Abstract
Tumors are composed of different types of cancer cells that contribute to tumor heterogeneity. Among these populations of cells, cancer stem cells (CSCs) play an important role in cancer initiation and progression. Like their stem cells counterpart, CSCs are also characterized by self‐renewal and the capacity to differentiate. A particular population of CSCs is constituted by mesenchymal stem cells (MSCs) that differentiate into cells of mesodermal characteristics. Several studies have reported the potential pro‐or anti‐tumorigenic influence of MSCs on tumor initiation and progression. In fact, MSCs are recruited to the site of wound healing to repair damaged tissues, an event that is also associated with tumorigenesis. In other cases, resident or migrating MSCs can favor tumor angiogenesis and increase tumor aggressiveness. This interplay between MSCs and cancer cells is fundamental for cancerogenesis, progression, and metastasis. Therefore, an interesting topic is the relationship between cancer cells, CSCs, and MSCs, since contrasting reports about their respective influences have been reported. In this review, we discuss recent findings related to conflicting results on the influence of normal and CSCs in cancer development. The understanding of the role of MSCs in cancer is also important in cancer management. Stem Cells Translational Medicine 2017;6:2115–2125
Keywords: Mesenchymal stem cells, Cancer progression, Microenvironment, Epithelial to mesenchymal transition, Drug resistance
Significance Statement.
There is no doubt that mesenchymal stem cells (MSCs) can have strong effects on the outcome of tumor development and progression. The reasons by which the effects have been seen as suppressive or stimulating of cancerogenesis, also remain controversial. MSCs may act on all phases of carcinogenesis such as the generation of cancer stem cells (CSCs), epithelial‐to‐mesenchymal transition (EMT), angiogenesis, drug resistance, and metastasis. On the other hand, there are several studies that reported suppressive effects of MSCs on cancer cells. The discrepancy between these results may arise from issues that are related to tissues origin, individual genetic variability of patients, and cancer typology. Moreover, it is important to consider also the experimental variability due to different cancer cell lines used, MSCs origin, and different models of CSCs. Thus, clarifying the key role of MSCs in cancer development, or determining their potential use in cancer treatment, appears to be challenging. In this regard, in depth knowledge of key factors or mechanisms that control the pro‐ or anticancer effects of MSCs on cancer progression will certainly provide answers to the above questions. In addition, it is important to evaluate the significance of resident MSCs in cancer. In summary, to achieve a better treatment of patients, future clinical approaches will need to use strategies that inhibit or modulate the dialog between MSCs and cancer cells.
Introduction: Stem Cells and Cancer Stem Cells
What Are Stem Cells and Mesenchymal Stem Cells?
Stem cells are characterized by the capacity to self‐renew and to generate differentiated progenies. The regulation of these processes is fundamental for the maintenance of the stem cell pool within a tissue 1. Cells capable to differentiate into mesodermal‐derived tissues, such as adipocytes, chondrocytes, and osteoblasts, are called mesenchymal stem cells (MSCs) and they are suggested to reside in all human organs and tissues 2. Several studies report also that MSC can circulate in the peripheral blood 3 and are detected in tissues other than bone marrow, such as subcutaneous fat (adipose stem cells [ASCs]) 4, 5, periodontal ligament 6, umbilical cord blood 7, fetal tissues 8, lymph nodes 9, and adult spleen and thymus 10, thus hypothesizing a “mesenchymal organization,” virtually present in all post‐natal organs and tissues 11. Some reports describe that MSCs can also differentiate in non‐mesodermal cell types, such as gut and skin epithelial cells, hepatocytes, pneumocytes, and neuronals 12, 13, 14, 15. However, there is a lack of accuracy regarding to both terminology and biological characteristics. Many authors state that MSCs are considered different from so‐called multipotent adult progenitor cells that are able to differentiate into neurons, epithelial cells, as well as in cells of mesenchymal origin 12. Another typology of stem cells, different from MSCs, are multipotent mesenchymal stromal cells from which derive only cells belonging to mesodermal tissues, such as fat, muscle, bone, and cartilage cells 16. Such differences both in terminology and biological characteristics home probably in the variability of experimental methodologies, rather than in the existence of different stem cells of mesenchymal origin, although it is possible to hypothesize that it can exist a gradient of MSC differentiation as well as demonstrated for hematopoietic stem cell precursors. MSCs are rare with 1/105 cells in bone marrow and lose their differentiation potential after 40 doublings 17. These cells are also able to migrate from the circulation to different tissues in response to a variety of signals. This process is called “homing” and is regulated by a specific pattern of chemokines and chemokine's receptors 2. Indeed, MSCs are recruited to the site of wound healing to repair injured tissues in a similar process than the one found in tumors. On the other hand, inflammation contributes to tumourigenesis and metastasis through the homing process. In this contest, it is also important to consider the role of immune cells, including macrophages, lymphocytes, monocytes, and dendritic cells. In fact, the immune system has both stimulatory and inhibitory effects on tumor initiation, development, and metastasis formation, and the balance of these responsiveness can depend on the different tumor microenvironments 18. Thus, the interplay among epithelial cancer and stromal cells and immune compartment is fundamental for cancerogenesis, progression, and metastasis, and may lead to a different biological behavior of tumor cells during the cancer progression 19.
What Are ASCs?
An interesting source of MSCs is the adipose tissue that originates adipose‐derived mesenchymal stem cells (ASCs). These cells are similar to bone marrow‐derived MSCs, are multipotent and are capable to differentiate into mesenchymal lineages 19. Nevertheless, there are little differences in their immunophenotype, differentiation ability, proteome, immunomodulatory properties, and transcriptome. Some differences about specific characteristics of MSCs and ASCs, other than intrinsic heterogeneity, can be due to different isolation and culture experimental methodologies. ASCs are more stable in long‐term cultures, show a major viability and minor senescence, have a higher proliferation ability and maintain a high rate of differentiation in long‐term culture with respect to MSCs 20, 21. Moreover, it has been demonstrated that ASCs sustain hematopoiesis with major efficacy compared to MSCs 21. Regarding to immunophenotype, ASCs express the CD34 marker decreasing during differentiation as well as CD54 and CD49d. MSCs do not express CD34, show low expression of CD54 and CD49d 22, 23, 24 (Supporting Information Table S1). Moreover, the same ASCs can be different on the basis of their origin (visceral vs. subcutaneous fat). Some studies reported that subcutaneous ASCs are different from visceral ASCs in terms of morphology, and multipotent differentiation potential 25 and, consequently, have a different biological behavior with cancer cells. Subcutaneous ASCs show a fibroblast like shape and are able to home to cancer cells, while visceral ASCs are similar to epithelial cells and more able to differentiate. Then, visceral ASCs are more able to proliferate, induce epithelial to mesenchymal transition activating the PI3K/AKT signaling, improve migration, and invasion of breast cancer cells secreting IL‐6 and IL‐8 compared to subcutaneous ASCs 25. This is important in delineating the correlation between ASCs origin and cancer progression.
What Are Cancer Stem Cells?
Observations dating back to more than 50 years have evidenced similarities between cancer and embryonic development and that led to the hypothesis of the existence of cancer stem cells (CSCs). Recent growing evidences suggest that the tumor is composed of heterogeneous populations of cells with different levels of malignity and the tumor development is driven by a specialized cell subset, characterized by self‐renewing, multi‐potent, and tumor‐initiating properties 26 (Supporting Information Table S2). These malignant cells are called CSCs and their maintenance is tightly ensured by the microenvironment and the stroma. They are probably generated from normal stem or precursor cells within tissues after mutations occur and are typically resistant to conventional treatments 26, 27 (Fig. 1). This model has been studied and demonstrated especially for hematological diseases. For solid tumors, it is very difficult to establish a detailed tumor hierarchy due to the loss of specific markers for CSCs. Many of markers used to define the CSCs of solid tumors fail in selecting this subpopulation. For example, CD133 marker has been the first marker used for isolating CSCs from colon carcinoma. Recently, some studies reported that metastatic colon carcinoma cells negative to CD133 are able to recapitulate the tumor as well as those positive for CD133 28, 29. Then, Brabletz et al. 30 have demonstrated that in colon cancer there are two stem subpopulations: one, able to initiate the tumor, defined “resident cancer stem cells” subset and another, able to propagate the tumor forming metastasis defined “migrating stem cells” subpopulation. Thus, several studies demonstrated that within of CSCs could exist a specific hierarchy 26, 27, 28, 29, 30. Consequently and on the basis of these uncertainties, a different terminology in defining CSCs is used as “tumor initiating cells” or “tumor propagating cells.” Moreover, it has been hypothesized that there is an alternative, but not exclusive, model of carcinogenesis based on the fact that the tumor could be made up of different heterogeneous clones of tumor cells with different mutational profiles that represent different phases of tumourigenesis 31. This model takes in consideration the effect of microenvironment on each clone in terms of growth and development. On the other hand, late relapse of cancer provides a direct confirmation for the existence and the persistence of tumor initiating cells at a subclinical level 32. This is defined cancer dormancy. In this context, Zimmerlin et al. 32 reported that MSCs signals have different effects on dormant and resting tumor initiating breast cancer cells. In particular, they demonstrated that ASCs enhance the growth of active, but not resting cells. However, the CSC's model still is a controversial theme and object of debate. In these scenarios, the CSCs field needs to be deeply explored for understanding the carcinogenesis and address new therapeutic strategies. ASCs have been used as tissue repair promoters and in several clinical fields, including cardiac, orthopedic, plastic, bone, and breast surgery 33, 34, 35. They have been identified as optimal and potential candidates for tissue reconstruction in patients with oncological history. Delay et al. 36 reported a cohort of 880 breast reconstruction using autologous fat. This cohort included also patients with history of breast cancer and no cancer development was detectable with a follow up of 10 years 36. The same results were reported by other studies 37, 38, 39. However, despite optimal esthetic results, some reports have suggested that MSCs could promote cancer recurrence 19, 40, 41. In fact, ASCs could active and improve the growth of resting and residual breast cancer cells after surgery in breast cancer patients. In this context, an interesting and still poorly explored research topic is the one dealing with the relationship between cancer cells, CSCs, and MSCs. Since we have contrasting reports about their respective influences, some evidences suggest an antagonistic effect of normal cells on cancer cells, while others have evidenced that MSCs can favor cancer proliferation, invasion, and metastasis. In this review, we aim to discuss and provide up‐to date data on this exciting topic.
Figure 1.
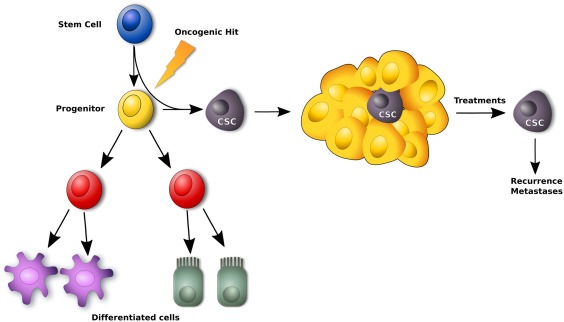
Model of CSCs theory: only CSCs are able to form and sustain tumor and are resistant to conventional therapies. CSCs are generated from normal stem cells or precursor/progenitor cells where epigenetic mutations are occurred. Abbreviation: CSC, cancer stem cell.
The Role of MSCs in Neoplastic Microenvironment
It is well‐known that interactions between cancer cells and stroma are of fundamental importance in promoting both the development and invasiveness of tumors. For instance, cancer cells may lead to modifications of topography and molecular composition of stroma during early tumor development and this, in turn, can affect the properties of the cancer cells 42. Therefore, the bidirectional interplay between cancer cells and cells of stroma, including MSCs, endothelial, immune, and fibroblast‐like stromal cells, plays a key role in tumor progression and metastasis and creates a complex microenvironment called tumor niche (Fig. 2). In normal stroma, predominant cells are fibroblasts that secrete an extracellular matrix (ECM) providing a natural barrier against tumor progression 43, 44. On the other hand, the ECM is able to support and promote tumor progression by modifications of the same ECM 45. In this context, both fibroblasts and myofibroblasts, denominated cancer‐associated fibroblasts (CAFs) produce proteins such as collagen, fibronectin, α‐smooth muscle actin, and others, creating alterations of ECM architecture. As a result, the cancer cells start to change their morphology becoming invasive and metastatic 46 as it has been described in some studies of breast 47 and pancreatic cancers 48. In these processes, MSCs can be fundamental. It has been reported that MSCs can originate from tumor resident stroma progenitors, or can be recruited from other tissues as bone marrow by circulation 49. Interestingly, MSCs have the tendency to migrate into damaged tissues or organs, driven by chemotactic gradients of cytokines/chemokines released from same damaged tissues. Once arrived in injured sites, MSCs provide structural support and secrete factors for tissue repair 50. Therefore, this physiological behavior happens also for the tumor that can be considered as a “wounds that never heal” 51. Circulating MSCs from bone marrow, adipose tissue or MSCs derived from tumor stroma cells that are able to differentiate in CAFs 52.
Figure 2.
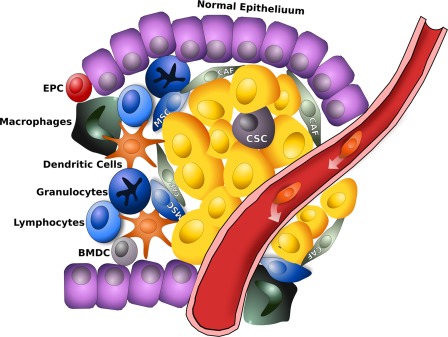
Tumor microenvironment consists not only of tumor cells and CSCs but are involved several types of cells and or processes, such as fibroblasts, migration of immune cells, angiogenesis, and matrix remodeling, MSCs thereby generating the so‐called tumor niche. Therefore, cancer cells and CSCs are intimately in contact with these cells, promoting tumourigenesis and cancer progression through several mechanisms among which tissue remodeling through matrix metalloproteinases, deposition of different extracellular matrix, liberation of pro‐angiogenic molecules, and secretion of soluble factors. Abbreviations: BMDC, bone‐marrow derived cells; CAF, cancer‐associated fibroblast; CSC, cancer stem cell; EPC, endothelial progenitor cells; MSC, mesenchymal stem cell.
In a model of inflammation‐induced gastric cancer, MSCs generated CAFs that were recruited to tumor microenvironment in a process that was mediated by TGF‐β and SDF‐1α 53. In an osteosarcoma model, it was found that cancer cells were able to inhibit the osteogenic differentiation of MSCs through TGF‐β/Smad2/3 signaling and to increase their production of vascular endothelial growth factor (VEGF) and IL‐6 and other pro‐tumor cytokines. In this case, MSCs derived from femur fracture patients undergoing orthopedic surgery. Moreover, TGFβ‐mediated inhibition of osteogenic differentiation was developed through increased expression of β‐catenin 54. Interestingly in breast cancer, the axis SDF‐1α/CXCR4 is crucial in the interaction between breast cancer cells and MSCs of bone marrow. Breast cancer cells are able to attract marrow derived MSCs and in turn, breast cancer cells preferentially metastasize to the bone marrow. In both cases, SDF‐1α seems to be involved 55. The same axis is probably important also to guide the interaction between cancer cells and adipose‐derived stem cells 56.
Another key feature of cancer is that the metabolism is an anaerobic glycolysis even under aerobic conditions. It has been demonstrated that osteosarcoma cells induce oxidative stress with reactive oxygen species (ROS) production in adipose MSCs. This also induces a shift to aerobic glycolysis with MSCs producing lactates. Furthermore, cancer cells can increase their mitochondria mass and activity and consequently increase their energetic metabolism 57, 58. MSCs can also regulate the metabolism of cancer cells through secretion of exosomes 59. In a recent study, exosomes produced from the prostate cancers can inhibit the adipogenic differentiation of MSCs, favoring the differentiation of MSCs into myofibroblasts 60. In turn, the exosome differentiated MSCs stimulate angiogenesis and tumor growth 60. Some studies describe two classes of polarized MSCs depending on expression of Toll like receptors (TLR). TLR4 primed‐MSCs are defined MSCs1 and are polarized into pro‐inflammatory phenotype. They are able to inhibit tumor growth and metastasis. TLR3 primed MSCs are defined MSCs2 and have the classic immunosuppressive phenotype. They are capable to improve the tumor growth and favor metastasis formation. This classification depends on the cytokine profile expressed from specific MSCs and include over‐expression of TGFβ, and SMAD3–4 61.
Different types of immune cells are also identifiable within the tumor microenvironment. These cells play both stimulatory and inhibitory roles on cancer growth. Both T‐cells and B‐cells infiltrations may represent an important favorable prognostic factor, as it was demonstrated in melanoma, colorectal, breast, and ovarian cancers 62, 63.
During tumor progression, another class of immune cells to be considered are macrophages. In fact, monocytes and macrophages can be recruited into tumors site altering the tumor microenvironment and accelerating tumor progression 64. Macrophages shift their phenotypes in response to various microenvironmental signals generated both from tumor and stromal cells. Macrophages can be subdivided into two categories: classic M1 and alternative M2 macrophages 65. The M1 macrophage is involved in the inflammatory response, pathogen clearance, and antitumor immunity. On the contrary, the M2 macrophage is involved in an anti‐inflammatory response, wound healing, and has pro‐tumorigenic properties. The tumor‐associated macrophages (TAMs) closely resemble the M2‐polarized macrophages and are critical modulators of the tumor microenvironment. Several studies have suggested that TAM accumulation in tumors correlates with a poor clinical outcome and provide a favorable microenvironment to support tumor development and progression regulating tumor angiogenesis, invasion, metastasis, immunosuppression, and chemotherapeutic resistance 64, 65, 66. Together, MSCs and TAMs promote tumor growth. In fact, MSCs can promote tumor progression increasing recruitment of TAMs in tumor site via CCR2 64. Another chemokine produced by MSCs, able to recruit the TAM is CCL2 67. Thus, MSCs and TAMs can engage in a bidirectional interaction resulting in tumor promotion and progression. Last, a very important characteristic to consider is that tumor microenvironment is not static but is actually dynamic by being the result of continuous tissue remodeling, tumor metabolic changes, recruitment of circulating stromal and immune cells, and a result of changes induced by anticancer agents.
Interaction Among MSCs, Cancer Cells, and CSCs
CSCs features are mainly represented by tumor‐initiating capacity, metastatic potential, and drug resistance. Some studies have reported that MSCs can increase the CSCs population within a tumor 68. Bone morphogenetic proteins (BMPs) are among the molecules that are responsible for stemness and drug resistance. In ovarian cancer, the number of CSCs can be increased by MSCs isolated from human ovarian tumor ascites via BMP2 and BMP4 68. The latter could also be produced in response to Hedgehog (Hh) secretion by ovarian cancer cells with potential mediation in resistance to chemotherapeutic drugs 69. In breast cancer cells, MSCs induce an increase of mir‐199 and mir‐214 leading to a repression of FoxP2 expression. This, in turn, promotes metastasis and maintenance of cancer stemness phenotype of breast cancer cells 70. There are several chemokines and cytokines produced by MSCs that get a key role in modulating CSCs and cancer cells. MSCs produce PGE2 after stimulation of IL‐1α and IL‐1β secreted by colon cancer cells. This leads to a secretion of IL‐6, CXCL1, and CXCL8 by MSCs increasing stemness characteristics of colon cancer cells 71. Other studies have reported that MSCs are able to increase the number of breast cancer cells positive to aldehyde dehydrogenases (ALDH) by secretion of CXCR2 ligands 72. Conditioned media derived from MSCs culture includes specific cytokines such as IL‐6 and CXCL8 able to induce expression of Oct4 and Sox2 in colorectal cancer cells 73. Moreover, another study demonstrated that IL‐6 secreted by MSCs leads to an increase of CSCs expressing CD133 in colorectal cancer cells by JAK2‐STAT3 pathway 74. Moreover, in prostate cancer, it has been observed that after bone marrow‐mesenchymal stem cells (BM‐MSCs) infiltration in tumor, CCL5 increases, leading to a strong expansion of CSCs, that, in turn, induces an over‐expression of matrix metalloproteinase 9, ZEB‐1, CD133, and CXCR4 molecules. CCL5 secreted by MSCs promotes also proliferation of breast cancer cell lines 75 and invasion 76. Irradiated breast cancer cells increase the release of TGFβ1, VEGF, and platelet‐derived growth factor BB (PDGF‐BB), which trigger the migration of MSCs (in this case from a murine model) to cancer cells through the upregulation of CCR2 77. Other factors secreted by MSCs, important in the interaction of cancer cells can be CXCL1, CXCL5, 6 and 7, IL4, IL8, IL10, IL17b, S100A4, and EGF (Fig. 3). The pattern of chemokines axis is strictly dependent on tumor cell types and niches. Besides chemokines effects, there are other types of interactions that exist between cancer cells and MSCs. These are carried out through exosomes and small secreted vesicles that are implicated in intercellular communications which may also have a role in cancerogenesis, either directly or in cooperation with chemokines. For instance, exosomes secreted by cholangiocarcinoma cells promote the migration of human bone‐marrow derived MSCs to cancer cells via the release of CXCL1 and other cytokines from the same MSCs 78. Another mechanism involves the urokinase plasminogen activator, that is expressed and released by a variety of solid tumor cell lines (brain, lung, prostate, and breast) and which plays a role in the migration of MSCs to the tumor site 75. On the other hand, there are some observations in a murine model underlining the possibility that MSCs could have also an antiproliferative effect on cancer cells by upregulating the expression of p21 and caspase‐3 and therefore leading to G0‐G1 arrest and apoptosis of cancer cells 79. Adipose‐derived MSCs from human healthy donors were shown to produce interferon‐β (IFN‐β) which, through STAT1 activation, significantly induced apoptosis and suppressed the proliferation of some breast and lung cancer cell lines 80. We reported that in cocultures of breast and osteosarcoma cell lines (MCF7 and SAOS2, respectively) with adipose‐derived MSCs derived from patients undergoing esthetic surgery, the latter did not differentiate and maintain the stemness phenotype in vitro, whereas the co‐injection of MSCs and MCF7 in murine models led to an increase of tumor size and vascularization compared to controls 19. Moreover, cancer cells increased the proliferation of adipose‐derived MSCs that led us to hypothesize that cancer cells may contribute to the maintenance of the resident stem population, which could give them an advantage in terms of aggressiveness. The maintaining of stemness features of resident stem cells was confirmed by the absence of epithelial‐mesenchymal‐transition (EMT). EMT and mesenchymal‐epithelial‐transition (MET) are processes by which the cells undergo molecular and structural changes and migrate to other sites in the body. Therefore, it has been demonstrated that there are different types of stem cell populations: one resident and defined as stationary stem cell population that is involved in the maintenance of tissues homeostasis, and the other defined as migratory stem cells that able to migrate and invade. Based on these considerations, we hypothesize that our stem cells are stationary and are not undergoing EMT. We could consider that cancer cells could directly maintain the adipose‐derived MSCs population present in the tumor microenvironment, independently from the activation of homing/migration signals and without the necessity to recruit bone‐marrow derived MSCs. Endothelial/pro‐angiogenic factors were downregulated in cocultured cells in vitro, thus stressing the concept of a specific cancer‐induced stemness maintenance. In vivo assays, we have demonstrated that adipose‐derived MSCs, co‐injected with MCF7 breast cancer cells, were integrated in tumor microenvironment, thus leading to more proliferating, bigger and clearly vascularized tumors in nude mice 19. Therefore, although it is well recognized that there is a bidirectional interplay between MSCs and cancer cells, specific mechanisms involved in promoting or inhibition of tumor growth from MSCs remain poorly established.
Figure 3.
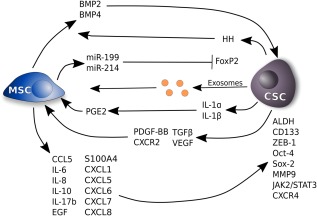
MSCs secretome and tumor stemness. MSCs release several factors that can induce stemness and drug resistance or maintain the CSCs phenotype. MSCs produce BMP4 and 2 increasing CSCs number in ovarian cancer. CSCs, in turn, activate Hh pathway. MSCs can induce an increase of mir‐199 and mir‐214 leading to a repression of FoxP2 promoting metastasis and maintenance of CSCs phenotype. IL‐1α and IL‐1β secreted by cancer cells induce the production of PGE2, IL6, and IL8 by MSCs. This leads to a secretion of IL‐6, CXCL1, and CXCL8 by MSCs increasing stemness characteristics. MSCs are able to increase the number of breast cancer cells positive to ALDH by secretion of CXCR2 ligands inducing expression of Oct4 and Sox2. IL‐6 secreted by MSCs leads to an increase of CSCs expressing CD133 by JAK2‐STAT3 pathway. Irradiated breast cancer cells increase the release of TGFβ1, VEGF, and PDGF‐BB, which activate the migration of MSCs to cancer cells. Moreover, cocultures of MSCs with breast cancer cells increases the production of CXCR2 ligands including CXCL1, 5, 6, 7, and 8 that are able to increase the percentage of CSCs. Also, IL‐10, IL‐17b, and EGF, secreted by MSCs, are involved in increasing breast CSCs number. MSCs can also lead to an increase of CCL5 expression that, in turn, induce an increase of CSCs by upregulation of ZEB1, MMP9, and CD133 in prostate cancer. MSCs can also regulate the metabolism of CSCs through secretion of exosomes in breast cancer and cholangiosarcomas. Abbreviations: ALDH, aldehyde dehydrogenases; CSC, cancer stem cell; EGF, endothelial growth factor; HH, hedgehog; MSC, mesenchymal stem cell; PDGF‐BB, platelet‐derived growth factor BB; VEGF, vascular endothelial growth factor.
Immune System and CSCs: Implications for Immunotherapy
A lot of research has focused on the role of the immune system in preventing tumor growth and on the other hand on the ability of cancer cells to escape the immune system 81. The investigational efforts have led to the clinic several drugs targeting immune check‐points such as cytotoxic T lymphocyte antigen‐4 (CTLA‐4) and programmed cell death/ligand‐1 (PD‐1/PD‐L1), with impressive results 82. Nevertheless, in many cases, these drugs do not act against the tumor or they are effective for short times. CSCs, besides being chemo‐ and radio‐resistant, are also immune‐resistant. For example, in many cases, they lack the expression of human leukocyte antigen (HLA) class I, so escaping the killing mediated by T lymphocytes 81. Moreover, CSCs have been demonstrated to release soluble factors with immunomodulatory properties, such as IL‐10, IL‐13, TGF‐β, and others that can make the tumor microenvironment insensible to the effector immune cells 83. The same tumor niche can host Treg cells and suppressive M2 macrophages. On the other hand, some studies have identified on CSCs the expression of activator natural killer (NK) receptors ligands, thus implying a probable sensitivity of CSCs by NK cells 84. These findings could open the way to new therapeutic approaches, exploiting NK. Besides CSC themselves, human MSCs have been reported to partially express major histocompatibility complex class I and to lack the expression of HLA class II antigens, that may result in a non‐immunogenic phenotype. Moreover, MSCs have been described as having immunosuppressive properties by modulating both T‐cell and B‐cell functions 2. It seems reasonable to speculate that one of the mechanism of interaction between cancer cells and CSCs could be influenced by the immune system. In the last years, a lot of research has focused on the immunomodulatory effects of MSCs, since these cells can be found around vascular areas of the bone marrow, which could have a negative effect on cytotoxic T cells 85. Although these mechanisms are misunderstood, MSCs have been reported to exert an immune‐suppressive effect. A probable mechanism may involve the demonstrated capacity of MSCs to migrate from bone marrow, adipose tissue and other sites, and to the tumor where they directly influence tumor microenvironment and tumor growth. Another mechanism may involve MSCs‐mediated recruitment and maintenance of regulatory T cells (Tregs) resulting in cytotoxic T cells negative regulation, as it was demonstrated using bone marrow aspirate from healthy subjects 85. This expansion has been attributed to the secretion of TGF‐β by MSCs 85. Besides favoring the expansion of Tregs, it has also been demonstrated that MSCs can induce a switch in favor of Th2‐type CD4+ T cells that increases expression levels of IL‐10 and decreases the activity of NK cells 85, 86.
Effect of MSCs on EMT and Metastasis Formation
EMT is a process through which cancer cells acquire an invasive phenotype that leads to metastasis. Some reports indicate a key role of MSCs in causing EMT. Human bone marrow‐derived MSCs have been reported to promote EMT in pancreatic cancer cells through Notch signaling 87. In luminal breast cancer, adipose‐derived MSCs from women undergoing breast reconstruction could induce an overexpression of EMT related genes as they expressed several TGF‐β‐related BMP 88. The way through which MSCs exert a role on tumor invasion, is not completely elucidated. In a coculture model of breast cancer cells with MSCs, it was shown that the latter enhanced the elongation, directional migration, and traction of cancer cells. This process was mediated by human MSCs‐secreted TGF‐β, migratory proteins rho‐associated kinase, focal adhesion kinase, and matrix metalloproteinases 88. During the metastatic process, MSCs can promote cancer progression by using homing and chemokines axis. Indeed, in a model of prostate cancer, it was demonstrated that prostate cancer cells secrete CXCL16 which recruits bone marrow‐derived MSCs via the axis CXCL16/CXCR6. Subsequently MSCs differentiate into CAFs which, in turn, through the other axis CXCL12/CXCR4, induce EMT 89 (Fig. 4). By contrast, some reports have suggested an inverse role played by MSCs on the metastatic potential. For example, although human MSCs increased tumor growth, they also significantly downregulated TGF‐β with effects on the invasive and metastatic potential and as demonstrated in a model of hepatocellular carcinoma 90.
Figure 4.
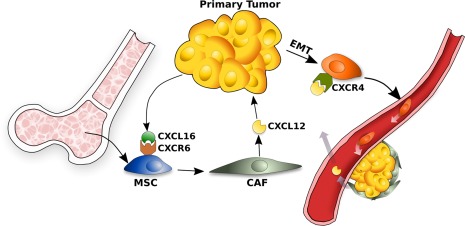
Model of a possible mechanism that enables cancer cells to migrate and form new tumors. Cancer cells produce CXCL16 that, in turn, induce the recruitment of MSCs in tumor site. CXCL16 binds its receptor, CXCR6 on MSCs. The latters are converted in CAFs producing high levels of CXCL12. CXCL12, in turn, induces cancer cells to undergo to an EMT that heightens CXCR4 expression in cancer cells. CXCR4 expression enables metastasis. Abbreviations: CAF, cancer‐associated fibroblast; EMT, epithelial‐to‐mesenchymal transition; MSC, mesenchymal stem cell.
Tumor Angiogenesis and CSCs
Tumors create their own vascularization through different processes that are associated with angiogenesis, remodeling of pre‐existing vessels, recruitment, vascular mimicry (VM), and differentiation of bone marrow endothelial precursors 46.
The vessel network is also a key component of the niche, where CSCs can play a role following radio‐ and chemo‐therapies. As an example, treatment with the VEGF‐A‐inhibitor drug, such as Bevacizumab, has been demonstrated to deplete the CSCs subpopulation in an orthotopic model of glioma, which leads to significant inhibition of tumor growth 91.
Another example is head‐and‐neck squamous cell carcinoma, where CSCs have been shown to localize in the proximity of tumor vessels, and where they are maintained by growth factors that are secreted by endothelial cells 92. On the other hand, in colorectal cancer, it seems that CSCs population is better maintained by hypoxic conditions, leading to the formulation of the hypoxic niche hypothesis 93. Moreover, MSCs that produce VEGF, Angiopoietin‐1 (Ang‐1) and other pro‐angiogenic factors, could differentiate into pericytes and endothelial cells, which supports tumor vascularization and growth. In a colorectal cancer model, it has been shown that primary human MSCs secrete a series of pro‐angiogenic factors, such as interleukin‐6 (IL‐6) and angiopoietin‐1, inducing also cancer cells to produce endothelin‐1 (ET‐1), thereby promoting tumor angiogenesis (Fig. 5). ET‐1 activated Akt and ERK pathways in endothelial cells which led to the induction of tumor angiogenesis 93. In a breast cancer model, both endothelial cells and adipose‐derived MSCs interplayed to give rise to pericytes and mature vessels 94. This interconnection could act via intercellular or paracrine signals, and probably through angiopoietins signal, since adipose‐derived MSCs have been shown to express Angiopoietin‐1 while endothelial cells expressed their corresponding receptors Tie1 and 2 94. However, there are opposite demonstrations of a negative role of MSCs on angiogenesis. It has been showed that murine MSCs could release reactive oxygen species which damage endothelial cells, and that MSCs could affect vessels formation in a melanoma model 95.
Figure 5.
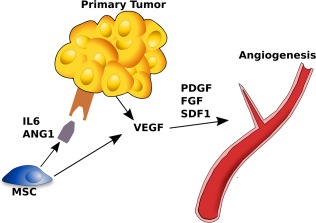
Model of a mechanism inducing tumor angiogenesis and involving MSCs. These cells can produce a series of angiogenic factors such as angiopoietin‐1 and IL‐6 that induce the secretion of VEGF and other angiogenic molecules promoting tumor angiogenesis. Abbreviations: FGF, fibroblast growth factor; MSC, mesenchymal stem cell; PDGF, platelet‐derived growth factor; VEGF, vascular endothelial growth factor.
In the case of the severe hypoxia within the tumor, several growth factors such as PDGF and VEGF are strongly expressed representing crucial chemotactic and mitogenic factors for MSC. Thus, MSC migrate toward tumor and promote vasculogenesis by an autonomous VEGF production and, therefore, further empower the pro‐angiogenic potential of tumors. Another process that deserves to be cited is the so called “vascular mimicry.” In this, cancer creates itself channels for fluid transport independent of typical modes of angiogenesis. It has been demonstrated that uveal melanoma cells are able to dedifferentiate in endothelial like cells losing the specific melanoma markers and acquiring those of endothelial cells. Considering the CSCs model, it has been also hypothesized that a fraction of CSCs could differentiate in cells with VM phenotype. Vartanian et al. 96 have demonstrated that melanoma cells were able to increase the vasculogenic potential of MSCs by VM. In fact, MSCs derived from adipose tissues of C57BL/6 mice in cocultured with melanoma cells formed vascular‐like network on Matrigel. MSCs alone was not able to form capillary like structures. This is the first direct evidence that melanoma cells instruct MSCs to participate in VM. Now, the concept of VM and its importance in interaction of MSCs and cancer cells is receiving improved attention in the field of angiogenesis especially for angiogenic therapies.
Influence of CSCs and MSCs on Multidrug Resistance
Among several mechanisms, one of major importance is the multidrug resistance (MDR) one. Indeed, the inefficacy of anticancer treatment may be ascribed to a reduced drug uptake, increase in drug extrusion from the cancer cell, increase drug inactivation or decreased activation, decrease in the formation of drug‐activated complex, and increase of repair of drug induced damage. Several studies reported that CSCs are resistant to conventional therapies in many types of solid tumors. CSCs present some transmembrane transporters such as ABC transporters (ATP‐binding cassette) family of molecules that actively pump the drug outside the cell. This unique property is also used as a method to isolate stem‐like cells from tumor cells 1, 97. CSCs also possess some enzymes, like ALDH and glutathione transferase (GST), that are capable to metabolize and inactivate anticancer agents such as platinum salts and others. Several signaling pathways have been linked to the drug resistance of CSCs, among which Wnt, Notch, Hedgehog. A number of studies confirmed the capacity of MSCs to confer drug and radiation resistance to cancer cells. In a model of breast cancer, adipose‐derived MSCs provide resistance to trastuzumab via the activation of c‐Src and the downregulation of the phosphatase and tensin homolog 98. The methylation of the tumor suppressor genes promoters has been shown to transform MSCs (from human healthy donors' bone marrow and umbilical cord blood) into CSCs, that have tumor‐initiating and drug resistance capacities in in vivo models 99. In general, MSCs can modulate the sensitivity of cancer cells to chemotherapeutic agents through the production of factors like polyunsaturated fatty acids, PDGF‐C, hepatocyte growth factor, nitric oxide, and interleukin‐17A (IL‐17A) 100.
Perspectives: Exploiting MSCs for Cancer Therapy
It has been demonstrated that, after murine MSCs transplantation in animal models, sarcoma developed 101. In a same manner, it has been reported a case of a late local recurrence of human osteosarcoma which occurred 13 years after the initial pathology and 18 months after a lipofilling procedure 40. Therefore, it is unclear if MSCs have an effect mainly tumor promoting or suppressive. Discrepancy between these results may arise also from isolation techniques and growth conditions of MSCs, experimental design in phenotype characterization, heterogeneity in MSCs population, individual donor variability, and injection time of MSCs in each experiment. Thus, it is important to standardize experimental protocols. Although MSCs could be a promising cell source in cell therapy, these observations and results question the safety and therapeutic procedure in clinical applications of MSCs grafting, and particularly for patients with cancer history. Therefore, the clinical application of MSCs for cancer treatment is still challenging. Due to contrasting results regarding the roles played by MSCs in cancer, both in animal and human models, and it remains difficult to establish what are the mechanisms implicated in cancer development due to MSCs. Some studies have showed a tumor suppressive effect of MSCs, others reported a tumor supportive potential. Indeed, in an interesting paper exploring in vivo and in vitro effects of adipose‐derived MSCs on melanoma and glioblastoma cell lines, MSCs have been shown to produce similar pro‐inflammatory and pro‐angiogenic soluble factors; however, consequent effects were different with demonstrated pro‐survival effect on melanoma cells and antitumor effect on glioblastoma cells 102. It seems that the result could depend on the specific response of tumor cells to MSCs paracrine signals and on the type of tumor 103. Moreover, cancer cells can release factors that induce and maintain the stem cell phenotype in MSCs, block their differentiation, and therefore support tumor proliferation, angiogenesis and metastases formation. These lead to the creation of a stem cell microenvironment that could help reinforce drug resistance. In fact, an important issue to consider derives from what we have demonstrated on the influence of cancer cells on MSCs 19. We could hypothesize that when cancer cells remained present within the tissue after surgery, these cells could recruit resident or bone‐marrow derived MSCs that promote recurrence, tumor induction by disrupting cancer “dormancy.” In efforts to find new therapeutic strategies against tumors, it has been proposed to use MSCs as vehicles to deliver drugs directly to cancer cells 104. For example, human MSCs transduced with an adenoviral expression vector carrying the human IFN gene, suppressed the growth of lung metastasis in an in vivo model of breast and lung cancers. MSCs were isolated from the bone marrow of healthy donors undergoing bone marrow harvest for use in allogeneic bone marrow transplantation. Another consequence of such a strategy is the possibility to deliver directly tumor agents using other routes of administration which would be more toxic to tumors 104. Currently, several phase I and phase II trials utilizing MSCs as anticancer treatment are ongoing 105. As an example, a phase I trial is currently recruiting patients with localized prostate cancer, using allogeneic bone marrow as source of MSCs [NCT01983709], while another phase I/II trial, which uses adipose derived MSCs, is now active although not yet recruiting for patients with recurrent ovarian cancer [NCT02068794]. Thus, it is crucial that researchers continue to examine the roles and mechanisms of MSCs in tumor progression to harness the therapeutic potential of MSCs and to control cancer progression.
Conclusion
Looking at available evidence, we do not have clear data about the activity of MSCs on cancer cells, due to contradicting effects that could be favorable or unfavorable for tumor's growth. Unfortunately, the processes are complicated by the nature of cellular interactions between MSCs and cancer cells that include membrane fusion or mitochondria exchange, growth factors, or metabolites that shape the relationship of MSCs with tumor cells even more enigmatic. In this context, there is no doubt that caution should be used in the field of regenerative medicine when for example adipose tissue is used in patients with cancer history. In fact, if cancer cells persist following surgery, they will most likely induce resident MSCs to promote tumor angiogenesis, thus favoring tumor growth. Although, there are no methods which can allow the microscopic detection of diseases, clinicians must use MSCs grafting only after meticulous analyses of possible cancer. Future challenges will focus on understanding how MSCs are able to affect all phases of carcinogenesis, from CSC arising, angiogenesis, tumor growth, and metastasis, to the MDR. Moreover, due to the fact that MSCs migrate toward tumor sites, it will be interesting and attractive to perform studies that consider MSCs as drug carriers. To achieve a better treatment of patients, future clinical approaches will need to use strategies that inhibit the dialog and the relationship between MSCs and cancer cells.
Author Contributions
F. Papaccio and F. Paino: conception and design, assembly of data, analysis, and interpretation, manuscript writing; V.D., T.R., and V.T.: assembly of data, analysis, and interpretation, manuscript writing; V.T. and G.P.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Table 1
Supporting Information Table 2
Acknowledgments
Funding for this review was provided by PON03PE_00060_7 “Sviluppo preclinico di nuove terapie e di strategie innovative per la produzione di molecole ad azione farmacologica” Campania Bioscience Technological District financed by MIUR PON R&C 2007–2013.
References
- 1. Reya T, Morrison SJ, Clarke MF et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111. [DOI] [PubMed] [Google Scholar]
- 2. Chamberlain G, Fox J, Ashton B et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 3. Roufosse CA, Direkze NC, Otto WR et al. Circulating mesenchymal stem cells. Int J Biochem Cell Biol 2004;36:585–597. [DOI] [PubMed] [Google Scholar]
- 4. Lee RH, Kim B, Choi I et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004;14:311–324. [DOI] [PubMed] [Google Scholar]
- 5. De Francesco F, Tirino V, Desiderio V et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One 2009;4:e6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trubiani O, Di Primio R, Traini T et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol 2005;18:213–221. [DOI] [PubMed] [Google Scholar]
- 7. Erices A, Conget P, Minguel JJ. Mesenchymal progenitor cells in human umbelical cord blood. Br J Haematol 2000;109:235–242. [DOI] [PubMed] [Google Scholar]
- 8. In't Anker PS, Scherjon SA, Kleijburg‐van der Keur C et al. Isolation of mesenchymal stem cells of fetal and maternal origin from human placenta. Stem Cells 2004;22:1338–1345. [DOI] [PubMed] [Google Scholar]
- 9. Ame‐Thomas P, Maby‐El Hajjami H, Monvoisin C et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumour B‐cell growth: Role of stromal cells in follicular lymphoma pathogenesis. Blood 2007;109:693–702. [DOI] [PubMed] [Google Scholar]
- 10. Krampera M, Sartoris S, Cosmi L et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev 2007;16:797–810. [DOI] [PubMed] [Google Scholar]
- 11. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post‐natal organs and tissues. J Cell Sci 2006;119:2204–2213. [DOI] [PubMed] [Google Scholar]
- 12. Jiang Y, Jahagirdar BN, Reinhardt RL et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 13. Smith JR, Pochampally R, Perry A et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 2004;22:823–831. [DOI] [PubMed] [Google Scholar]
- 14. Sato Y, Araki H, Kato J et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005;106:756–763. [DOI] [PubMed] [Google Scholar]
- 15. Kotton DN, Ma BY, Cardoso WV et al. Bone marrow‐ derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 16. Horwitz EM, Le Blanc K, Dominici M et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393–395. [DOI] [PubMed] [Google Scholar]
- 17. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: Paradoxes of passaging. Exp Hematol 2004;32:414–425. [DOI] [PubMed] [Google Scholar]
- 18. Shiao SL, Ganesan AP, Rugo HS et al. Immune microenvironments in solid tumours: New targets for therapy. Genes Dev 2011;25:2559–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paino F, La Noce M, Di Nucci D et al. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: Implication for autologous fat grafting. Cell Death Dis 2017;8:e2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kern S, Eichler H, Stoeve J et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 21. Izadpanah R, Trygg C, Patel B et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006;99:1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noel D, Caton D, Roche S et al. Cell specific differences between human adipose‐derived and mesenchymal‐stromal cells despite similar differentiation potentials. Exp Cell Res 2008;314:1575–1584. [DOI] [PubMed] [Google Scholar]
- 23. De Ugarte DA, Morizono K, Elbarbary A et al. Comparison of multi‐lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101–109. [DOI] [PubMed] [Google Scholar]
- 24. Dominici L, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Citotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 25. Ritter A, Friemel A, Fornoff F et al. Characterization of adipose‐derived stem cells from subcutaneous and visceral adipose tissues and their function in breast cancer cells. Oncotarget 2015;6:34475–34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tirino V, Desiderio V, Paino F et al. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumourigenicity in vivo. FASEB J 2011;25:2022–2030. [DOI] [PubMed] [Google Scholar]
- 27. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 28. Shmelkov SV, Butler JM, Hooper AT et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133‐ metastatic colon cancer cells initiate tumours. J Clin Invest 2008;118:2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren F, Sheng WQ, Du X. CD133: A cancer stem cells marker, is used in colorectal cancers. World J Gastroenterol 2013;19:2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brabletz T, Jung A, Spaderna S et al. Opinion: Migrating cancer stem cells—An integrated concept of malignant tumour progression. Nat Rev Cancer 2005;5:744–749. [DOI] [PubMed] [Google Scholar]
- 31. Diaz‐Cano SJ. Tumour heterogeneity: Mechanisms and bases for a reliable application of molecular marker design. Int J Mol Sci 2012;13:1951–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimmerlin L, Donnenberg AD, Rubin PJ et al. Regenerative therapy and cancer: In vitro and in vivo studies of the interaction between adipose‐derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A 2011;17:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kozlik M, Wójcicki P. The use of stem cells in plastic and reconstructive surgery. Adv Clin Exp Med 2014;23:1011–1017. [DOI] [PubMed] [Google Scholar]
- 34. Longo UG, Rizzello G, Berton A et al. Potential of adipose derived stem cells in orthopaedic surgery. Curr Stem Cell Res Ther 2013;8:418–421. [DOI] [PubMed] [Google Scholar]
- 35. Gimble MJ, Bunnell BA, Guilak F. Human adipose‐derived cells: An update on the transition to clinical translation. Regen Med 2012;7:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delay E, Garson S, Tousson G et al. Fat injection to the breast: Technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 2009;29:360. [DOI] [PubMed] [Google Scholar]
- 37. Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: A personal technique with 25 years of experience. Aesthet Plast Surg 2009;33:706. [DOI] [PubMed] [Google Scholar]
- 38. Yoshimura K, Asano Y, Aoi N et al. Progenitor‐enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 2010;16:169. [DOI] [PubMed] [Google Scholar]
- 39. Donnenberg VS, Zimmerlin L, Rubin PJ et al. Regenerative therapy after cancer: What are the risks? Tissue Eng Part B Rev 2010;16:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perrot P, Rousseau J, Bouffaut AL et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One 2010;5:e10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaput B, Foucras L, Le Guellec S et al. Recurrence of an invasive ductal breast carcinoma 4 months after autologous fat grafting. Plast Reconstr Surg 2013;131:123e–124e. [DOI] [PubMed] [Google Scholar]
- 42. Calorini L, Bianchini F. Environmental control of invasiveness and metastatic dissemination of tumour cells: The role of tumour cell‐host cell interactions. Cell Commun Signal 2010;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kunz‐Schughart LA, Knuechel R. Tumour‐associated fibroblasts (Part II): Functional impact on tumour tissue. Histol Histopathol 2002;17:623–637. [DOI] [PubMed] [Google Scholar]
- 44. Maffini MV, Soto AM, Calabro JM et al. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 2004;117:1495–1502. [DOI] [PubMed] [Google Scholar]
- 45. Park CC, Bissell MJ, Barcellos‐Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today 2000;6:324–329. [DOI] [PubMed] [Google Scholar]
- 46. Junttila MR, de Sauvage FJ. Influence of tumour micro‐environment heterogeneity on therapeutic response. Nature 2013;501:346–354. [DOI] [PubMed] [Google Scholar]
- 47. Yamashita M, Ogawa T, Zhang X et al. Role of stromal myofibroblasts in invasive breast cancer: Stromal expression of alpha‐smooth muscle actin correlates with worse clinical outcome. Breast Cancer 2012;19:170–176. [DOI] [PubMed] [Google Scholar]
- 48. Fujita H, Ohuchida K, Mizumoto K et al. α‐smooth muscle actin expressing stroma promotes an aggressive tumour biology in pancreatic ductal adenocarcinoma. Pancreas 2010;39:1254–1262. [DOI] [PubMed] [Google Scholar]
- 49. Krampera M, Franchini M, Pizzolo G et al. Mesenchymal stem cells: From biology to clinical use. Blood Transfus 2007;5:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu Y, Chen L, Scott PG et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659. [DOI] [PubMed] [Google Scholar]
- 51. Dvorak HF. Tumours: Wound that do not heal. N Engl Med 1986;315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 52. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: Key players in cancer progression. Mol Cancer 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quante M, Tu SP, Tomita H et al. Bone marrow‐derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumour growth. Cancer Cell 2011;19:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tu B, Peng ZX, Fan QM et al. Osteosarcoma cells promote the production of pro‐tumour cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF‐β/Smad2/3 pathway. Exp Cell Res 2014;320:164–173. [DOI] [PubMed] [Google Scholar]
- 55. Karnoub AE, Dash AB, Vo AP et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;7162:557–556. [DOI] [PubMed] [Google Scholar]
- 56. Muehlberg FL, Song YH, Krohn A et al. Tissue‐resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 2009;30:589–597. [DOI] [PubMed] [Google Scholar]
- 57. Bonuccelli G, Avnet S, Grisendi G et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumour cells. Oncotarget 2014;5:7575–7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fotia C, Massa A, Boriani F et al. Hypoxia enhances proliferation and stemness of human adipose‐derived mesenchymal stem cells. Cytothecnology 2015;6:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Konala VBR, Mamidi MK, Bhonde R et al. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell‐free regeneration. Cytotherapy 2016;18:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chowdhury R, Webber JP, Gurney M et al. Exosomes trigger mesenchymal stem cell differentiation into pro‐angiogenic and pro‐invasive myofibroblasts. Oncotarget 2015;6:715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waterman RS, Tomchuck SL, Henkle SL et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro‐inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One 2010;5:e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fridman WH, Pagès F, Sautès‐Fridman C et al. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. [DOI] [PubMed] [Google Scholar]
- 63. Nielsen JS, Sahota RA, Milne K et al. CD20+ tumour‐infiltrating lymphocytes have an atypical CD27‐ memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 2012;18:3281–3292. [DOI] [PubMed] [Google Scholar]
- 64. Mantovani A. MSCs, macrophages, and cancer: A dangerous ménage‐à‐trois. Cell Stem Cell 2012;11:730–732. [DOI] [PubMed] [Google Scholar]
- 65. Jia XH, Feng GW, Wang ZL et al. Activation of mesenchymal stem cells by macrophages promotes tumour progression through immune suppressive effects. Oncotarget 2016;7:20934–20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chanmee T, Ontong P, Konno K et al. Tumour associated macrophages as major players in the tumour microenvironment. Cancers (Basel) 2014;6:1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C‐C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst 2010;102:522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McLean K, Gong Y, Choi Y et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumourigenesis via altered BMP production. J Clin Invest 2011;121:3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coffman LG, Choi YJ, McLean K et al. Human carcinoma‐associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget 2016;7:6916–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cuiffo BG, Campagne A, Bell GW et al. MSC‐regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell 2014;15:762–774. [DOI] [PubMed] [Google Scholar]
- 71. Li HJ, Reinhardt F, Herschman HR et al. Cancer‐stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov 2012;2:840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu S, Ginestier C, Ou SJ et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011;71:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu XB, Liu Y, Wang GH et al. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR‐mediated NF‐κB activation. Sci Rep 2016;6:21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tsai KS, Yang SH, Lei YP et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology 2011;141:1046–1056. [DOI] [PubMed] [Google Scholar]
- 75. Lazennec G, Lam PY. Recent discovieries concerning the tumour‐mesenchymnal stem cell interactions. Biochim Biophys Acta 2016;1866:290–299. [DOI] [PubMed] [Google Scholar]
- 76. Pinilla S, Alt E, Abdul Khalek FJ et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett 2009;284:80–85. [DOI] [PubMed] [Google Scholar]
- 77. Klopp AH, Spaeth EL, Dembinski JL et al. Tumour irradiation increases the recruitment of circulating mesenchymal stem cells into the tumour microenvironment. Cancer Res 2007;67:11687–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haga H, Yan IK, Takahashi K et al. Tumour cell derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles 2015;4:24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu YR, Yuan Y, Wang XJ et al. The growth inhibitory effect of mesenchymal stem cells on tumour cells in vitro and in vivo. Cancer Biol Ther 2008;7:245–251. [DOI] [PubMed] [Google Scholar]
- 80. Ryu H, Oh JE, Rhee KJ et al. Adipose tissue‐derived mesenchymal stem cells cultured at high density express IFN‐β and suppress the growth of MCF‐7 human breast cancer cells. Cancer Lett 2014;352:220–227. [DOI] [PubMed] [Google Scholar]
- 81. Maccalli C, Parmiani G, Ferrone S et al. Immunomodulating and immunoresistance properties of cancer‐initiating cells: Implications for the clinical success of immunotherapy. Immunol Invest 2017;46:221–238. [DOI] [PubMed] [Google Scholar]
- 82. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yoshimura A, Muto G. TGF‐beta function in immune suppression. Curr Top Microbiol Immunol 2011;350:127–147. [DOI] [PubMed] [Google Scholar]
- 84. Tallerico R, Todaro M, Di Franco S et al. Human NK cells selective targeting of colon cancer‐initiating cells: A role for natural cytotoxicity receptors and MHC class I molecules. J Immunol 2013;190:2381–2390. [DOI] [PubMed] [Google Scholar]
- 85. Ramasamy R, Tong CK, Seow HF et al. The immunosuppressive effects of human bone marrow‐derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol 2008;251:131–136. [DOI] [PubMed] [Google Scholar]
- 86. Di Ianni M, Del Papa B, De Ioanni M et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008;36:309–318. [DOI] [PubMed] [Google Scholar]
- 87. Kabashima‐Niibe A, Higuchi H, Takaishi H et al. Mesenchymal stem cells regulate epithelial‐mesenchymal transition and tumour progression of pancreatic cancer cells. Cancer Sci 2013;104:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McAndrews KM, McGrail DJ, Ravikumar N et al. Mesenchymal stem cells induce directional migration of invasive breast cancer cells through TGF‐β. Sci Rep 2015;5:16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maxwell PJ, Neisen J, Messenger J et al. Tumour‐derived CXCL8 signaling augments stroma‐derived CCL2‐promoted proliferation and CXCL12‐mediated invasion of PTEN‐deficient prostate cancer cells. Oncotarget 2014;5:4895–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li GC, Ye QH, Xue YH et al. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97‐H cell line. Cancer Sci 2010;101:2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Calabrese C, Poppleton H, Kocak M et al. A perivascular niche for brain tumour stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- 92. Krishnamurthy S, Dong Z, Vodopyanov D et al. Endothelial cell‐initiated signaling promotes the survival and self‐renewal of cancer stem cells. Cancer Res 2010;70:9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mao Q, Zhang Y, Fu X et al. A tumour hypoxic niche protects human colon cancer stem cells from chemotherapy. J Cancer Res Clin Oncol 2013;139:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Orecchioni S, Gregato G, Martin‐Padura I et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res 2013;73:5880–5891. [DOI] [PubMed] [Google Scholar]
- 95. Otsu K, Das S, Houser SD et al. Concentration‐dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 2009;113:4197–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vartanian A, Karshieva S, Dombrovsky V et al. Melanoma educates mesenchymal stromal cells towards vasculogenic mimicry. Oncol Lett 2016;11:4264–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tirino V, Desiderio V, Paino F et al. Cancer stem cells in solid tumours: An overview and new approaches for their isolation and characterization. FASEB J 2013;27:13–24. [DOI] [PubMed] [Google Scholar]
- 98. Daverey A, Drain AP, Kidambi S. Physical intimacy of breast cancer cells with mesenchymal stem cells elicits trastuzumab resistance through Src activation. Sci Rep 2015;5:13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Teng IW, Hou PC, Lee KD et al. Targeted methylation of two tumour suppressor genes is sufficient to transform mesenchymal stem cells into cancer stem/initiating cells. Cancer Res 2011;71:4653–4663. [DOI] [PubMed] [Google Scholar]
- 100. Shi Y, Du L, Lin L et al. Tumour‐associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat Rev Drug Discov 2017;16:35–52. [DOI] [PubMed] [Google Scholar]
- 101. Tolar J, Nauta AJ, Osborn MJ et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007;25:371–379. [DOI] [PubMed] [Google Scholar]
- 102. Kucerova L, Matuskova M, Hlubinova K et al. Tumour cell behaviour modulation by mesenchymal stromal cells. Mol Cancer 2010;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gomes CM. The dual role of mesenchymal stem cells in tumour progression. Stem Cell Res Ther 2013;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Studeny M, Marini FC, Dembinski JL et al. Mesenchymal stem cells: Potential precursors for tumour stroma and targeted‐delivery vehicles for anticancer agents. J Natl Cancer Inst 2004;96:1593–1603. [DOI] [PubMed] [Google Scholar]
- 105. Zhang C, Yang SJ, Wen Q et al. Human‐derived normal mesenchymal stem/stromal cells in anticancer therapies. J Cancer 2017;8:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1
Supporting Information Table 2


