Abstract
An empirical model of temperament that assessed transactional and cascade associations between respiratory sinus arrhythmia (RSA), negative affectivity, and the caregiving environment (i.e., maternal intrusiveness) across 3 time points during infancy (N= 388) was examined. Negative affectivity at 5 months was associated positively with maternal intrusiveness at 10 months, which in turn predicted increased negative affectivity at 24 months. RSA at 5 months was associated positively with negative affectivity at 10 months, which subsequently predicted greater RSA at 24 months. Finally, greater RSA at 5 months predicted greater negative affectivity at 10 months, which in turn predicted greater maternal intrusiveness at 24 months. Results are discussed from a biopsychosocial perspective of development.
Keywords: temperament, parenting, RSA, negative affect
Recent theoretical and empirical work has provided updated perspectives regarding the nature of temperament (Rothbart, 2011; Shiner et al, 2012), defined as early emerging, relatively stable individual differences in the realms of affectivity, activity level, attention, and self-regulation (Shiner et al, 2012). It is now widely acknowledged that temperament is not as stable as previously conceptualized. As biological systems mature and new skills come on-line, especially those that play a role in the inhibition of behavior, the expression of infant's affect and their behavioral reactivity may decrease (Rothbart, 2011). Thus, although temperament is believed to be biologically-based and early emerging, many traits can change as a function of the reciprocal associations between infants' rapidly developing biological systems and increasingly sophisticated behavioral control. Traditional temperament perspectives posit that temperament traits are biologically driven and become more open to environmental influences over time. Current temperament perspectives, however, suggest that this dichotomy between biology and the environment is not plausible; instead, temperament is shaped by complex relations that occur between the infant and her environment starting at conception and continuing across childhood (Rothbart, 2011; Shiner et al., 2012).
Two hypotheses are at the center of these modified views of temperament; the first is that change occurs in the expression of temperament across infancy and early childhood, and the second is that these changes result from complex associations among children's biological functioning and behavioral control, as well as the environmental contexts in which children develop (Rothbart, 2011; Shiner et al., 2012; Zentner & Bates, 2008). In the current study, we sought to identify potential associations among biological, behavioral, and environmental processes that are theorized to underlie change in temperament dispositions. As opposed to past perspectives that have approached studying the development of biological and behavioral functioning in a dichotomized way, we align with current temperament perspectives and acknowledge that biology and behavior influence one another in a reciprocal fashion across development, and are truly integrated processes (Dennis, Buss, & Hastings, 2012; Shiner et al., 2012). However, for the purposes of studying the integration of these levels of influence across development it is necessary to create artificial boundaries given that it is impossible to tease apart what is “biological”, “behavioral”, and “environmental”.
Although it is widely accepted that there is a transactional nature between biological, behavioral, and environmental factors in development (Calkins, 2011; Gottlieb & Lickliter, 2007), there is a lack of empirical work directly examining the bi-directional developmental influences on children's temperamental traits. Thus, in an effort to better understand how infants' biological functioning influences their behavioral expression of temperament and how these behavioral manifestations of temperament affect the development of infants' biological systems, we consider both observed and physiological measures of temperament in the current study as an important empirical step in this area of inquiry. Moreover, we aim to better understand how the caregiving environment influences infants' temperament characteristics both biologically and behaviorally, as well as identify the way in which infants' characteristics may impact the caregiving they receive. To address these issues, we employ tenets of a biopsychosocial theoretical perspective (Calkins, 2011; Calkins, Perry, & Dollar, 2015) and build a developmental model of temperament that acknowledges the interdependence among biological systems, behavioral expression, and environment at 3 time points from 5 to 24 months of age.
Biopsychosocial Model of Temperament
A biopsychosocial framework underscores the importance of integrating across biological, behavioral, and social levels of analysis when accounting for variation in patterns of child adjustment and maladjustment (Calkins et al., 2015). By doing so, this theoretical approach provides greater specificity into the process mechanisms that help to explain developmental phenomena. Further, this framework underscores the important role of biology in developmental pathways to and from the complex behaviors we observe, while also highlighting that the social context may alter or constrain biobehavioral associations. In accordance with this perspective, we examine indicators of children's biological and behavioral functioning, as well as their social context, in an effort to identify specific pathways that contribute to the change in temperament expression over time.
For decades, temperament researchers have acknowledged the importance of children's developing biological systems in the emergence, continuity, and change of individual differences in temperament (Goldsmith et al., 1987; Rothbart, 2011). Research in this area has identified biomarkers, or biological indicators of a specific developmental process, that correlate with observable behaviors reflecting the experience and expression of emotion (Dennis et al., 2012). Many temperament researchers have focused specifically on young children's parasympathetic nervous system (PNS) functioning. From this work, scientists have been able to identify PNS biomarkers and highlight the important role they play in individuals' ability to modulate their state, emotions, and arousal in ways that allow them to react in a socially appropriate manner (Kreibig, 2010; Porges, 2003).
One way that parasympathetic influences on cardiac activity can be assessed is by measuring respiratory sinus arrhythmia (RSA), which reflects heart rate variability that occurs at the frequency of breathing. Specifically, through an integrated system of feedback loops the vagus cranial nerve sends input to the heart and causes changes in cardiac activity that allow the body to transition between sustaining metabolic processes and generating responses to the environment (Calkins, 2011; Porges, 2007). It is because of this process that RSA is theorized to contribute to individual differences in children's experience and modulation of arousal in emotionally-charged contexts.
Temperament researchers have consistently identified baseline RSA, or RSA during a resting state, as a key biomarker of an individual's characteristic level of arousal or physiological reactivity. As such, baseline RSA is commonly considered to be a temperamental, trait-like measure that is associated with an individual's capacity for responding to the environment (Blandon, Calkins, Keane, & O'Brien, 2010; Stifter & Fox, 1990). The majority of studies conducted with children find that higher baseline RSA is associated with better developmental outcomes such as greater sociability and appropriate emotional expressivity (Cole, Zhan-Waxler, Fox, Usher, & Welsh, 1996; Gottman & Katz, 2002), better regulation/soothability (Calkins, 1997), and greater attentional control (Suess, Porges, & Plude, 1994). In a number of studies using infant samples, however, greater RSA has been found to be associated with greater irritability (DiPietro, Larson, & Porges, 1987), frustration (Calkins, Dedmon, Gill, Lomax, & Johnson, 2002), fear (Stifter & Fox, 1990), and stress (Gunnar, Porter, Wolf, Rigatuso, & Larson, 1995). One possible explanation for these mixed findings is that greater reactivity to the environment during infancy may afford infants increased opportunities to gain and practice regulatory skills, subsequently contributing to more appropriate behavioral expression of emotion in childhood. It is also possible that greater engagement in environmental contexts that elicit negative emotion contribute to greater flexibility of biological responding in various social contexts, thereby influencing more socially appropriate responses in childhood. Thus, understanding the way in which developmental changes in baseline RSA are associated with individual differences in behavioral outcomes from early infancy through toddlerhood is imperative.
Although there are differing opinions regarding which behavioral traits should be considered under the rubric of temperament, the dimension of negative affectivity is arguably the most widely studied. Negative affectivity refers to the dispositional tendency toward experiencing and expressing the emotions of frustration/anger, fear, and sadness (Goldsmith et al., 1987). Infants and children who experience and express high negative affectivity are inclined to cry, fuss, and show more negative emotions overall. Further, these children show intense emotional reactions in a range of situations, highlighting characteristic levels of negativity (Rothbart & Bates, 2006).
Given that infants high in negative affectivity tend to get more upset and display negative emotions more often, it is not surprising that negative affect has important implications for children's social and psychological well-being, including psychopathology and social competence (Rothbart & Bates, 2006; Stifter & Dollar, 2016). For instance, negative affectivity has been highlighted repeatedly as an important predictor of behavior problems in both the internalizing and externalizing realms (Eisenberg et al., 2005; Mesman et al., 2009). In addition, numerous studies have reported that negative affectivity is inversely associated with the development of appropriate social skills and overall social competence in childhood (Rydell, Thorell, & Bohlin, 2007). Therefore, a greater understanding of how infants' negative affectivity changes, as well as how it is associated bidirectionally and transactionally with developmental changes in baseline RSA and environmental factors, is critical.
One of the most salient environmental factors believed to influence changes in the expression of temperament is input from primary caregivers. Indeed, the quality of interactions with the child's proximal environment, namely the parents, has been shown to play a role in the development of some temperament traits, including negative affectivity (Rothbart & Bates, 2006). Sensitive parents who promptly respond to and correctly interpret their child's cues (Ainsworth, Blehar, Waters, & Wall, 1978) may create a context in which their child is able to develop, use, and practice the ability to control their negative emotions (Kopp, 1989). In contrast, parental intrusiveness, or an exertion of parental control over children, preempts the child's development of autonomy (Ainsworth et al., 1978), and has been linked with a host of poor child outcomes, including more negativity and aggressive behavior (Belsky, Putnam, & Crnic, 1996; Egeland, Pianta, O'Brien, 1993).
Historically, research on the direction of effects between children's temperament and parenting behaviors focused on unidirectional associations; some research suggests that children's biological and behavioral indicators of temperament affect subsequent parenting behavior, and other work suggests the opposite direction of effects. For instance, multiple studies have reported that highly negative children are more likely to evoke parenting that is more rejecting, controlling and power assertive than less negative children (e.g., Clark et al., 2000). Similarly, lower levels of baseline RSA, thought to indicate less biological flexibility in environmental responding, have been found to predict more negative maternal behavior at a later age (Kennedy, Rubin, Hastings, & Maisel, 2004).
In contrast, researchers have also found that harsh parenting is predictive of changes in toddlers' expression of negative emotions (Scaramella, Sohr-Preston, Mirabile, Callahan, & Robinson, 2008), and disengaged and intrusive parenting is predictive of sustained or increased levels of infants' negative affectivity (Belsky, Fish, & Isabella, 1991). Moreover, a number of studies have found negative parenting behaviors to be associated with less adaptive patterns of children's physiological functioning over time (Calkins, Graziano, Berdan, Keane, & Degnan, 2008; Calkins, Smith, Gill, & Johnson, 1998). Thus, there is consistent empirical support indicating a potential bidirectional association between parenting behaviors that are less affectionate and supportive, and both infant negative affectivity and physiological responding. This work underscores the importance of assessing these bidirectional relations in the context of one another in developmental models of temperament. However, almost all empirical work on this topic presents correlational findings that only measure one direction of effects, and is therefore preliminary in nature. Considering the simultaneous bidirectional associations between these constructs across infancy greatly extends this area of research.
Bidirectional, Transactional, and Cascade Associations
In addition to positing that developmental processes are biological, behavioral, and social in nature, inherent in a biopsychosocial perspective is the notion that these developmental processes do not function in isolation; instead, they change one another continuously across the course of development (Calkins et al., 2015). Empirical work guided by this model attempts to elucidate coactions and transactions across and within multiple levels to gain a better understanding of the association between biology and the development of complex behavior to influence social adaptation (Sameroff, 2010).
To our knowledge, no study has examined the complex associations among biological and behavioral indicators of temperament in conjunction with the parenting context, across infancy. However, longitudinal support for bidirectional and transactional associations across biological and behavioral functioning been documented. For example, Pesonen, Räikkönen, Heinonen, Komsi, Järvenpää, and Strandberg (2007) found evidence of a bidirectional relation between negative affectivity and maternal stress across a 5-year period; higher infant negative affectivity at 6 months contributed to an increase in stress 5 years later, and maternal stress when infants were 6 months contributed to an increase in child negative affectivity over the same period. Although parenting stress is not a direct representation of parent behavior, parenting stress has been linked to less sensitivity and more intrusiveness (Belsky, 1984). Thus, these negative parenting behaviors are likely to be one mechanism through which greater maternal stress is associated with increased negative emotionality. In another study, Perry, Mackler, Calkins, and Keane (2014) demonstrated a transactional association between child physiological functioning and maternal sensitivity from age 2.5 to age 5.5 such that sensitive responding by mothers when children were 2.5 was associated with greater increases in physiological functioning by age 4.5, which in turn was associated with higher levels of sensitive parenting when children were 5.5 years-old. Taken together, these studies highlight that young children's biological and behavioral characteristics have an influence on, and are influenced by, the social environment.
Although beneficial in improving our understanding of the relations between biological, behavioral, and environmental aspects of temperament, these studies only addressed the associations between two of these three aspects at a given time. Examining each of these aspects in one model allows for a more nuanced understanding of how biological and behavioral processes are simultaneously associated with one another and with the environmental context in which they are both situated. Moreover, to our knowledge, researchers have yet to investigate the potential bi-directional association between children's biological and behavioral temperament traits. For example, theoretical and empirical work has suggested that behavioral affectivity is at least partially dependent on underlying physiological mechanisms (Calkins, 2011; Porges, 2003); it is also possible, however, that the behaviors children engage in when reacting to their environment facilitate or influence the development of their biological systems.
Multi-level developmental models such as these are particularly informative when investigating developmental change in temperament traits because they account for the well-established stability in parenting, emotional affectivity, and physiological functioning. That is, associations that emerge reflect bi-directional and transactional relations among biological, behavioral, and environmental factors after acknowledging the significant role of each processes' prior functioning. In addition, including biological, behavioral, and environmental processes allows for a better test of previously theorized cascade effects from biology to environment. Developmental cascades models are based on the expectation that development in one domain will shape development in other domains in a progressive cascade (e.g., Gottlieb, 2007; Calkins et al., 2015). For example, research has indicated that infants' physiological capabilities predict observed behavior (Perry, Calkins, & Bell, 2015; Stifter & Fox, 1990), and that children's observed behavior has an impact on the parenting that they receive (Clark & Kochanska, 2000; Davidov & Grusec, 2006). Therefore, empirically testing the potential cascade effects from biology to environment over an extended developmental time period during infancy is an important next step.
The Current Study
Our study uses a biopsychosocial model and builds on previous work by examining the transactional and cascade associations among biological, behavioral, and environmental aspects of infant temperament at 5 months, 10 months, and 24 months of age. Specifically, we consider the longitudinal indirect pathways by which RSA, negative affectivity, and maternal intrusiveness influence, and are influenced by, one another across infancy. We chose to focus our investigation on maternal intrusiveness because we believed that intrusive parenting behavior specifically may be more strongly related to infant negativity and baseline RSA over time, than mother's general sensitivity. Intrusive parenting limits children's autonomy and as a result can also limit their opportunities to modulate their own negative affect during emotionally charged situations. Moreover, the limiting of autonomy is frustrating for infants and may also hinder their ability to control physiological arousal in a way that may facilitate greater PNS functioning and flexibility (as indicated by baseline RSA).
As presented throughout our literature review, a number of significant associations may arise when considering RSA, negative affectivity, and maternal intrusiveness in combination with one another across infancy. Because we are the first to look at the associations among these constructs in this way, we can only speculate directionality on a few potential transactional pathways. We outline these paths below but also acknowledge that a significant strength of our design is that it allows for additional and alternative paths to emerge.
Because maternal intrusiveness may limit children's ability to be autonomous, and a lack of autonomy often proves to be frustrating for infants, maternal intrusiveness at 5 months may be associated with greater negative affectivity at 10 months. Previous work has shown that highly negative children are more likely to evoke parenting that is more controlling and power assertive. Thus, greater negative affectivity at 10 months may subsequently predict an increase in maternal intrusiveness at 24 months.
Baseline RSA is often considered to be a trait-like measure associated with reactivity to the environment. Therefore, higher baseline RSA at 5 months may be associated with greater negative affectivity at 10 months. However, increased opportunities in which infants respond to and engage with the environment may facilitate the development of greater biological flexibility across various contexts. Thus, greater negative affectivity at 10 months may be associated with greater increases in baseline RSA at 24 months.
Finally, because restricted autonomy associated with greater maternal intrusiveness may have a negative influence on developing biological systems such that it also restricts children's opportunities to practice up- and down-regulating their own arousal, maternal intrusiveness at 5 months may be associated with decreases in baseline RSA at 10 months. Less biological flexibility in environmental responding may make it harder for children to behave in socially appropriate ways and therefore be more difficult to parent. Therefore, decreases in baseline RSA at 10 months may be associated with increases in maternal intrusiveness at 24 months.
Because analyses regarding cascade effects across biological, behavioral, and social processes were exploratory in nature, we did not speculate any specific pathways.
Methods
Participants
The current study utilized data from infants and their mothers who are part of a larger, ongoing longitudinal study examining psychobiological processes in cognitive and emotional development (N = 410). Study participants were recruited by two research locations (Greensboro, North Carolina, and Blacksburg, Virginia), with each location recruiting half of the total sample. The Blacksburg sample was assessed between September 2002 and November 2009, the Greensboro sample between April 2007 and September 2009. The demographics of the participants recruited from each site reflected the demographics of the area in which each research laboratory was located. Participants at each site did not differ in terms of sex, χ2 (1, N = 388) = 2.26, p = ns. However, the Blacksburg site had mothers with higher levels of education on average, t (378) = -3.26, p < .001, and the Greensboro site had a greater number of ethnic minority participants, χ2 (1, N = 384) = 26.65, p < .001. Infants were recruited via commercial mailing lists, flyers, newspaper advertisements, and word of mouth. Of the original 410 study participants, 22 were removed from the dataset because they were low birth-weight (i.e., weighing less than 5 lbs 8oz (2500 grams), premature (born more than 28 days early), or were diagnosed with a developmental delay by age 3. Thus, the final sample included a total of 388 healthy, full-term infants. Mothers were, on average, 29 years old (SD = 6) when the infants were born. Demographics of the sample broken down by assessment can be found in Table 1.
Table 1. Sample characteristics by assessment.
| 5-month assessment | 10-month assessment | 24-month assessment | |
|---|---|---|---|
| Total N | 388 | 352 | 320 |
| Infant age | 162 days(SD=7.8); 5.4 months | 314 days(SD=11.4); 10.4 months | 758 days(SD=28.8); 25.2 months |
| Infant sex | 51% female | 51% female | 51% female |
| Infant race | 13% African American 78% European American 9% multiracial or other |
13% African American 78% European American 9% multiracial or other |
12% African American 80% European American 8% multiracial or other |
| Mother education | 2% did not complete high school 26% completed high school 6% had a technical degree 44% had a bachelor's degree 22% had a graduate degree |
2% did not complete high school 26% completed high school 6% had a technical degree 44% had a bachelor's degree 22% had a graduate degree |
2% did not complete high school 26% completed high school 6% had a technical degree 44% had a bachelor's degree 22% had a graduate degree |
Families lost to attrition included those who could not be located, moved out of the area, declined participation, or did not respond to phone and letter requests to participate. There were no significant differences between families who did or did not participate at every time point in terms of child sex or race. Families who did and did not participate at every time point also did not differ with regards to any of the primary variables of interest, with one exception; mothers who did not participate at every assessment were slightly more intrusive at 5 months than mothers who participated at every assessment, t (357) = -2.21, p < .05. A chi-square test revealed that mothers who did not participate at every assessment were no different than mothers who did participate at every assessment in terms of education.
Procedures
Data were collected in both research locations using identical protocols. Research assistants from each location were trained together by the project's Principal Investigator on protocol administration, as well as on behavioral and psychophysiological coding. To ensure that identical protocol administration was maintained between the labs, the Blacksburg site periodically viewed DVD recordings and psychophysiology files collected by the Greensboro site. To ensure that identical coding criteria were maintained between labs, the Blacksburg site provided reliability coding for behavioral data and verification of artifact screening for psychophysiology data collected and coded by the Greensboro site.
Upon arrival at the research laboratory, participants were greeted by a research assistant who explained the study procedures and obtained signed consent from the mother. After a brief warm-up period, a researcher applied ECG (electrocardiogram) electrodes and infants participated in a variety of behavioral laboratory tasks assessing cognitive, emotional, and maternal-child interaction processes. The session was digitally recorded for later behavioral coding. Parents were paid $50 for each laboratory visit.
Measures
Maternal intrusiveness
Intrusive behavior was defined as insensitive or over-controlling behavior during which mothers ignored or over-rode infants' behavioral cues and initiations of play in favor of their own behavioral agenda. Intrusive maternal behavior was observed at the 5 and 10-month laboratory assessments during a peek-a-boo task in which mothers were instructed to play peek-a-boo with their infants for 2 minutes using their hands or a provided washcloth to cover their own eyes. Examples of intrusive behaviors included mothers failing to modulate their own behavior in response to infants turning away or expressing negative affect, and overwhelming or over stimulating infants with a continuous barrage of play instead of letting infants set the pace of play. Behavior was also scored as intrusive if mothers physically manipulated infants' hands or face to make them perform an action or manipulation. At 24-months, infants and their mothers were given puzzles and asked to work together for 2 minutes to put the puzzles together. Examples of maternal intrusive behaviors included mothers taking over putting together the puzzle, mothers taking puzzle pieces out of their infant's hands, and mothers following their own agenda rather than following the lead of the infant.
For both tasks, intrusive behavior was coded in 30 second epochs using a coding scheme adapted from previous work (Calkins, Hungerford, & Dedmon, 2004) and was rated on a 1 to 4 scale with a 1 indicating no evidence of intrusiveness and a 4 being consistent, high levels of intrusive behavior across the epoch. Scores were summed across epochs and divided by the total number of epochs to create an average maternal intrusiveness score for each task. Reliability coding was accomplished on 22% of the sample at 5 and 24 months and 30% of the sample at 10-months. The inter class correlation (ICC) between each pair of coders was acceptable at 5-months (ICC=.96), 10-months (ICC=.83), and 24-months (ICC=.95).
Baseline respiratory sinus arrhythmia (RSA)
To measure infant RSA, 5 and 10-month continuous ECG data was recorded for 1 minute while infants were seated on their mother's laps and watched a research assistant manipulate a toy containing brightly colored balls on a testing table 1.1 meters in front of them. Mothers were instructed not to talk or interact with their infant. At 24-months, continuous ECG data was recorded for 1 minute while infants watched an emotionally neutral video. These procedures quieted the infant and yielded minimal gross motor movements and have been used in prior work with this age group (e.g., Bell, 2012).
ECG data was recorded from two neonatal disposable electrodes using modified lead II alignment (right collarbone and lower left rib; Stern, Ray, & Quigley, 2001). The cardiac electrical activity was amplified using a James Long Company Bioamp (Caroga Lake, NY) and bandpassed from 0.1 to 100 Hz. The QRS complex was displayed on the acquisition computer monitor and digitized at 512 samples per second. The acquisition software was Snapshot-Snapstream (HEM Corporation, Southfield, MI) and the raw data were stored for later R-wave detection and analyses.
ECG data were examined and analyzed using IBI Analysis System software developed by James Long Company (Caroga Lake, NY). First, R waves were detected offline with a four-pass peak detection algorithm, resulting in a data file with onset times for each detected R-wave. To edit ECG artifact, the ECG signal was viewed alongside tick marks representing the times of software-detected R-waves. If an R-wave was not detected by the software, a tick mark was inserted into the graphical ECG record. If the undetected R-wave was visible in the ECG, it was marked manually. If the R-wave was not visible, the tick mark was placed based on the specific editing rules of Byrne and Porges (1993). Movement artifact was designated by the absence of at least three consecutive R-waves. These artifact-scored epochs were eliminated from all calculations.
The edited R-wave was converted to heart period (i.e., time between heart beats). Spectral analysis was used to calculate high frequency variability (i.e., RSA) in the heart period data, using a discrete Fourier transform with a 16-second Hanning window and 50% overlap. The frequency band for quantification of RSA at each age was 0.24 – 1.04 Hz. This frequency band is appropriate for all age groups between infancy and early childhood (Bar-Haim, Marshall, & Fox, 2000). The RSA data were transformed using natural log to normalize the distribution.
Negative Affectivity
Mothers reported on infants' negative affectivity at 5 and 10 months using the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003). The IBQ-R is a 191-item questionnaire assessing 3- to 12-month old infants' emotional and behavioral responses across a number of situations, measuring 14 domains of infant temperament. Parents were asked to report on specific infant behaviors in the last one to two weeks (e.g., when introduced to an unfamiliar adult, how often did the baby cling to a parent), using a 7-point Likert scale with response options that range from never (1) to always (7). Although all IBQ-R temperament subscales were collected, the Negative Affectivity factor was of interest in the current study. The Negative Affectivity factor of the IBQ included the Sadness (general low mood and activity specifically related to personal suffering; 14 items), Distress to Limitations (fussing, crying or showing distress; 16 items), Fear (startle or distress to sudden changes in stimulation; 16 items), and reverse coded Falling Reactivity (rate of recovery from peak distress, excitement, or general arousal; 13 items) subscales. Taken together, these subscales reflected infants' tendency to experience negative emotions and their behavioral expressions of increased arousal. Thus, we believe this measure most accurately reflected infant's negative affectivity across a range of emotions in a variety of situations. In our data, the 59-item factor had an internal reliability (Cronbach's Alpha) of .92 at 5 months and .89 at 10 months.
At 24 months, mothers reported on infants' negative affectivity using the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, 2006). The ECBQ is a 201-item questionnaire assessing 1.5- to 3-year old children's emotional and behavioral responses across a variety of contexts, measuring 18 dimensions of child temperament. Parents were again asked to report on specific child behaviors (e.g., “When another child took away his/her toy, how often did your child become angry?”), using a 7-point Likert scale with response options that range from never (1) to always (7). Although all 18 of the ECBQ temperament subscales were collected, the Negative Affectivity factor was again of primary interest in the current study. The Negative Affectivity factor included the Discomfort (10 items), Sadness (12 items), Fear (11 items), Frustration (12 items), and reverse coded Soothability (9 items) subscales. Similar to the IBQ-R, these subscales reflected children's tendency to experience negative emotions and their ability to modulate the expression of these emotions. In our data, the 54-item factor had an internal reliability of .88.
Results
Analytic Approach
Preliminary analyses were conducted to examine descriptive information for study variables and correlations among study variables (see Table 2). Data screening was conducted to assess for normality and outliers. Associations between negative affectivity, RSA, and maternal intrusiveness were estimated using Mplus (Version 7; Muthen & Muthen, 2012) and full information maximum likelihood (FIML) estimation was used to handle incomplete data (please see Table 2 for Ns of each variable). Because there were different questionnaires and different mother-child tasks given at each age to align with the varying developmental levels spanning the study, negative affectivity and maternal intrusiveness were standardized across time points. Finally, given the well-established association between maternal education and parenting behavior (e.g., Fox, Platz, & Bently, 1995), maternal education was included as a covariate in all models.
Table 2. Descriptive Statistics and Correlations among Model Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Negative affectivity 5m | -- | ||||||||
| 2. Negative affectivity 10m | .56** | -- | |||||||
| 3. Negative affectivity 24m | .38** | .45** | -- | ||||||
| 4. RSA 5m | .07 | .20** | .03 | -- | |||||
| 5. RSA 10m | .01 | .09 | .00 | .30** | -- | ||||
| 6. RSA 24m | .02 | .14* | .01 | .18** | .28** | -- | |||
| 7. Maternal intrusiveness 5m | -.01 | -.07 | .08 | -.05 | -.06 | -.03 | -- | ||
| 8. Maternal intrusiveness 10m | .09 | .15** | .15* | .01 | .03 | -.11 | .26** | -- | |
| 9. Maternal intrusiveness 24m | .14* | .23** | .32** | .07 | .11 | .07 | .07 | .24** | -- |
|
| |||||||||
| Mean | 2.98 | 3.38 | 3.07 | 3.97 | 4.64 | 5.43 | 1.28 | 1.21 | 1.38 |
| Standard Deviation | .65 | .62 | .54 | 1.21 | 1.13 | 1.30 | .45 | .41 | .45 |
| Minimum | 1.54 | 1.90 | 1.70 | 1.06 | .99 | 2.40 | 1.00 | 1.00 | 1.00 |
| Maximum | 4.92 | 5.09 | 4.76 | 11.11 | 8.87 | 12.28 | 3.25 | 3.67 | 3.25 |
| Skew (SE) | .29(.13) | .13 (13) | .32(.14) | 1.55(.13) | .41(.13) | .87(.15) | 2.16(.13) | 2.81(.13) | 1.52(.14) |
| N | 374 | 344 | 320 | 357 | 336 | 273 | 359 | 344 | 303 |
Note: values are prior to standardization;
p < .05,
p < .01
As suggested by de Jonge, Dormann, Janssen, Dollard, Landeweerd, & Nijhuis (2001), a stability model was compared to the more complex, cross-lagged model. Therefore, the following models were estimated: a stability model for maternal intrusiveness, infant RSA, and infant negative affectivity with no cross-lag paths, and a full reciprocal model for maternal intrusiveness, RSA, and negative affectivity. Across models, concurrent associations among constructs were estimated. Given that the stability and cross-lagged models were nested, a chi-square difference test was used to evaluate if the cross-lagged model explained the data above and beyond the stability model (de Jonge et al, 2001).
Structural Model Comparisons
Stability model
Autoregressive coefficients were constant over time for maternal intrusiveness, RSA, and negative affectivity in the stability model (see Figure 1. for standardized estimates). Negative affectivity showed moderate stability from 5- to 24-months (5m to 10m: B = .55, p < .001; 10m to 24m: B= .41, p < .001). Maternal intrusiveness (5m to 10m: B = .30, p < .001; 10m to 24m B= .24, p < .001), and RSA (5m to 10m: B = .28, p < .001; 10m to 24m: B= .30, p < .001), showed low to moderate stability from 5- to 24-months. There were no significant within-time correlations between negative affectivity, RSA, and maternal intrusiveness at the 5-month time point. RSA was also not correlated with maternal intrusiveness or negative affectivity at the 10- and 24-month time point. However, maternal intrusiveness was correlated positively with negative affectivity at both 10-months (r = .15, p<.01) and 24-months (r = .19, p<.01), indicating that greater maternal intrusiveness was associated with children's decreased ability to control their emotional experiences and regulate negative emotional states. The stability model had adequate fit to the data, χ2 (21) = 53.40, p < .00, CFI = 0.91, RMSEA = 0.06 [CI .04-.09], SRMR = 0.05.
Figure 1. Stability model across constructs (standardized estimates).
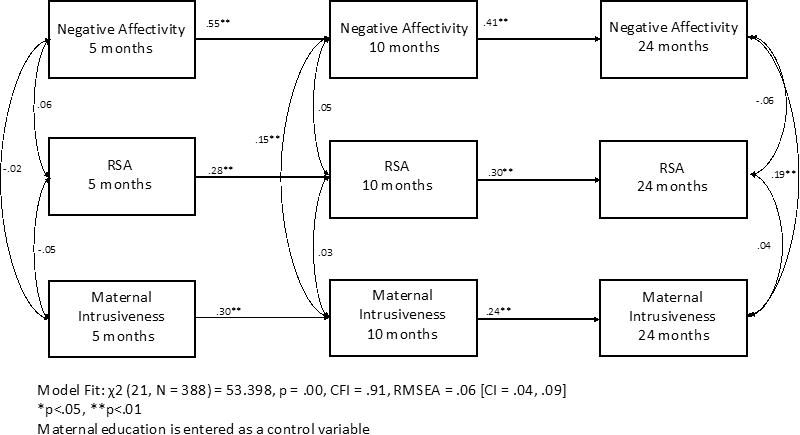
Cross-lagged model
The cross-lagged model demonstrated excellent model fit, χ2 (9) = 12.38, p = .20, CFI = 0.99, RMSEA = 0.03 [CI .00-.07], SRMR = 0.02, and the chi-square difference test revealed that the cross-lagged model significantly improved fit over the stability model, Δ χ2 (12) = 41.02, p < .001. As such, the cross-lagged model accounting for the cross-construct relations better explained the associations between negative affectivity, RSA, and maternal intrusiveness from 5-months to 24-months than the model containing just stability pathways. Within the cross-lagged model (see Figure 2. for standardized estimates) all autoregressive stability paths remained positive and significantly different from zero. The cross-lagged paths revealed that greater negative affectivity at 5-months and 10-months was associated with greater maternal intrusiveness at 10-months (B = .11, p < .05) and 24-months (B = .18, p < .001), supporting previous literature indicating that specific child characteristics may influence the type of parenting they receive. However, greater maternal intrusiveness at 10-months also predicted greater negative affectivity at 24-months (B = .10, p <.05), indicating a potential transactional association. In addition, infants' RSA at 5-months was associated positively with their negative affectivity at 10-months (B = .11, p < .01). Again, this finding aligns with prior research suggesting that RSA during a resting state is an indicator of children's reactivity and may therefore be associated with greater negative reactivity in challenging emotional contexts. However, negative affectivity at 10-months was also associated with greater RSA at 24-months (B = .19, p < .05), again suggesting a transactional association between the development of biological systems and observed behavior.
Figure 2. Cross-lagged model across constructs (standardized estimates).
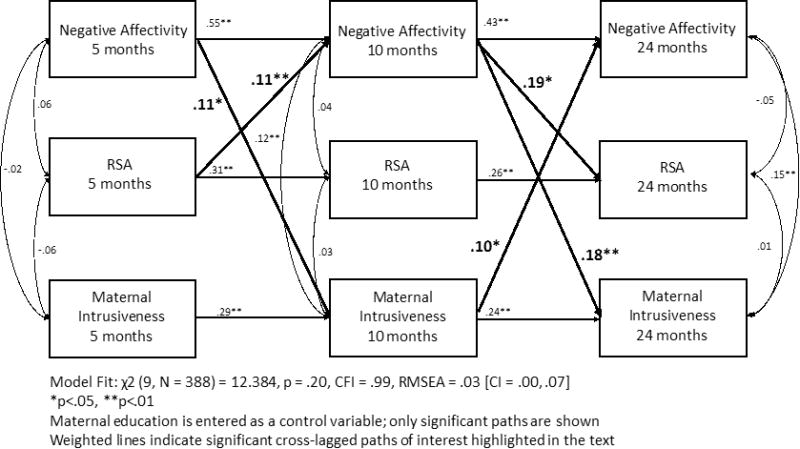
Within the cross-lagged model, we used a bias-corrected bootstrapping procedure (10,000 draws) to test the indirect effects across negative affectivity, RSA, and maternal intrusiveness from 5-months to 24-months. This approach has been shown to generate the most accurate confidence intervals for indirect effects, reducing Type 1 error rates and increasing power over other similar tests (MacKinnon, Lockwood, & Williams, 2004). For the purposes of interpretation, we will focus only on indirect effects that did not include stability paths.
Two indirect pathways supported the potential transactional associations highlighted by the direct effects presented above. The first transactional pathway from negative affectivity at 5-months to maternal intrusiveness at 10-months to negative affectivity at 24-months was significant (unstandardized estimate = .02, 95% BC Bootstrap CI [.001, .04]). This finding indicates that infant negative affectivity may elicit greater maternal intrusiveness which subsequently may also facilitate a greater tendency for children to display negative affectivity over time. The second indirect pathway from 5-month RSA to 10-month negative affectivity to 24-month RSA was also significant (unstandardized estimate = .03, 95% BC Bootstrap CI [.005, .05]), suggesting that although physiological functioning may contribute to the behavior we observe, behavior infants engage in may also further facilitate changes in the development of physiological systems. Finally, there was one significant cascade effect from 5-month RSA to 10-month negative affectivity to 24-month maternal intrusiveness (unstandardized estimate = .02, 95% BC Bootstrap CI [.005, .05]). This effect was evident across biological, behavioral and social indicators and suggested that infant's physiological functioning was associated with subsequent behavioral reactivity, and the behavior infants displayed had a direct effect on the later parenting that they received.
Discussion
Updated conceptualizations of temperament theories have altered our view of the nature of temperament, especially in regards to the stability of certain temperament traits and how the expression of temperament influences and is influenced by the infant's environment (Rothbart, 2011; Shiner et al, 2012). Specifically, recent perspectives emphasize that the expression of temperament is shaped by complex and inextricably integrated associations among indicators of biological and behavioral functioning, and the environmental context, that occur over time (Rothbart, 2011; Shiner et al., 2012). Even though these important modifications to theoretical conceptualizations of temperament are now widely accepted, there is a lack of empirical work addressing the complex interplay that is theorized to exist between one's biobehavioral temperament traits and their social environment across early development. In an attempt to fill some of these gaps in the developmental literature, the current study created arbitrary, yet analytically necessary, lines among potential biological, behavioral, and environmental influences associated with developmental changes in temperament traits. In doing so, we focused on potential bidirectional and reciprocal associations that may emerge. Specifically, the transactional and cascade associations among baseline RSA, negative affectivity, and maternal intrusiveness across infancy were examined.
As hypothesized, we demonstrated that greater negative affectivity at 5 and 10 months was associated with greater maternal intrusiveness at 10 and 24 months, respectively. These findings support previous research suggesting that infants' negative affectivity may evoke parenting behaviors that are higher in control or negativity (e.g., Coplan, Reichel, & Rowan, 2009). Children with greater negative affectivity can experience a high degree of arousal and can easily become overwhelmed. In turn, it may be more difficult for parents to calm and assist them in transitioning from one context to the next, therefore taxing the parent's patience and increasing the likelihood that they would respond in a more controlling or intrusive way. Moreover, highly aroused children who are unable to manage their emotional experiences or expressions in effective ways may direct angry outbursts or oppositional behaviors toward parents, which is also likely to increase parents' attempts to control children's behavior and emotions in more adverse and negative ways (Kiff, Lengua, & Zalewski, 2011).
In accordance with prior literature (e.g., Bridgett et al., 2009), we also found that greater maternal intrusiveness at 10 months predicted greater negative affectivity at 24 months, indicating that parenting behaviors also have an impact on infant's temperament traits. Taken together, these findings suggest a transactional relation between infant negative affectivity and maternal intrusiveness across the infancy period. Specifically, infants who are higher in negative affectivity may be more difficult to soothe and therefore evoke more intrusive and controlling parenting. The increase in intrusive parenting, however, may deprive children opportunities in which they can practice managing their arousal and behavior on their own, therefore exacerbating infants' negative emotional responses. Thus, the significant indirect effect from negative affectivity at 5 months to greater negative affectivity at 24 months through maternal intrusiveness at 10 months highlights a potential negative and escalating cycle of mutual influence between infants and their caregivers. This cycle may have significant implications for infant's developing self-regulatory skills. Self-regulatory development occurs over a gradual transition from almost sole reliance on the caregiver to increasing levels of independence (Sameroff, 2010). Intrusive behavior may not allow mothers to appropriately attune to their child's needs and serve as effective co-regulators who are able to scaffold the skills needed to appropriately modulate emotional experiences and behavior.
Contrary to expectations, there was no association between maternal intrusiveness and infants' RSA in either direction. Although previous research has demonstrated that parenting behaviors directly affect the development of infants' physiological functioning (Calkins et al., 2008; Perry et al., 2015), the majority of this work has found these associations by assessing changes in RSA from a baseline state to an emotional task. Unlike these studies, we focused our investigation on baseline RSA given its hypothesized role in infants' trait-like ability to respond to the environment. We believe that combining an indicator of potential environmental responding (i.e., baseline RSA) with an indicator of observed behavioral reactions to environmental stimuli (i.e., negative affectivity), provided us with multiple aspects of temperament that are necessary when attempting to build a more cohesive developmental model. A number of existing studies investigating the association between parenting and baseline RSA fail to find an association. For example, maternal sensitivity during free play and the still-face reunion was not significantly related to infants' baseline RSA in multiple studies (e.g., Conradt & Alblow, 2010; Propper et al., 2008). Similarly, Perry and colleagues (2012) reported that maternal emotional support was not related to trajectories of baseline RSA from age 3 to age 5. However, one study examining the bidirectional association between maternal negative behavior and baseline RSA found that lower levels of baseline RSA at age 2 did predict more negative maternal behavior at age 4, although negative maternal behavior at age 2 was not associated with baseline RSA at age 4 (Kennedy et al., 2004). The stable nature of baseline RSA that is accounted for in our model, and the lack of association between maternal intrusiveness and infant RSA, suggests that although baseline RSA is thought to be indicative of potential physiological responding, it may be relatively less sensitive to environmental variation than measures of physiological functioning that are hypothesized to be more regulatory in nature, such as changes in RSA from a baseline measure to a task measure. That is, parenting behaviors may be more predictive of how an infant controls their arousal and responds to emotionally-charged situations rather than a more characteristic measure of whether they are more or less equipped to respond.
Given that no studies to our knowledge have investigated the bidirectional bio-behavioral associations among temperament traits that are biological and behavioral in nature across infancy, our hypothesis that higher RSA at 5 months would be associated with greater negative affectivity at 10 months, and greater negative affectivity at 10 months would facilitate greater increases in RSA at 24 months, was based solely on work examining the unidirectional links between RSA and negative affectivity. A significant number of studies have found higher RSA to be associated with better developmental outcomes in children (e.g., Calkins, 1997; Gottman & Katz, 2002). In infancy, however, this pattern does not always emerge. For example, DiPietro and colleagues (1987) found that newborn infants with greater RSA were more reactive and irritable according to the Neonatal Behavioral Assessment Scale. Similarly, Gunnar and colleagues (1995) reported that newborns with higher baseline RSA showed greater cortisol levels following a heelstick blood draw, indicating a greater negative reaction to stress. Stifter and Fox (1990) found that newborns with greater RSA were rated by their mothers at 5-months as being more easily frustrated and fearful. Finally, in 6 month olds, Calkins, and colleagues (2002) found that more easily frustrated infants displayed higher RSA scores than did less easily frustrated infants. Thus, our finding that greater baseline RSA at 5 months is associated with greater maternal-reported negative affectivity at 10 months not only supports previous research, but it also provides an important extension to prior work in this area within this developmental period.
The current study also found evidence of the reciprocal association such that greater negative affectivity at 10 months was associated with greater RSA at 24 months. Thus, the significant indirect effect from 5-month baseline RSA to 24-month baseline RSA through infants' negative affectivity at 10-months suggests a bio-behavioral transactional association across the first two years of life. Empirical work has shown that infants with greater RSA display greater sustained attention and visual discrimination (Linnemeyer & Porges, 1986; Richards, 1987), and show increased emotion to novel events (Stifter, Fox, & Porges, 1989). In addition, Calkins and colleagues (2002) found that not only were infants with greater RSA more easily frustrated, they sought help from their mothers more frequently than children with lower RSA. When engaging with a sensitive caregiver, this increased assistance may facilitate co-regulatory patterns that are instrumental to the later development of children's independent self-regulatory skills (Kopp, 1982; 1989). Thus, there is a theoretical change in the function of RSA from infancy through early childhood that is supported by the current findings. During infancy, when children lack regulatory skills to manage their reactivity to the environment, a greater propensity for physiological responding to emotionally taxing environmental stimuli (i.e., higher baseline RSA) was associated with greater negativity, irritability, and distress. Thus, greater RSA in infancy may function to elicit greater interactions with the environment by increasing opportunities for infants to attend to the environment, experience a wide range of emotional intensities, refine skills needed to manage those emotions, and facilitate greater flexibility of parasympathetic responding by continuously requiring the up and down-regulation of arousal. Greater RSA in early childhood, that is likely in part a result from these experiences, may then function to provide children with increased physiological flexibility to challenging environments and allow them to utilize a range of self-regulatory strategies more effectively, thereby supporting the well-established link between higher baseline RSA and optimal outcomes in childhood.
Finally, we found support for one multi-level cascade association, providing an important next step for the biopsychosocial literature. A test of indirect effects revealed that 5-month RSA predicted 10-month negative affectivity, which in turn predicted 24-month maternal intrusiveness. That is, infant's physiological functioning affected the behaviors that they later employed, which had a direct impact on the parenting that they received, a critical environmental context for the development of young children's later cognitive, social, and emotional functioning (Bates & Pettit, 2015; Fay-Stammbach, Hawes, & Meredith, 2014). This cascade effect highlights that although the previously described association between 10-month negative affect and 24-month RSA does emerge, negative affect at 10-months is also associated with greater maternal intrusiveness. Thus, if early biological reactivity influences greater negative affect, intrusiveness that may result from infant's expressed negativity may have even more detrimental effects on infant's self-regulatory development if toddler's biological systems are more reactive to environmental input (i.e., greater RSA). In contrast, however, greater flexibility in biological responding could also buffer the effects of intrusive parenting behavior.
Although many developmental scientists theorize cascade effects across biological, behavioral, and environmental processes (i.e., Gottlieb, 2007; Calkins et al., 2015), research investigating developmental processes across that incorporate functioning in each of these domains is severely lacking. Our study is one of the first to provide empirical support for the notion that one way in which children's biological characteristics influence the environment in which they are embedded is through the influence physiological mechanisms have on observable behavior. Specifically, biology is hypothesized to be a driving force behind the development of behavior such that the behavioral manifestations of multiple developmental psychopathologies are thought to be at least partially dependent on underlying biological mechanisms (Rutter & Sroufe, 2000). However, the environment plays a critical role in that both biological and behavioral traits influence which environments individuals put themselves in and how those environments respond to them. Thus, considering biological and behavioral processes isolation is problematic and does not allow a comprehensive picture of important developmental phenomena including temperament. We believe that the current study provides evidence that invaluable insight into complex multi-level constructs can be gained by incorporating biological, behavioral, and environmental processes and assessing the longitudinal associations among them.
Limitations and Future Directions
Despite the many strengths of this study, it is not without limitations. First, the current study examined baseline RSA as a measure of temperamental biological reactivity and did not account for changes in RSA in response to emotionally eliciting events. Change in RSA in response to emotionally taxing tasks is most often hypothesized to assess regulatory functioning, or children's ability to modulate or control the emotional arousal that they experience (El-Sheikh, Hinnant, & Erath, 2015; Perry et al., 2015). As previously mentioned, we felt that having a measure of observed negative affectivity, in which infant's ability to manage their emotions is reflected, combined with their physiological capacity for responding (i.e., baseline RSA), was ideal given our focus on individual differences in the expression of temperament over time. Nonetheless, a measure of change in RSA during emotionally-charged situations would have complimented the current findings. Unfortunately, RSA was not assessed during emotion-eliciting tasks when children were 24 months old and thus this data was not available. Building upon the current findings, future work should examine the bidirectional, transactional, and cascade effects of RSA change, negative affectivity, and parental intrusiveness during infancy.
A second limitation of the current work is that the measure of infant negative affectivity at each time point was reported by mothers, not observed in the laboratory. It is well-documented that many factors may affect how parents rate their child's temperament (e.g., maternal depression, mood, or personality), potentially leading to a biased portrayal of their child's behavior (Stifter & Dollar, 2016); thus, an important next step is for future work to examine the associations found in the current study while employing observations of negative affectivity. Although an important extension, it should be noted that there are also many advantages of employing parent reports of infant temperament. In the current study, a reported measure of negative affectivity was particularly advantageous over an observed measure given that we aimed to assess negative affectivity across multiple time points. Without having the same reported measure at each time point, it would be difficult to ascertain which aspects of change were due to actual developmental changes within the infant versus differences attributed to variation in the behavioral task employed. In addition, use of parent report of infant negative affectivity presented the opportunity for us to utilize three differing methods (parent-report, physiological, and observed), thereby adding to the strength of the findings. Moreover, the measure of negative affectivity used in the current study allowed us to gather information on children's experience and expressions of multiple emotions across multiple environmental contexts, an advantage a behavioral laboratory task would not have been able to provide.
The unequal time points between assessments were also a limitation. The larger ongoing longitudinal study from which these data were pulled is focused on cognition and emotion integration across early development. Infants were seen at 5 and 10 months because of the emphasis placed on the emergence of the executive attention system. After the 10-month assessment, infants were not seen again until they were 24 months-old, a time point in which we anticipated seeing the emergence of self-regulatory skills. Moreover, interacting with an infant and interacting with a toddler are qualitatively different in terms of the child's cognitive development, regulatory skills, and language abilities, and therefore should be considered when interpreting intrusiveness in mother-child interactions.
Finally, although the sample used in the current study is relatively diverse, the majority of infants were from families in which at least one parent had a college education or higher. We did control for maternal education in the current analyses. However, given that parental education and income level are known to be associated with the constructs examined in the current study (Fox et al., 1995), future work is needed to determine whether associations found in this study function similarly with more at risk and diverse socioeconomic samples. In normative samples containing highly educated and high income moms, such as the one used in the current study, there is often less variability in measures of general sensitivity. Thus, another important direction for future work utilizing samples with more diversity is to assess the role of specific sensitive behaviors on the development of infant's temperamental traits. Our sample also lacked fathers. Fathers are taking a much more active role in child rearing and we are not able to address how their specific parenting behaviors may compliment mothers' parenting styles to affect infant temperament.
Conclusion
In conclusion, this study extends our understanding of how negative affectivity develops across infancy. Further, this work provides preliminary evidence that this development occurs at least in part because of a complex association that exists between children's biological and behavioral functioning within their social context. Although preliminary support exists for transactional and cascade associations among physiological and behavioral manifestations of temperament traits and environmental factors during infancy, to our knowledge this is the first study to consider these associations in one model. Using a biopsychosocial model of development, we provided evidence of transactional associations between infant negative affectivity and maternal intrusiveness from 5 months to 24 months, as well as between infant baseline RSA and infant negative affectivity. In addition, a cascade effect emerged across all levels of analysis highlighting the need for future work to consider the complex associations that exists both within the individual and between the individual and the environment.
Acknowledgments
This research was supported by Grants HD049878 and HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to the last author. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research and to our research teams at Blacksburg and Greensboro for their assistance with data collection and coding.
References
- Ainsworth MS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Oxford, England: Lawrence Erlbaum; 1978. [Google Scholar]
- Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37(1):44–56. doi: 10.1002/1098-2302(200007)37:1<44∷AID-DEV6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bates JE, Pettit GS. Temperament, parenting, and social development. In: Grusec JE, Hastings PD, Grusec JE, Hastings PD, editors. Handbook of socialization: Theory and research. 2. New York, NY, US: Guilford Press; 2015. pp. 372–397. [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83:251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Belsky J. The determinants of parenting: A process model. Child Development. 1984;55(1):83–96. doi: 10.2307/1129836. [DOI] [PubMed] [Google Scholar]
- Belsky J, Fish M, Isabella RA. Continuity and discontinuity in infant negative and positive emotionality: Family antecedents and attachment consequences. Developmental Psychology. 1991;27(3):421–431. doi: 10.1037/0012-1649.27.3.4212008. [DOI] [Google Scholar]
- Belsky J, Putnam S, Crnic K. Coparenting, parenting, and early emotional development. In: McHale JP, Cowan PA, McHale JP, Cowan PA, editors. Understanding how family-level dynamics affect children's development: Studies of two-parent families. San Francisco, CA, US: Jossey-Bass; 1996. pp. 45–55. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O'Brien M. Contributions of child's physiology and maternal behavior to children's trajectories of temperamental reactivity. Developmental Psychology. 2010;46(5):1089–1102. doi: 10.1037/a0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne EA, Porges SW. Data-dependent filter characteristics of peak-valley respiratory sinus arrhythmia estimation: A cautionary note. Psychophysiology. 1993;30(4):397–404. doi: 10.1111/j.1469-8986.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31(2):125–135. doi: 10.1002/(SICI)1098-2302(199709)31:2<125∷AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Biopsychosocial models and the study of family processes and child adjustment. Journal of Marriage and Family. 2011;73(4):817–821. doi: 10.1111/j.1741-3737.2011.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE, Gill KL, Lomax LE, Johnson LM. Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy. 2002;3(2):175–197. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50(8):751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Hungerford A, Dedmon SE. Mothers' interactions with temperamentally frustrated infants. Infant Mental Health Journal. 2004;25:219–239. doi: 10.1002/imhj.20002. [DOI] [Google Scholar]
- Calkins SD, Perry NB, Dollar JM. A biopsychosocial model of the development of self-regulation in infancy. In: Balter L, Tamis-LeMonda C, editors. Child Psychology: A Handbook of Contemporary Issues. 3rd Vol. 3. in press. [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral, and physiological regulation during toddlerhood. Social Development. 1998;7(3):350–369. doi: 10.1111/1467-9507.00072. [DOI] [Google Scholar]
- Clark LA, Kochanska G, Ready R. Mothers' personality and its interaction with child temperament as predictors of parenting behavior. Journal of Personality and Social Psychology. 2000;79(2):274–285. doi: 10.1037/0022-3514.79.2.274. [DOI] [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105(4):518–529. doi: 10.1037/0021-843X.105.4.518. [DOI] [PubMed] [Google Scholar]
- Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants' early regulatory behavior. Infant Behavior & Development. 2010;33(3):251–265. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Reichel M, Rowan K. Exploring the associations between maternal personality, child temperament, and parenting: A focus on emotions. Personality and Individual Differences. 2009;46(2):241–246. doi: 10.1016/j.paid.2008.10.011. [DOI] [Google Scholar]
- Davidov M, Grusec JE. Untangling the Links of Parental Responsiveness to Distress and Warmth to Child Outcomes. Child Development. 2006;77(1):44–58. doi: 10.1111/j.1467-8624.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- De Jonge J, Dormann C, Janssen PPM, Dollard MF, Landeweerd JA, Nijhuis FJN. Testing reciprocal relationships between job characteristics and psychological well-being: A cross-lagged structural equation model. Journal of Occupational and Organizational Psychology. 2001;74:29–46. doi: 10.1348/096317901167217. [DOI] [Google Scholar]
- Dennis TA, Buss KA, Hastings PD. Introduction to the monograph: Physiological measures of emotion from a developmental perspective: State of the science. Monographs of the Society for Research in Child Development. 2012;77(2):1–5. doi: 10.1111/j.1540-5834.2011.00654.x. [DOI] [Google Scholar]
- di Pietro JA, Larson SK, Porges SW. Behavioral and heart rate pattern differences between breast-fed and bottle-fed neonates. Developmental Psychology. 1987;23(4):467–474. doi: 10.1037/0012-1649.23.4.467. [DOI] [Google Scholar]
- Egeland B, Pianta R, O'Brien MA. Maternal intrusiveness in infancy and child maladaptation in early school years. Development and Psychopathology. 1993;5(3):359–370. [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valiente C, … Shepard SA. The Relations of Problem Behavior Status to Children's Negative Emotionality, Effortful Control, and Impulsivity: Concurrent Relations and Prediction of Change. Developmental Psychology. 2005;41(1):193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath SA. Sleep and development: Advancing theory and research: VI. Marital conflict, vagal regulation, and children's sleep: A longitudinal investigation. Monographs Of The Society For Research In Child Development. 2015;80(1):89–106. doi: 10.1111/mono.12146. [DOI] [PubMed] [Google Scholar]
- Fay-Stammbach T, Hawes DJ, Meredith P. Parenting influences on executive function in early childhood: A review. Child Development Perspectives. 2010;8(4):258–264. doi: 10.1111/cdep.12095. [DOI] [Google Scholar]
- Fox RA, Platz DL, Bentley KS. Maternal factors related to parenting practices, developmental expectations, and perceptions of child behavior problems. The Journal of Genetic Psychology: Research and Theory on Human Development. 1995;156(4):431–441. doi: 10.1080/00221325.1995.9914835. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior & Development. 2003;26(1):64–86. doi: 10.1016/S0163-6383(02)00169-8991.tb01623.x. [DOI] [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, McCall RB. What is temperament? Four approaches. Child Development. 1987;58(2):505–529. doi: 10.2307/1130527. [DOI] [PubMed] [Google Scholar]
- Gottlieb G, Lickliter R. Probabilistic epigenesis. Developmental Science. 2007;10(1):1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF. Children's emotional reactions to stressful parent-child interactions: The link between emotion regulation and vagal tone. Marriage & Family Review. 2002;34(3-4):265–283. doi: 10.1300/J002v34n03_04. [DOI] [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Child Development. 1995;66(1):1–13. doi: 10.2307/1131186. [DOI] [PubMed] [Google Scholar]
- Kennedy AE, Rubin KH, Hastings PD, Maisel B. Longitudinal Relations between Child Vagal Tone and Parenting Behavior: 2 to 4 Years. Developmental Psychobiology. 2004;45(1):10–21. doi: 10.1002/dev.20013. [DOI] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M. Nature and nurturing: Parenting in the context of child temperament. Clinical Child and Family Psychology Review. 2011;14(3):251–301. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. doi: 10.1037/0012-1649.18.2.199. [DOI] [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25(3):343–354. doi: 10.1037/0012-1649.25.3.343. [DOI] [Google Scholar]
- Kreibig SD, Samson AC, Gross JJ. The psychophysiology of mixed emotional states. Psychophysiology. 2013;50(8):799–811. doi: 10.1111/psyp.12064. [DOI] [PubMed] [Google Scholar]
- Linnemeyer SA, Porges SW. Recognition memory and cardiac vagal tone in 6-month-old infants. Infant Behavior & Development. 1986;9(1):43–56. doi: 10.1016/0163-6383(86)90037-8. [DOI] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman J, Stoel R, Bakermans-Kranenburg MJ, van IJzendoorn MH, Juffer F, Koot HM, Alink LA. Predicting growth curves of early childhood externalizing problems: Differential susceptibility of children with difficult temperament. Journal Of Abnormal Child Psychology. 2009;37(5):625–636. doi: 10.1007/s10802-009-9298-0. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 7th. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Perry NB, Calkins SD, Bell MA. Indirect effects of maternal sensitivity on infant emotion regulation behaviors: The role of vagal withdrawal. Infancy. 2015 doi: 10.1111/infa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Mackler JS, Calkins SD, Keane SP. A transactional analysis of the relation between maternal sensitivity and child vagal regulation. Developmental Psychology. 2014;50(3):784–793. doi: 10.1037/a0033819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A, Räikkönen K, Heinonen K, Komsi N, Järvenpää A, Strandberg T. A transactional model of temperamental development: Evidence of a relationship between child temperament and maternal stress over five years. Social Development. 2008;17(2):326–340. doi: 10.1111/j.1467-9507.2007.00427.x. [DOI] [Google Scholar]
- Porges SW. Social Engagement and Attachment: A Phylogenetic Perspective. In: King JA, Ferris CF, Lederhendler II, King JA, Ferris CF, Lederhendler II, editors. Roots of mental illness in children. New York, NY, US: New York Academy of Sciences; 2003. pp. 31–47. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Cox M. Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development. 2008;79(5):1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior & Development. 2006;29(3):386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Development. 1987;58(2):488–496. doi: 10.2307/1130525. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality in development. New York, NY, US: Guilford Press; 2011. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, Damon W, Lerner RM, Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol 3, Social, emotional, and personality development. 6th. Hoboken, NJ, US: John Wiley & Sons Inc; 2006. pp. 99–166. [Google Scholar]
- Rothbart MK, Ellis LK, Posner MI. Temperament and self-regulation. In: Vohs KD, Baumeister RF, Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2nd. New York, NY, US: Guilford Press; 2011. pp. 441–460. [Google Scholar]
- Rutter M, Sroufe LA. Developmental psychopathology: Concepts and challenges. Development and Psychopathology. 2000;12(3):265–296. doi: 10.1017/S0954579400003023. [DOI] [PubMed] [Google Scholar]
- Rydell A, Thorell LB, Bohlin G. Emotion regulation in relation to social functioning: An investigation of child self-reports. European Journal of Developmental Psychology. 2007;4(3):293–313. doi: 10.1080/17405620600783526. [DOI] [Google Scholar]
- Sameroff A. A unified theory of development: A dialectic integration of nature and nurture. Child Development. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Scaramella LV, Sohr-Preston SL, Mirabile SP, Robison SD, Callahan KL. Parenting and children's distress reactivity during toddlerhood: An examination of direction of effects. Social Development. 2008;17(3):578–595. doi: 10.1111/j.1467-9507.2007.00439.x. [DOI] [Google Scholar]
- Shiner RL, Buss KA, McClowry SG, Putnam SP, Saudino KJ, Zentner M. What is temperament now? Assessing progress in temperament research on the twenty-fifth anniversary of Goldsmith et al. (1987) Child Development Perspectives. 2012;6(4):436–444. [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. 2nd. New York: Oxford University Press; 2001. [Google Scholar]
- Stifter CA, Dollar JM. Temperament and Developmental Psychopathology. In: Cicchetti D, editor. Developmental Psychopathology. 3rd Vol. 3. in press. [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn and 5-month temperament. Developmental Psychology. 1990;26(4):582–588. doi: 10.1037/0012-1649.26.4.582. [DOI] [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in 5- and 10-month-old infants. Infant Behavior & Development. 1989;12(2):127–137. doi: 10.1016/0163-6383(89)90001-5. [DOI] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31(1):17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]


