Abstract
The G-protein coupled receptor (GPCR) family of genes represents one of the largest druggable families of genes in the human genome. This is evident by the fact that approximately 30% of currently marketed drugs target GPCRs. However, many of these drugs are limited in their clinical potential as they are associated with debilitating side effects – a consequence of our incomplete understanding of their pharmacology and the signaling pathways regulated by GPCRs. Because of the limited range of tools available to resolve these issues, integrated approaches are required to fully understand the pharmacological action of drugs and the biochemical repertoire regulated by GPCRs. In this review we will focus on the action of antipsychotic drugs on certain monoamine GPCRs in the central nervous system (CNS) and the approaches being developed to elucidate their distinct pharmacology.
Introduction
The major monoamine neurotransmitters in the brain are dopamine (DA), serotonin (5-HT), and norepinephrine (NE) [1]. Monoamine dysfunctions have been implicated in various CNS disorders such as attention-deficit/hyperactivity disorder, drug abuse, Parkinson’s disease (PD), schizophrenia, depression and bipolar disorder [2–6]. Antipsychotics are a major class of psychotropic drugs that are used in the treatment of schizophrenia and mood disorders. These antipsychotic drugs are GPCR ligands (agonists, antagonists, inverse agonists and partial agonists) that have been used extensively to study the effects of monoamine GPCR activation or inhibition. Several in vitro and in vivo assays to study antipsychotic action at GPCRs have been developed and we will suggest how these can be integrated with novel approaches to give us a better understanding of GPCR mechanisms of action.
The “typical” antipsychotics discovered in the 1950s, including chlorpromazine and haloperidol, are clinically effective but have serious side effects, such as extrapyramidal symptoms (EPS) and hyperprolactinemia. However another drug, clozapine, belonging to the later “atypical” antipsychotic category, has comparable clinical efficacy but no EPS [7], although it does display a suite of other adverse effects. The prevalent hypothesis prior to the 1970s was that dopamine was involved in the mechanism of action of these drugs, yet no GPCR targets were identified. Pioneering work by Seeman et al. and Creese et al. [8,9] using competition binding experiments with [3H]-haloperidol and [3H]-dopamine showed that the common property of all antipsychotics was their ability to bind to DA receptors in striatal homogenates, supporting the “DA hypothesis” of schizophrenia. Several years later Meltzer et al. [10] showed that antipsychotics could be classified as typical or atypical based on their binding affinities to DA D2 and serotonin type 2A (5-HT2A) receptors. However, antipsychotics have also been shown to bind to other GPCRs such as serotonin 5-HT1A [11], alpha-adrenergic α1 and β2, histamine H1 and muscarinic acetylcholine M1 receptors [12]. While the efficacy of most clinically effective antipsychotics can be attributed to D2 and 5-HT2A receptors, compounds with distinct pharmacology such as the specific 5-HT2A receptor inverse agonist pimavanserin may find applications in particular conditions such as PD psychosis [13]. Owing to the diverse pharmacological profile of antipsychotics, a better understanding of their action is essential. Although clinical studies in humans provide invaluable information, both cellular and animal models are necessary to elucidate the mechanisms of action of antipsychotics.
In vitro approaches to elucidate antipsychotic action at GPCRs
Historically, the classification of antipsychotics has been made by interpreting data from binding studies of DA or 5-HT2 receptors in tissue. However, the discovery of several families of G proteins [14], the cloning of GPCRs and the observation that a GPCR can activate multiple G proteins [15] have in recent years caused an explosion in the development of assays that can reveal the regulation of multiple downstream signaling cascades. Several high-throughput screening (HTS) assays have been developed such as aequorin-based fluorescent assays [16] or cyclic adenosine monophosphate (cAMP) response element-directed reporter assays [17] to measure Gαq, Gαi, and Gαs signaling events. Additionally, fluorescence resonance energy transfer assays, such as a protein kinase A (PKA) cAMP sensor [18] and an EPAC-based sensor [19], have been developed to study Gαi and Gαs signaling downstream of several GPCRs. Recent advances have been made through development of bioluminescence resonance energy transfer (BRET) [20] assays to study GPCR-dependent cAMP signaling [21]. Using a reporter-based assay to measure G protein signaling at DA and 5-HT2 receptors, Weiner et al. [22] have revealed that most antipsychotics are antagonists or inverse agonists at DA D2 and 5-HT2A receptors, supporting the earlier observations from binding studies. Moreover, recent findings have shown that several antipsychotics are partial agonists at the 5-HT1A receptor Gi pathway [23], and this property might provide an explanation for the better side effect profile of atypical antipsychotics [24].
Interestingly, in recent years a new paradigm in GPCR signaling has emerged, wherein GPCRs mediate their cellular functions by two distinct mechanisms, the canonical G protein-dependent signaling pathway and a G protein-independent β-arrestin-mediated form of signaling [25]. This new paradigm emerged from observations that beta-arrestin, which normally mediates desensitization [26] and internalization [27] of GPCRs, can mediate GPCR signaling through its ability to scaffold kinase complexes [28,29]. β-Arrestin-dependent signaling is both temporally and spatially separated from G protein signaling [30,31]. The discovery of these two separate modes of signaling has given rise to the concept of biased signaling at GPCRs [32], although a similar but even broader concept termed “functional selectivity” had been proposed previously [33]. This new signaling paradigm, along with initial observations that β-arrestin2 knockout (βarr2KO) mice show reduced DA-dependent locomotion in response to psychotropic drugs such as morphine and amphetamine [34,35], has led to a reanalysis of cellular assays and screening technologies for antipsychotics. Historically, the effects of antipsychotics had been analyzed only at G protein-coupled responses; but since these discoveries, antipsychotic profiling now focuses on both G protein- and β-arrestin-dependent signaling pathways. Antipsychotics that were classified as agonists, antagonists or partial agonists now need to be reclassified based on their activity not only at the G protein pathway but also at the β-arrestin pathway. Some studies have determined the activity of antipsychotics at interactions of β-arrestin2 and the D2 DA receptor [36,37], but no similar studies of the 5-HT2A receptor-arrestin pathway have yet been published, probably due to the fact that ligands at these receptors function via both arrestin-independent and -dependent mechanisms in different cell types [38,39]. Using a BRET-based approach, Masri et al. [36] showed that all clinically effective antipsychotics uniformly display antagonist activity at D2 receptor-βarr2 interactions. This study correlates well with the observation that amphetamine-induced hyperlocomotion is reduced in βarr2KO mice and highlights the need to associate in vitro and in vivo observations (Table 1).
Table 1.
Adapted and modified from Masri et al (39). EC50 and Emax of antipsychotics for both intrinsic and antagonist activity are presented. These in vitro data are compared to behavioral data in the last column.
| Compounds | Intrinsic activity for Gi/o Pathway |
Intrinsic activity for β-arrestin 2 pathway |
Antagonistic activity for Gi/o pathway |
Antagonistic activity for β-arrestin 2 pathway |
Inhibition of PCP or Amph locomotion in βarr2 signaling deficient mice |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| pEC50 | Emax (%) | pEC50 | Emax (%) | KB (nM) | Emax (%) | KB (nM) | Emax (%) | |||
| Quinpirole | 8.25 ± 0.12 | 66.02 ± 1.05 | 7.01 ± 0.04 | 100 ± 1.01 | N.A. | N.A. | N.A. | N.A. | N.A. | |
| Haloperidol | 7.32 ± 0.39 | −42.6 ± 4.5 | N.A. | N.A. | 0.28 ± 0.16 | 104 ± 4.5 | 0.12 ± 0.03 | 83.7 ± 2.5 | Y66, a | |
| Clozapine | 6.97 ± 0.52 | −56.3 ± 9.8 | N.A. | N.A. | >10,000 | N.A. | 67.7 ± 14.0 | 85.7 ± 8.2 | Y52,a | |
| Aripiprazole | 9.45 ± 0.24 | 49.0 ± 3.9 | N.A. | N.A. | 0.12 ± 0.05 | 29.9 ± 5.6 | 1.07 ± 0.32 | 69.6 ± 2.8 | N66, a | |
| Risperidone | 9.25 ± 0.35 | −16.4 ± 2.8 | N.A. | N.A. | 1.08 ± 0.9 | 96.3 ± 7.0 | 0.02 ± 0.01 | 83.0 ± 3.0 | Y a | |
The behavioral data represent inhibition of either amphetamine (amph) or phencyclidine (PCP) induced locomotion in βarr2 signaling deficient mice (i.e βarr2−/− or D2GSK3β−/− mice);
Urs et al Society for Neuroscience 2011 poster;
N.A not applicable; N=No inhibition, Y=lnhibition.
In vivo approaches to understanding antipsychotic action at GPCRs
Rodent models remain the most prevalent experimental paradigm for in vivo studies. Pharmacological rodent models that have been used to screen for antipsychotic efficacy are based on amphetamine- (Amph) and phencyclidine- (PCP) induced hyperactivity. Although these models do not recapitulate all the symptoms of schizophrenia observed in humans-including the positive (hallucinations, delusions and disorganization), negative (alogia, avolition and anhedonia) and cognitive symptoms - they have both reliability and predictive validity when assessing antipsychotic action [40]. These pharmacological models are analyzed in common behavioral tests corresponding to schizophrenia endophenotypes, such as inhibition of hyperlocomotion, reversing prepulse inhibition (PPI) disruption, social behavior, conditional avoidance response, and latent inhibition ;and additionally in determining the side effect profile by measuring catalepsy (as a measure of EPS), prolactin secretion, agranulocytosis and glucose levels [24,41–43]. These pharmacological models have revealed that all antipsychotics are efficacious in reversing hyperlocomotion and PPI [42], which are considered endophenotypes of positive symptoms of schizophrenia [41]. However, although some atypical, but no typical, antipsychotics are moderately efficacious in reversing endophenotypes of negative symptoms in animal models, contradictory evidence in human patient studies [44–46] suggests that reversal of negative symptoms in schizophrenia is a largely unmet need and requires further investigation. Recent evidence from human studies and certain animal models has suggested a role for prefrontal cortical DA D1 receptors in the manifestation of negative symptoms and cognitive deficits in schizophrenia [45,47]. Interestingly, most antipsychotics have weak binding to DA D1 receptors, suggesting an avenue for further research.
In addition to these pharmacological models, genetic models have also been used to screen for antipsychotics. Several genetic models, such as DA transporter knockout [48], NMDA receptor subunit 1 knockdown [49] and disrupted in schizophrenia (DISC1) knockout [50] mice, recapitulate positive and/or negative symptoms of schizophrenia, and some of these models can be used to screen for novel antipsychotics. One of the advantages of using animal models is to delete or overexpress a particular gene of interest to test its role in endophenotypes of schizophrenia. However, a genetic approach requires a valid rationale, perhaps established through association or pathology studies, to target a particular gene, since generating a mouse model can be an expensive and time-consuming endeavor. But a genetic targeting strategy leading to amelioration of schizophrenia endophenotypes could provide new targets for antipsychotic therapies. Several ligands against novel targets such as partial agonists to β-arrestin2 (βarr2) at D2 receptors [51] and agonists to neurotensin receptor NTR1 [52], metabotropic glutamate receptor mGluR2 [53] and nicotinic acetylcholine receptors [54], which could potentially lead to new antipsychotic therapies, have been identified through genetic animal models.
Several studies have used receptor knockout mice such as 5-HT2A, 5-HT1A and D2 knockout mice to study the effects of antipsychotics on schizophrenia endophenotypes [55–58]. However global receptor knockout studies do not allow facile interrogation of cell-specific effects of drugs on receptor signaling pathways or behaviors. To study the role of cell-specific signaling pathways in schizophrenia endophenotypes and antipsychotic action, several strategies have emerged over the past few years by combining genetic targeting approaches. A technique further developed by the Gene Expression Nervous System Atlas (GENSAT) project, termed bacterial artificial chromosome (BAC) transgenic technology, targets GPCR-specific neuronal populations with promoter-specific reporters that label [59,60] or Cre recombinase that deletes [61] any gene of interest when combined with a mouse line expressing a “floxed” gene. Several studies with these Cre lines have provided useful insights into the action of antipsychotics. Bateup et al. [62] showed that mice with a deletion of DARPP32 (dopamine and cAMP regulated phosphoprotein of 32kDa molecular weight) in either D1 or D2 receptor-expressing neurons had a reduced cataleptic response when treated with haloperidol. DARPP32, as its name implies, is a canonical downstream G-protein effector that is regulated by DA receptors through the cAMP/PKA pathway [63], although its phosphorylation is regulated in opposing fashion by D1 and D2 DA receptors upon haloperidol treatment [64]. Thus these studies suggest that haloperidol, which is an antagonist at both D2 and D1 receptors, probably causes catalepsy due to inhibition of the G-protein pathway at both receptors. A similar study by our group analyzed the effect of deletion of the other arm of the GPCR pathway, i.e. the beta-arrestin pathway in specific neuronal populations [65]. We showed that in either D1 or D2 neuron-specific knockouts of GSK3β [65], which is downstream of D2R-βarr2 signaling [35,66,67] haloperidol still caused catalepsy. These combined results suggest that antagonism of the G protein pathway and not the βarr2 pathway (through GSK3β) is predominantly responsible for the cataleptic response caused by haloperidol. Moreover, in mice with GSK3β deletion in D2 neurons we found that aripiprazole, but not haloperidol, lost its ability to antagonize Amph-induced locomotion, suggesting that aripiprazole acts predominantly through the βarr2 pathway to alleviate this endophenotype of schizophrenia. The atypical antipsychotic aripiprazole, unlike haloperidol, is a partial agonist at the G-protein pathway (Table 1) but, similar to haloperidol, is an antagonist at D2/βarr2 interactions. These data therefore suggest that antagonizing the βarr2 pathway at the D2 receptor might alleviate psychosis without causing catalepsy. Interestingly, 5-HT1A and 2A receptor agonists have been shown to attenuate haloperidol-induced catalepsy in rodents [68] and several “atypical” antipsychotics are partial agonists at 5HT1A receptors. These results furthermore highlight the fact that functional selectivity can be observed at the behavioral level as well (Figure 1A). In a recent study Allen and colleagues [51] generated novel β-arrestin-biased ligands based on the aripiprazole scaffold and showed that these novel β-arrestin biased antipsychotic-like compounds are efficacious at inhibiting Amph- and PCP-induced hyperlocomotion without inducing catalepsy.
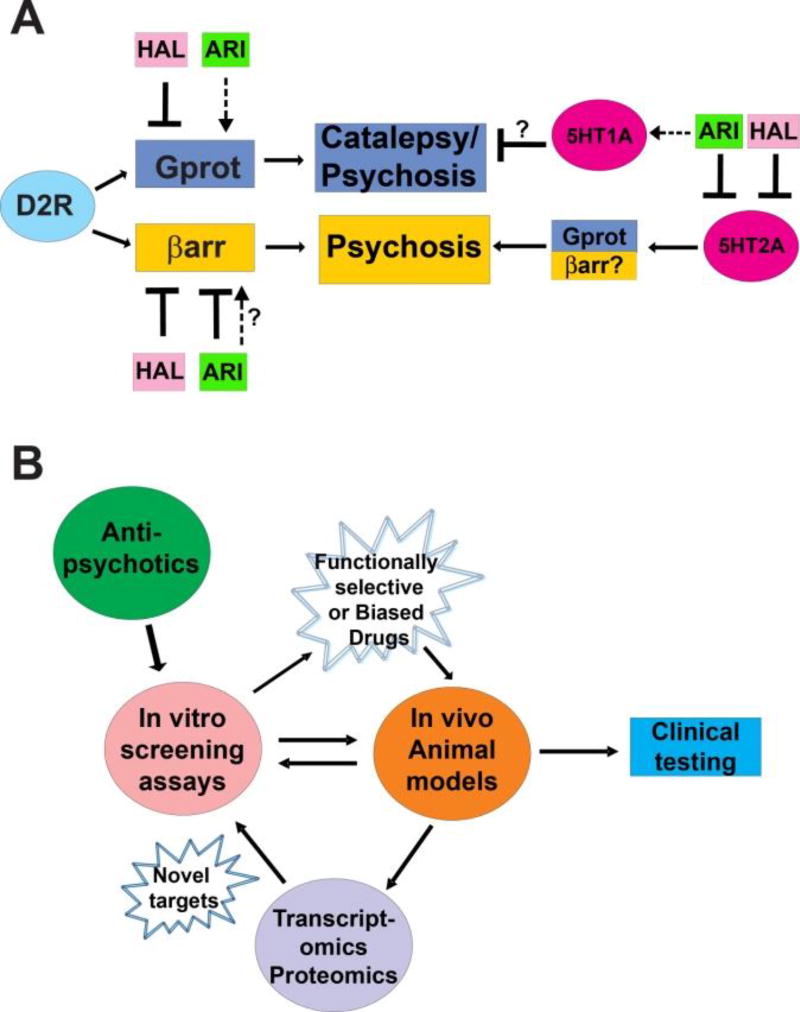
Figure 1.
A) Schematic representation of antipsychotic (APS) action on G-protein (Gprot) and β-arrestin (βarr) signaling pathways and its effect on catalepsy and psychosis.
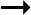 Activation;
Activation;
 inhibition/antagonist;
inhibition/antagonist;
 partial agonist. Haloperidol (HAL) and aripiprazole (ARI) are used as representative typical and atypical APS, respectively. HAL and ARI act on D2 dopamine (D2R) and 5-HT2A serotonin receptors. Unlike HAL, ARI is a partial agonist at the D2-Gprot pathway and at 5-HT1A receptors, which might explain its non-cataleptic properties. Moreover, antagonist activity at the D2-βarr pathway and at 5-HT2A receptors might be largely responsible for reducing psychosis.
partial agonist. Haloperidol (HAL) and aripiprazole (ARI) are used as representative typical and atypical APS, respectively. HAL and ARI act on D2 dopamine (D2R) and 5-HT2A serotonin receptors. Unlike HAL, ARI is a partial agonist at the D2-Gprot pathway and at 5-HT1A receptors, which might explain its non-cataleptic properties. Moreover, antagonist activity at the D2-βarr pathway and at 5-HT2A receptors might be largely responsible for reducing psychosis.
B) Schematic representation of the various integrated approaches used to decipher antipsychotic action including novel techniques in development.
Transcriptomic and proteomic approaches
A concerted research effort involving both in vitro and in vivo studies can be successfully employed to identify novel signaling pathways. However, cellular assays and animal behavior models cannot be efficiently used to characterize the comprehensive molecular effects of drugs throughout the brain. Recently a translational profiling approach called Translational Ribosome Affinity Purification (TRAP) was developed to identify the molecular determinants of different cell types in the brain [69,70]. This technology uses a BAC mouse line that expresses an EGFP-tagged L10a subunit of the ribosomal machinery in a cell-specific manner, which can then be used to affinity purify L10a-bound actively translating mRNA transcripts. An alternative but similar approach involves the use of a mouse line expressing an HA-tagged L22 ribosome subunit that is Cre-inducible [71]. Such techniques provide more sensitive and cell-specific data compared to microarrays, and many more targets can be identified by using the latest whole-transcriptome sequencing technologies such as RNAseq [72,73]. Although mRNA studies have been done before [74,75], TRAP can potentially be used to assess the effects of psychotropic drugs on the mRNA profile in various cell types in the brain. One potential application would be a comparative study of cell-specific mRNA profiles induced by endogenous versus functionally selective GPCR ligands. Such studies might provide an opportunity to identify previously unacknowledged novel targets for the development of more selective therapies with fewer side effects.
Transcriptional profiles provide valuable information about the genes that are active under particular conditions but they do not necessarily translate into similar protein profiles. Several techniques have been utilized to assess protein profiles under different conditions. One of the most widely used techniques is mass spectrometric analysis of cellular or tissue samples [76]. A relevant example of mass spectrometric methods to identify proteomic profiles upon GPCR stimulation was done by Xiao et al. [77], where the authors aimed to identify binding partners to β-arrestins under basal or stimulated conditions at the angiotensin AT1a receptor (AT1aR). The authors found several previously known interactors of β-arrestins and in addition identified several novel partners, which they confirmed by co-immunoprecipitation experiments. Furthermore, in a separate study the same group analyzed the phosphoprotein profile when the AT1aR is activated by a β-arrestin biased ligand Sar(1), Ile(4), Ile(8)-angiotensin (SII) [78]. The success of these studies provides impetus to perform further cell-specific protein profiling in vivo similar to TRAP. By combining BAC technology, cell-specific protein labeling such as biotinylation [79,80] and mass spectrometry, cell- or receptor-specific protein profiles can be determined upon exposure to various drugs. Such integrated approaches may provide insights into the actions of drugs at the translational and post-translational level.
In summary we have reviewed the various approaches used to elucidate the effects of antipsychotic drugs on GPCR signaling in the brain, including novel techniques that have gained prominence in the past several years. Integrating all or some of these methods should allow investigators to not only validate signaling pathways but also to identify non-canonical ones (Figure 1B). In addition, these integrated approaches will continue to provide valuable additional insights into the mechanisms of antipsychotic action at therapeutic and non-therapeutic targets. Such techniques are critical in the development of novel therapies that are more pathway-specific and efficacious with minimal off-target effects.
Highlights.
Historical perspective of antipsychotics and screening technologies
Current developments in antipsychotic screening methods
In vivo approaches to study and develop antipsychotics
Developments in transcriptomic and proteomic approaches
How different approaches can be integrated to better understand antipsychotic action
References
- 1.Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annu Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- 2.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 3.Birkmayer W, Hornykiewicz O. The L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien Klin Wochenschr. 1961;73:787–788. [PubMed] [Google Scholar]
- 4.Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- 5.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer HY. What's atypical about atypical antipsychotic drugs? Curr Opin Pharmacol. 2004;4:53–57. doi: 10.1016/j.coph.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 9.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 10.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 11.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 13.McFarland K, Price DL, Bonhaus DW. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson's disease. Behav Pharmacol. 2011;22:681–692. doi: 10.1097/FBP.0b013e32834aff98. [DOI] [PubMed] [Google Scholar]
- 14.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 15.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 16.Sheu YA, Kricka LJ, Pritchett DB. Measurement of intracellular calcium using bioluminescent aequorin expressed in human cells. Anal Biochem. 1993;209:343–347. doi: 10.1006/abio.1993.1132. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald LR, Mannan IJ, Dytko GM, Wu HL, Nambi P. Measurement of responses from Gi-, Gs-, or Gq-coupled receptors by a multiple response element/cAMP response element-directed reporter assay. Anal Biochem. 1999;275:54–61. doi: 10.1006/abio.1999.4295. [DOI] [PubMed] [Google Scholar]
- 18.Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- 19.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Piston DW, Johnson CH. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci U S A. 1999;96:151–156. doi: 10.1073/pnas.96.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR. Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol Pharmacol. 2008;74:585–594. doi: 10.1124/mol.108.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. * One of the first studies to use G-protein based assays to suggest that antipsychotics are inverse agonists or antagaonists at 5HT2A and D2 receptors. [PubMed] [Google Scholar]
- 23.Newman-Tancredi A, Assie MB, Leduc N, Ormiere AM, Danty N, Cosi C. Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int J Neuropsychopharmacol. 2005;8:341–356. doi: 10.1017/S1461145704005000. [DOI] [PubMed] [Google Scholar]
- 24.Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl) 2011;216:451–473. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- 25.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 26.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. ** This paper describes the first evidence that the interaction of β-arrestin with a GPCR represnts the signal for receptor enfdocytosis. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. ** These 2 studies (27 and 28) showed that beta-arrestins have other functions in addition to desensitization and have the capacity to signal in a G-protein independent manner. [DOI] [PubMed] [Google Scholar]
- 29.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 30.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 31.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 32.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 34.Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. ** Breakthrough study showing a role for beta-arrestin2 in DA signaling and DA-dependent behaviors. This study led to the initial idea that antipsychotics might also act on beta-arrestins in addition to G-protein signaling at the D2 receptor. [DOI] [PubMed] [Google Scholar]
- 36.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. * This study showed in vitro evidence that all antipsychotics uniformly antagonize D2-beta-arrestin2 interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klewe IV, Nielsen SM, Tarpo L, Urizar E, Dipace C, Javitch JA, Gether U, Egebjerg J, Christensen KV. Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology. 2008;54:1215–1222. doi: 10.1016/j.neuropharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- 39.Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. * This study showed functional selectivity at 5HT2A receptor-induced behaviors such as head-twitching. The authors showed that this behavior is beta-arrestin2-dependent and ligand-specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell SB, Geyer MA. Overview of animal models of schizophrenia. Curr Protoc Neurosci. 2007;Chapter 9(Unit 9):24. doi: 10.1002/0471142301.ns0924s39. [DOI] [PubMed] [Google Scholar]
- 41.van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiner I, Lubow RE, Feldon J. Abolition of the expression but not the acquisition of latent inhibition by chronic amphetamine in rats. Psychopharmacology (Berl) 1984;83:194–199. doi: 10.1007/BF00429734. [DOI] [PubMed] [Google Scholar]
- 44.King DJ. Drug treatment of the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 1998;8:33–42. doi: 10.1016/s0924-977x(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 45.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. * This study showed that D2 over-expression in the striatum of mice leads to cognitive deficits similar to schizophrenia presumably through the prefrontal cortex D1 receptor function. [DOI] [PubMed] [Google Scholar]
- 48.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 49.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 50.Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res. 2009;179:75–86. doi: 10.1016/S0079-6123(09)17909-8. [DOI] [PubMed] [Google Scholar]
- 51.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. * This study was the first demonstration of a beta-arrestin biased ligand developed at the D2 receptor using aripiprazole as a scaffold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinkead B, Nemeroff CB. Neurotensin, schizophrenia, and antipsychotic drug action. Int Rev Neurobiol. 2004;59:327–349. doi: 10.1016/S0074-7742(04)59013-X. [DOI] [PubMed] [Google Scholar]
- 53.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto S, Jarskog LF, Fleischhacker WW. Alternative pharmacologic targets for the treatment of schizophrenia: results from phase I and II trials. Curr Opin Psychiatry. 2013;26:158–165. doi: 10.1097/YCO.0b013e32835d8296. [DOI] [PubMed] [Google Scholar]
- 55.McOmish CE, Lira A, Hanks JB, Gingrich JA. Clozapine-induced locomotor suppression is mediated by 5-HT2A receptors in the forebrain. Neuropsychopharmacology. 2012;37:2747–2755. doi: 10.1038/npp.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bay-Richter C, O'Callaghan MJ, Mathur N, O'Tuathaigh CM, Heery DM, Fone KC, Waddington JL, Moran PM. D-amphetamine and antipsychotic drug effects on latent inhibition in mice lacking dopamine D2 receptors. Neuropsychopharmacology. 2013;38:1512–1520. doi: 10.1038/npp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, Low MJ, Geyer MA. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knock-out mice. J Neurosci. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bortolozzi A, Masana M, Diaz-Mataix L, Cortes R, Scorza MC, Gingrich JA, Toth M, Artigas F. Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. Int J Neuropsychopharmacol. 2010;13:1299–1314. doi: 10.1017/S146114571000009X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. * A pioneering study that led to creation of GENSAT, a repository of transgenice mice. [DOI] [PubMed] [Google Scholar]
- 60.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 64.Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–939. doi: 10.1038/nn.2153. * This study used epitope-tagged DARPP32 expressed in specific neuronal pouplations to determine the phosphorylation status of DARPP32 under various conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci U S A. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. * In this study cell-specific deletion of GSK3β in 2R expressing neurons led to the finding that antipsychotics and lithium act on the same substrate but in different neurons, explaining their unique therapeutic properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wadenberg ML. Serotonergic mechanisms in neuroleptic-induced catalepsy in the rat. Neurosci Biobehav Rev. 1996;20:325–339. doi: 10.1016/0149-7634(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 69.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. * This study illustrates the power of the TRAP approach to profile the transcriptomes of different cell populations in the brain of mice and how it provides a more complete picture than microarray studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. ** In this original demonstration of the TRAP approach, the authors provide an enabling technology to compare even closely related cell types in the brain and demonstrate that some of the differences in transcriptional profiles have functional consequences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cloonan N, Forrest AR, Kolle G, Gardiner BB, Faulkner GJ, Brown MK, Taylor DF, Steptoe AL, Wani S, Bethel G, et al. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Methods. 2008;5:613–619. doi: 10.1038/nmeth.1223. [DOI] [PubMed] [Google Scholar]
- 73.Bottomly D, Walter NA, Hunter JE, Darakjian P, Kawane S, Buck KJ, Searles RP, Mooney M, McWeeney SK, Hitzemann R. Evaluating gene expression in C57BL/6J and DBA/2J mouse striatum using RNA-Seq and microarrays. PLoS One. 2011;6:e17820. doi: 10.1371/journal.pone.0017820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacDonald ML, Eaton ME, Dudman JT, Konradi C. Antipsychotic drugs elevate mRNA levels of presynaptic proteins in the frontal cortex of the rat. Biol Psychiatry. 2005;57:1041–1051. doi: 10.1016/j.biopsych.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas EA, George RC, Danielson PE, Nelson PA, Warren AJ, Lo D, Sutcliffe JG. Antipsychotic drug treatment alters expression of mRNAs encoding lipid metabolism-related proteins. Mol Psychiatry. 2003;8:983–993. 950. doi: 10.1038/sj.mp.4001425. [DOI] [PubMed] [Google Scholar]
- 76.Gygi SP, Aebersold R. Mass spectrometry and proteomics. Curr Opin Chem Biol. 2000;4:489–494. doi: 10.1016/s1367-5931(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 77.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, 3rd, Lefkowitz RJ. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. *This study used a LC tandem MS proteomic approach to demonstrate the feasibility of assessing the interactome of the tagged GPCR interacting proteins βarrestins in HEK-293 cells under various signaling conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, et al. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. * This study combined the SILAC approach with LS tandem MS to identify the profile of protein phosphorylation downstream of β-arrestin-dependent GPCR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi-Rhee E, Schulman H, Cronan JE. Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 2004;13:3043–3050. doi: 10.1110/ps.04911804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]