes, respectively.
Chronic Dry Eye
A modified Japanese dry eye criteria determined the dry eye status at one, three, six, and twelve months postoperatively (Table 1). The Japanese dry eye criteria referred to the results of Schirmer test without anesthesia whereas the present study utilized scores of Schirmer test with anesthesia.7 Participants with dry eye were treated appropriately with preservative-free artificial tear supplements and/or punctal plug insertion. Silicone plugs were inserted into lower punctum only.
Table 1.
Modified Japanese dry eye criteria.
| Normal | Probable dry eye | Definite Dry Eye |
|---|---|---|
|
|
|
| ||
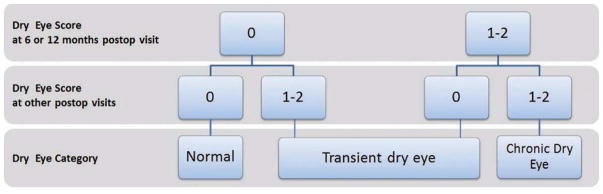
To determine the number of transient and chronic dry eye cases at the conclusion of the study, each eye was graded as follows: normal = 0, probable dry eye = 1 and definite dry eye = 2 and the following definitions were set: normal was defined as a having a score of 0 at all postoperative visits; chronic dry eye was defined as having a score of ≥1 at either six or twelve months plus another score of ≥1 at any postoperative visit; and transient dry eye was defined as those not meeting either criteria (Figure 1). Bilateral involvement of transient and chronic dry eye was also determined.
Figure 1.
Dry eye Categorization.
SPSS for windows version 21.0 (IBM Corp., Armonk, NY) was used for the statistical analysis. The Friedman test was performed to determine whether there was an overall statistically significant difference in dry eye parameters within the twelve-month follow up period. A P-value of <0.05 was considered significant. Post-hoc analysis with the Wilcoxon signed rank test was performed with a Bonferroni correction applied to compare post-surgical signs and symptoms to pre-surgical baseline. The significance level was set at P<0.013 for multiple comparison. Dry eye test results are presented as median and interquartile ranges (IQR). Linear regression analysis was performed to explore any significant associations between preoperative clinical findings, intraoperative application of mitomycin C (in PRK) and the development of chronic dry eye, as defined above.
RESULTS
A total of 143 participants comprised the study population, 73 of which were enrolled in the PRK group and 70 in the LASIK group. There were 34 men and 39 women in the PRK group; and 35 men and 35 women in the LASIK group. Demographic and preoperative refractive characteristics are presented in Table 2. Baseline preoperative dry eye parameters by treatment group are presented in Table 3.
Table 2.
Preoperative baseline characteristics by treatment group.
| PRK (n=146 eyes) | LASIK (n=139 eyes) | |
|---|---|---|
| * Age (years) | 31.0 ±5.2 | 28.8 ±5.1 |
| * Manifest Sphere (diopters, D) | −3.30 ±1.84 | −3.63 ±1.94 |
| * Manifest Cylinder (D) | −0.80 ±0.87 | −0.67 ±0.61 |
| * Manifest spherical equivalent (D) | −3.70 ±1.95 | −3.97 ±1.96 |
| * Ablation depth (microns) | 59.5 ±25.4 | 61.6 ±26.1 |
| Mitomycin C treated (%) | 23.8% | - |
Mean ± standard deviation
Table 3.
Median and interquartile ranges of preoperative dry eye parameters.
| PRK (n=146 eyes) | LASIK (n=139 eyes) | |||
|---|---|---|---|---|
| McMonnies score | 5.0 | (4.0–8.0) | 5.5 | (3.0–8.3) |
| Schirmer without anesthesia (mm) | 25.0 | (20.0–35.0) | 25.0 | (15.0–32.0) |
| Schirmer with anesthesia (mm) | 20.0 | (12.0–30.0) | 16.0 | (10.0–31.0) |
| Corneal sensation (g/mm2) | 0.40 | (0.40) | 0.40 | (0.40) |
| Tear breakup time (seconds) | 12.0 | (9.0–19.0) | 11.0 | (9.0–18.0) |
| Rose bengal staining score | 0 | (0) | 0 | (0) |
| Surface regularity index | 0.13 | (0.07–0.27) | 0.14 | (0.06–0.33) |
| Surface asymmetry index | 0.34 | (0.25–0.46) | 0.32 | (0.24–0.47) |
Tear Film
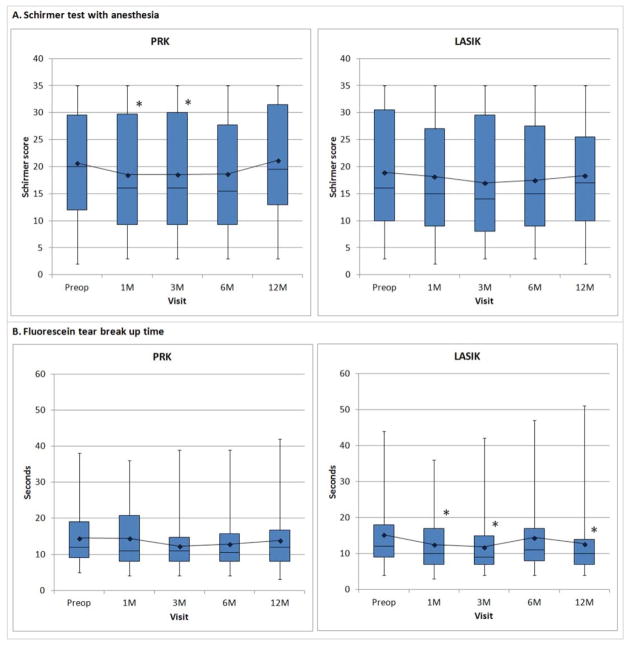
Two indices of the health of the tear film, Schirmer test with anesthesia and TBUT, were measured before and after surgery (Figure 2). There was a statistically significant difference in Schirmer test scores across time in PRK (P<0.001) but not in LASIK (P=0.134). Schirmer test scores were significantly lower only at one month (P=0.003) and three months (P=0.004) after PRK. There was no statistically significant difference in TBUT over time after PRK (P=0.378) however, there was a significant change after LASIK (P<0.001). TBUT was significantly faster at one month, three months and twelve months after LASIK (P<0.001) compared to preoperative baseline.
Figure 2.
Tear film after PRK and LASIK characterized by (A) Schirmer test with anesthesia and (B) tear breakup time after PRK and LASIK. Increased values indicate improvement. Boxplot shows median and interquartile ranges. Line graph shows mean values. Asterisk (*), statistically significant from preoperative value.
Ocular Surface
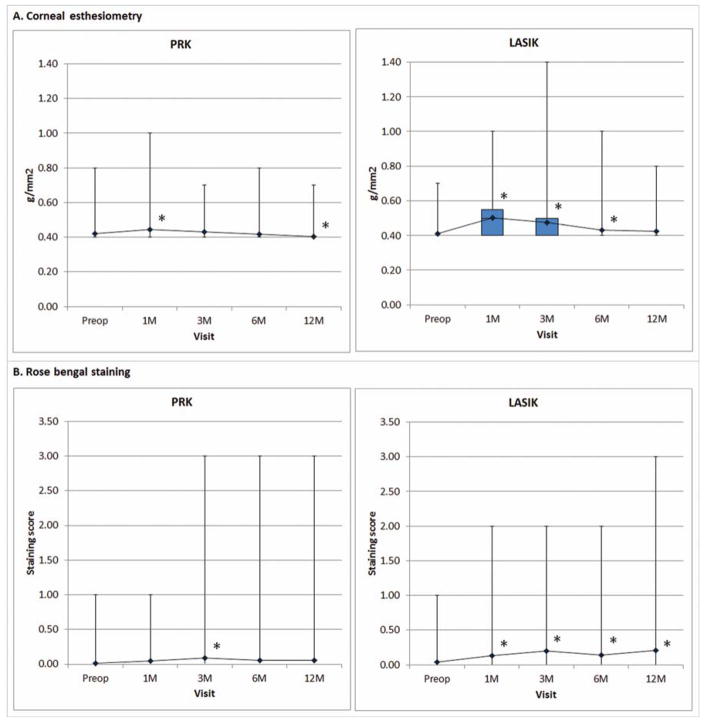
Two parameters of the health of the ocular surface, corneal nerve sensitivity and rose bengal staining, were evaluated before and after surgery (Figure 3). Corneal sensitivity changed significantly over time after either PRK or LASIK (P<0.001). Postoperative corneal sensitivity after PRK was significantly decreased at one month (P=0.010) but significantly increased at twelve months (P=0.007) postoperatively compared to baseline. It also decreased significantly at one month (P<0.001), three months (P<0.001) and six months (P=0.009) after LASIK. A statistically significant change in rose bengal staining scores was seen after either PRK (P=0.044) or LASIK (P=0.002). Post-hoc analysis revealed the postoperative rose bengal staining score was significantly higher only at three months after PRK compared to preoperative score (P=0.004). It was statistically significantly higher than preoperative value at all observed time points after LASIK (one month, P=0.005; three months, P<0.001; six months, P=0.006 and twelve months, P=0.001).
Figure 3.
Ocular surface characterized by (A) corneal esthesiometry and (B) rose bengal staining after PRK and LASIK. Decreased values indicate improvement. Boxplot shows median and interquartile ranges. Line graph shows mean values. Asterisk (*), statistically significant from preoperative value.
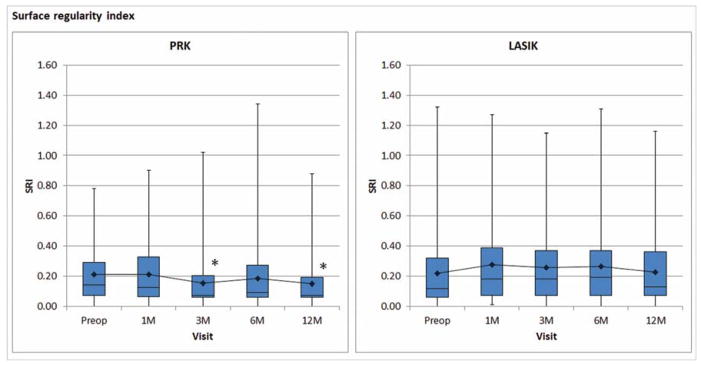
Corneal topography showed a statistically significant change in SRI in PRK (P<0.001) but not in LASIK (P=0.083). Post-hoc analysis showed postoperative SRI in PRK was significantly lower at three months (P=0.003) and twelve months (P=0.002) compared to baseline values (Figure 4).
Figure 4.
Corneal topographic indices (A) surface regularity index and (B) surface asymmetry index after PRK and LASIK. Decreased values indicate improvement. Boxplot shows median and interquartile ranges. Line graph shows mean values. Asterisk (*), statistically significant from preoperative value.
Dry Eye Symptoms
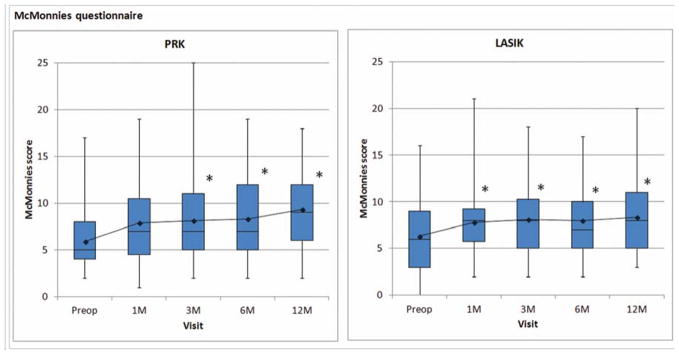
Figures 2–4 focused on the signs of dry eye. Figure 5 demonstrated the changes in the symptoms of dry eye: McMonnies scores significantly changed over time after either PRK (P<0.001) or LASIK (P<0.001). Scores were consistently higher than baseline at each postoperative timepoint in the PRK and LASIK groups. Symptoms of dry eye increased for both procedures and did not recover.
Figure 5.
Reported dry eye symptoms evaluated by McMonnies questionnaire. Decreased values indicate improvement. Boxplot shows median and interquartile ranges. Line graph shows mean values. Asterisk (*), statistically significant from preoperative value.
Chronic dry eye
Using a modified Japanese dry eye criteria, most eyes in the PRK and LASIK groups were classified as normal at all follow up visits. Definitive dry eye was uncommon following either PRK or LASIK (Table 4). Chronic dry eye, by our definition (Figure 1), occurred in 6 out of 120 PRK eyes (5.0%) and in 1 out of 123 LASIK eyes (0.8%). None of the participants had chronic dry eyes bilaterally (Table 5).
Table 4.
Dry eye status at each postoperative visit.
| 1 month | 3 months | 6 months | 12 months | ||
|---|---|---|---|---|---|
| PRK | Normal | 140 (95.9%) | 133 (95.0%) | 128 (95.5%) | 115 (91.3%) |
| Probable dry eye | 6 (4.1%) | 7 (5.0%) | 6 (4.5%) | 11 (8.7%) | |
| Definite dry eye | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| LASIK | Normal | 129 (92.8%) | 130 (94.9%) | 127 (96.9%) | 126 (99.2%) |
| Probable dry eye | 9 (6.5%) | 7 (5.1%) | 4 (3.1%) | 0 (0%) | |
| Definite dry eye | 1 (0.7%) | 0 (0%) | 0 (0%) | 1 (0.8%) |
Table 5.
Chronic dry eye cases after PRK and LASIK.
| Normal | Dry Eye | ||
|---|---|---|---|
|
| |||
| Transient | Chronic | ||
| PRK | 100 (83.3%) | 14 (11.7%) | 6 (5.0%) |
| Bilateral involvement | 3 (5.0%) | 0 (0%) | |
|
| |||
| LASIK | 105 (85.4%) | 17 (13.8%) | 1 (0.8%) |
| Bilateral involvement | 3 (4.9%) | 0 (0%) | |
Predictive factors for chronic dry eye
Preoperative findings in Schirmer test with anesthesia, TBUT, corneal esthesiometry, rose bengal staining and SRI were selected as predictors for chronic dry eye in PRK and LASIK. Correlation analysis revealed chronic dry eye in PRK is negatively correlated to preoperative Schirmer score (r=−0.235, P=0.005) and TBUT (r=−0.0.204, P=0.013) but positively correlated to preoperative SRI (r=0.167, P=0.035) whereas chronic dry eye in LASIK is negatively correlated with preoperative Schirmer score (r=−0.278, P=0.001) but positively correlated rose bengal staining score (r=0.243, P=0.003). Linear regression analysis showed the selected preoperative variables significantly accounted for the occurrence of chronic dry eye in PRK (R2=0.107, P=0.023) and in LASIK (R2=0.117, P=0.011). Among the selected variables, only Schirmer score in PRK and Schirmer and rose bengal staining scores in LASIK had significant regression weights (Table 6). There was no significant association between chronic dry eye and intraoperative use of mitomycin-C in PRK (R=0.94, P=0.308).
Table 6.
Predictive modeling for chronic dry eye following PRK and LASIK.
| Dependent Variable | Independent Variable | P-value | R2 |
|---|---|---|---|
| Chronic dry eye (PRK) | Preoperative Schirmer with anesthesia | 0.041* | 0.107* |
| Preoperative TBUT | 0.159 | ||
| Preoperative rose bengal staining score | 0.409 | ||
| Preoperative corneal sensitivity | 0.403 | ||
| Preoperative surface regularity index | 0.135 | ||
| Intraoperative mitomycin-C | 0.308 | 0.009 | |
|
| |||
| Chronic dry eye (LASIK) | Preoperative Schirmer with anesthesia | 0.014* | |
| Preoperative TBUT | 0.821 | 0.117* | |
| Preoperative rose bengal staining score | 0.048* | ||
| Preoperative corneal sensitivity | 0.351 | ||
| Preoperative surface regularity index | 0.778 | ||
P-value<0.05
DISCUSSION
Corneal denervation during PRK and LASIK impairs corneal sensation and feedback to the lacrimal gland causing a spectrum of ocular surface conditions associated with reduced tear production and secretion, tear film instability, corneal and conjunctival epitheliopathy and dry eye symptoms.1,8 The pattern of corneal nerve damage and recovery are different between PRK and LASIK9,10 thus ocular surface manifestations following the surgeries may differ as well. In this study, we found several significant changes in the tear film and ocular surface health after PRK and LASIK.
In PRK, corneal sensation significantly decreased in the first month after surgery before recovering to preoperative status at three months postoperatively. Schirmer scores significantly decreased only in the first three months, while ocular surface staining scores significantly increased at three months postoperatively. Improvement in corneal surface regularity was also observed starting at three months postoperatively. TBUT did not change significantly over time. At twelve months following PRK, a small but significant increase in corneal sensitivity was observed. Early recovery of corneal sensation may be indicative of a more rapid corneal nerve regeneration after PRK than after other types of corneal damage.11.12 This may also have prompted subsequent recovery of tear secretion thus limiting significant ocular surface epitheliopathy in the early postoperative period. Interestingly, there was a small but statistically significant increase in corneal sensitivity at twelve months following PRK. Although corneal hypersensitivity after PRK has been described previously,13 the current finding should be carefully interpreted as the difference between preoperative and twelve month postoperative values was very narrow (Figure 2A).
In LASIK, our findings supported previous reports of corneal sensitivity returning in LASIK at a later postoperative period than for PRK.14,15 We did not find a significant decrease in Schirmer test score despite a significant decrease in TBUT and increase in ocular surface staining score. These findings were suggestive of a qualitative change in tear film in LASIK.16 The compromised tear film stability as measured by TBUT in LASIK may be due to suppression of lacrimal gland fluid and conjunctival goblet cell mucin secretion by corneal nerve injury upon LASIK flap creation17,18 and the loss of conjunctival goblet cells from the application of the suction ring.19 Reduced blink rate and decreased release of neurotrophic factors may contribute to an alteration in the state of the ocular surface.16 Moreover, changes in corneal curvature postoperatively can also result in the abnormal distribution of tears during blinking.20
Patient-reported symptoms in both groups were found to be higher than baseline values up to twelve months postoperatively despite not having prior history or clinical findings of dry eye. The symptoms seemed to correlate with the postoperative clinical findings in LASIK but not in PRK since most clinical findings were improved to preoperative levels at six months following PRK. Symptoms of dry eye may be perceived while damaged corneal nerves regenerate. Healing nerves may have increased activity and activated corneal sensory nerves may produce irritation, stinging, and burning sensations.21 Although we found a small but significant increase in corneal sensitivity at twelve months after PRK, it is speculative at this point if this could be associated with the increased symptomatic findings at twelve months post-PRK. The aberrant firing and increased spontaneous activity of the regenerating corneal nerves were not detectable by the methods used in our study.
Although refractive surgery-related dry eye is well-studied, the incidence of chronic dry eye after refractive surgery is still unclear. Inconsistencies in published reports may be attributed to varying methodologies used in the investigations. Moreover, this may also be partly because dry eye has been defined in different ways both in literature and in practice. Some put emphasis more on symptoms while others focus more on objective findings. Previous reports on the incidence of dry eye after PRK and LASIK were based on one or two dry eye markers such as dry eye symptoms,22 corneal staining,4,23 or both.24
The present investigation applied a modified Japanese dry eye diagnostic criteria to determine the presence of dry eye after refractive surgery. The Japanese criteria described performing Schirmer test without anesthesia7 but the current study performed the test with anesthesia. Both techniques are commonly performed and accepted to assess dry eye, however, Schirmer test without anesthesia may give high false negative rate and therefore may be less reliable.25,26
The scoring system (normal = 0, probable dry eye = 1 and definite dry eye = 2) determined the dry eye status at every postoperative visit and subsequently differentiated those with transient post-surgical recovery versus chronic dry eye. Obtaining a score of ≥1 at either six or twelve months plus another score of ≥1 at any postoperative visit was considered chronic dry eye. The six month cut-off was selected as it was generally expected that most dry eye parameters return to baseline at six months postoperatively.23
By our dry eye definition, only a small percentage of eyes without a history of dry eye develop the disease after either PRK or LASIK Moreover, we found chronic dry eye was uncommon as the PRK group had 6 cases (5.0%) whereas the LASIK group had only one case (0.8%). The current findings were relatively lower than what was previously published. In a retrospective study, Shoja and associates23 reported 20% LASIK patients had chronic dry eye persisting six months or more after surgery. The discrepancy between the results could be attributed to differences in chronic dry eye definition and study population. Our study rates may be lower than previously reported due to relatively younger study population.
Correlation analysis indicated those with preoperatively lower Schirmer score, lower TBUT and higher SRI tend to develop chronic dry eye after PRK whereas those with preoperatively lower Schirmer score and higher rose bengal staining score tend to develop chronic dry eye after LASIK. Moreover, the regression model revealed that the selected preoperative findings influenced the development of chronic dry eye after PRK and after LASIK. In PRK, the selected variables only contributed to 10.7% of the prediction whereas in LASIK, the selected variables contributed to 11.7% of the prediction, suggesting there were other factors influencing post-refractive surgery chronic dry eye progression. The analysis also suggested signs of quantitative tear disturbance preoperatively such as lower Schirmer test score was expected to significantly contribute to chronic dry eye progression after either PRK or LASIK. Additionally, signs of epithelial damage preoperatively, such as higher staining score, significantly contribute to the development of chronic dry eye after LASIK. Although intraoperative use of mitomycin-C could potentially induce postoperative dry eye,27 our analysis did not show any significant association between mitomycin-C application and chronic dry eye in PRK.
To the best of our knowledge, this was the first prospective study to report the incidence of chronic dry eye after refractive surgery. The study design, robust sample size with high follow up rates up to twelve months postoperatively (82.2% PRK and 81.1% LASIK) are the main strengths of the investigation. The study was non-randomized because participants were allowed to select their preferred surgery thus no direct comparison between PRK and LASIK groups was performed. Dry eye was managed accordingly for the duration of the study, and thus could potentially underestimate the results reported. Nonetheless, this study may offer some insights on the occurrence of chronic dry eye, despite ample dry eye treatment, following two commonly performed laser refractive surgeries.
In conclusion, our study demonstrated that individuals without apparent dry eye were likely to have signs and symptoms of dry eye after either PRK or LASIK. A small percentage of these individuals would go on to develop chronic dry eye but a greater majority would experience it transiently and recover. Ocular surface and tear film abnormalities preoperatively could influence chronic dry eye progression after surgery.
WHAT WAS KNOWN
Dry eye is one of the most commonly reported complications of laser refractive surgery.
While dry eye is generally expected to occur transiently in the early postoperative period, it may also develop into a chronic condition.
The prevalence of LASIK-induced dry eye ranges between 0.25% and 48% but the specific incidence of chronic dry eye following PRK or LASIK has yet to be reported.
WHAT THIS PAPER ADDS
Changes in the ocular surface and tear film induced by PRK and LASIK were described and pre-defined criteria were used to identify chronic dry eye cases after PRK and LASIK.
We found chronic dry eye is uncommon following PRK and LASIK. Signs of quantitative tear film abnormality and ocular surface damage preoperatively could influence chronic dry eye development after either PRK or LASIK.
Intraoperative use of mitomycin-C is not associated with the development of chronic dry eye in PRK.
Acknowledgments
Financial support: CDMRP W81XWH-04-2-008
Footnotes
Publication and presentations: Portions of this material were published in Ophthalmology: Contreras-Ruiz L, Ryan DS, Sia RK, Bower KS, Dartt DA, Masli S. Polymorphism in THBS-1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology. 2014 Jul;121(7):1389–97. Epub 2014 Mar 27.
Portions of this material were presented as posters at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting in Ft. Lauderdale, Florida in May 2009 (Program # 574), 2010 (Program # 1920, # 2847, and # 3364) and 2012 (Program #1484), at the Military Health Research Symposium in Ft. Lauderdale, Florida in August 2012 and at the Tear Film & Ocular Surface Society Conference Florence, Italy Sept 22–25, 2010 and as presentations at ARVO 2009 (Program # 2547) and at the 4th Annual International Military Refractive Surgery Symposium. January 2010, San Antonio, Texas. The material has not otherwise been presented or published.
Proprietary interest: The authors have no financial interest in any product, drug, instrument, or equipment discussed in this manuscript.
Disclaimer The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense or U.S. Government.
References
- 1.Ang RT, Dartt DA, Tsubota K. Dry eye after refractive surgery. Curr Opin Ophthalmol. 2001;12(4):318–22. doi: 10.1097/00055735-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007 Apr;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 3.Nichols KK, Nichols JJ, Mitchell GL. The reliability and validity of McMonnies Dry Eye Index. Cornea. 2004 May;23(4):365–71. doi: 10.1097/00003226-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 4.De Paiva CS, Chen Z, Koch DD, Hamill MB, Manuel FK, Hassan SS, Wilhelmus KR, Pflugfelder SC. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006 Mar;141(3):438–45. doi: 10.1016/j.ajo.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.De Paiva CS, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology. 2003;110(6):1102–9. doi: 10.1016/s0161-6420(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Pflugfelder SC. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999 May;106(5):939–43. doi: 10.1016/S0161-6420(99)00513-8. [DOI] [PubMed] [Google Scholar]
- 7.Uchino Y, Uchino M, Dogru M, Ward S, Yokoi N, Tsubota K. Changes in dry eye diagnostic status following implementation of revised Japanese dry eye diagnostic criteria. Jpn J Ophthalmol. 2012 Jan;56(1):8–13. doi: 10.1007/s10384-011-0099-y. [DOI] [PubMed] [Google Scholar]
- 8.Quinto GG, Camacho W, Behrens A. Postrefractive surgery dry eye. Curr Opin Ophthalmol. 2008 Jul;19(4):335–41. doi: 10.1097/ICU.0b013e3283009ef8. [DOI] [PubMed] [Google Scholar]
- 9.Linna T, Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997;16:640–9. doi: 10.1076/ceyr.16.7.640.5058. [DOI] [PubMed] [Google Scholar]
- 10.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005 Dec;140(6):1059–64. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, Kumano Y, Zushi I, Yamada T, Matsui T, Nishida T. Corneal sensation after correction of myopia by photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2001 Mar;27(3):370–3. doi: 10.1016/s0886-3350(00)00756-2. [DOI] [PubMed] [Google Scholar]
- 12.Trabucchi G, Brancato R, Verdi M, Carones F, Sala C. Corneal nerve damage and regeneration after excimer laser photokeratectomy in rabbit eyes. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):229–35. [PubMed] [Google Scholar]
- 13.Ishikawa T, del Cerro M, Liang FQ, Kim JC, Aquavella JV. Hypersensitivity following excimer laser ablation through the corneal epithelium. Refract Corneal Surg. 1992 Nov-Dec;8(6):466–74. [PubMed] [Google Scholar]
- 14.Kumano Y, Matsui H, Zushi I, Mawatari A, Matsui T, Nishida T, Miyazaki M. Recovery of corneal sensation after myopic correction by laser in situ keratomileusis with a nasal or superior hinge. J Cataract Refract Surg. 2003 Apr;29(4):757–61. doi: 10.1016/s0886-3350(02)01840-0. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Santonja JJ, Sakla HF, Cardona C, Chipont E, Alió JL. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999 May;127(5):497–504. doi: 10.1016/s0002-9394(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 16.Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. 2010 Jul;8(3):135–45. doi: 10.1016/s1542-0124(12)70224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toda I, Asano-Kato N, Komai-Hori Y, Tsubota K. Dry eye after laser in situ keratomileusis. Am J Ophthalmol. 2001;132:1–7. doi: 10.1016/s0002-9394(01)00959-x. [DOI] [PubMed] [Google Scholar]
- 18.Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, Dartt DA. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008 Jan;49(1):168–74. doi: 10.1167/iovs.07-0337. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Prats JL, Hamdi IM, Rodriguez AE, Galal A, Alio JL. Effect of suction ring application during LASIK on goblet cell density. J Refract Surg. 2007 Jun;23(6):559–62. doi: 10.3928/1081-597X-20070601-04. [DOI] [PubMed] [Google Scholar]
- 20.Lee JB, Ryu CH, Kim J, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2000 Sep;26(9):1326–31. doi: 10.1016/s0886-3350(00)00566-6. [DOI] [PubMed] [Google Scholar]
- 21.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27(4):577–84. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 23.Shoja MR, Besharati MR. Dry eye after LASIK for myopia: Incidence and risk factors. Eur J Ophthalmol. 2007 Jan-Feb;17(1):1–6. doi: 10.1177/112067210701700101. [DOI] [PubMed] [Google Scholar]
- 24.Salomão MQ, Ambrósio R, Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: Mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009 Oct;35(10):1756–60. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Deng XG, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5(4):478–81. doi: 10.3980/j.issn.2222-3959.2012.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols KK, Mitchell GL, Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3):272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kymionis GD, Tsiklis NS, Ginis H, Diakonis VF, Pallikaris I. Dry eye after photorefractive keratectomy with adjuvant mitomycin C. J Refract Surg. 2006 May;22(5):511–3. doi: 10.3928/1081-597X-20060501-16. [DOI] [PubMed] [Google Scholar]