Abstract
Introduction
Osteogenesis imperfecta (OI) is characterized by skeletal fragility and muscle weakness. We investigated the effects of soluble activin type IIB receptor (sActRIIB-mFc) on muscle mass and function in two distinct mouse models of OI, osteogenesis imperfecta murine (oim) and +/G610C mouse.
Methods
Wildtype (WT), +/G610C, and oim/oim mice were treated from 2 to 4 months of age with Tris-Buffered Saline (vehicle) or sActRIIB-mFc and their hindlimb muscles evaluated for mass, morphology and contractile function.
Results
sActRIIB-mFc-treated WT, +/G610C and oim/oim mice had increased hindlimb muscle weights and myofiber cross-sectional area compared to vehicle-treated counterparts. sActRIIB-mFc treated oim/oim mice also exhibited increased contractile function relative to vehicle treated counterparts.
Discussion
Blocking endogenous ActRIIB was effective at increasing muscle size in mouse models of OI, and increasing contractile function in oim/oim mice. ActRIIB inhibitors may provide a potential mutation specific therapeutic option for compromised muscle function in OI.
Keywords: osteogenesis imperfecta murine, +/G610C, muscle, activin receptor type IIB, myostatin, peak tetanic force
Introduction
Sarcopenia is one of the most common pathophysiological outcomes associated with aging, resulting in reduced muscle mass with substantial changes in muscle fiber type from oxidative to glycolytic.1 Consequences of reduced muscle mass and strength often lead to increased fall risk and fractures, which are in part strongly influenced by cross-talk of the muscle-bone units. Frost’s mechanostat theory suggests that bone is mechanosensitive and that muscle mass and contractile force elicit peak voluntary mechanical load on bone.2 Indeed, many patients with sarcopenia are likely to develop osteoporosis and osteopenia as well.3–9 Sarcopenia and osteoporosis are not only associated with aging, but are also present in many disease states, including cancer cachexia, muscular dystrophies, and osteogenesis imperfecta (OI).10–12
Osteogenesis imperfecta, also known as brittle bone disease, is a heritable connective tissue disorder characterized by compromised biomechanical integrity in type I collagen containing tissues, including bone, vasculature, skin, and tendon.13 More than 85% of individuals with OI have mutations in their type I collagen genes, COL1A1 or COL1A2, while the remaining 15% have mutations in the genes of proteins associated with type I collagen assembly, mineralization or osteoblast function.13 The majority of the clinical and basic research in OI has been targeted towards improving bone directly. Although the correlation between muscle and bone strength is well established, muscle function and pathology in OI has only just begun to be investigated. The limited number of studies evaluating muscle function in type I and type III OI patients report reduced muscle strength.14–17 Muscle function has also been evaluated in two different mouse models of OI, and demonstrated that moderately severe recessive osteogenesis imperfecta murine (oim) mice have an inherent muscle pathology with reduced skeletal muscle mass and contractile function,12 while the milder dominant negative OI mouse model, +/G610C, does not.18 Due to the importance of the muscle-bone crosstalk to bone strength and quality, it is critical to understand muscle properties in OI; as improved muscle function is known to decrease fracture risk and may likely lead to improved bone strength and quality.
Myostatin, a well-known negative regulator of muscle growth, is a member of the TGF-β superfamily and binds to activin receptor type IIB to activate multiple intracellular signaling pathways to regulate gene expression.19 Myostatin knock-out mice exhibit muscle hypertrophy and hyperplasia.20 Due to myostatin’s impact on muscle, various therapeutic molecules have been developed to inhibit myostatin and have been evaluated in a diverse group of muscle-related disorders.21–26 Among these molecules, the soluble activin receptor type IIB-mFc has shown promising results in age-related sarcopenia, cancer cachexia, muscle dystrophy induced by hypoxic conditions, and muscular dystrophic disease.22–25,27,28
The presence of muscle weakness in OI not only negatively impacts activities of daily living, but it may also contribute to skeletal fragility and increased fracture risk. In the following study, we sought to investigate whether treatment with the soluble activin receptor type IIB decoy molecule (sActRIIB-mFc) would increase muscle mass and enhance contractile function in two molecularly distinct mouse models of OI, osteogenesis imperfecta murine (oim) and +/G610C. Homozygous oim (oim/oim) mice have a functional null mutation in their Col1a2 genes, and thus compensate by making homotrimeric type I collagen, α1(I)3, instead of normal heterotrimeric type I collagen, α1(I)2α2(I), while the +/G610C mice are heterozygous for a glycine to cysteine substitution at position 610 of the α2 chain of type I collagen.29,30 Genetically and phenotypically, the +/G610C mouse models osteogenesis imperfecta in affected family members of a large Amish kindred.31 The oim/oim and the +/G610C mice model moderately severe human OI type III and milder to moderate OI type IV individuals, respectively. As previously reported, oim/oim mice exhibit a muscle pathology, with reduced muscle mass and absolute and specific peak tetanic forces,12 while the +/G610C mice do not.18 In the following study, we investigated the impact of sActRIIB-mFc on muscle properties of +/G610C and oim/oim mice to test the hypothesis that blocking the ligands that bind the endogenous activin receptor IIB by the sActRIIB-mFc decoy will increase hindlimb skeletal muscle weights and absolute muscle contractile strength regardless of the presence of muscle pathologies associated with mutations in type I collagen (glycine substitution vs. functional null Col1a2).
Methods
Animals
Both OI mouse models, G610C (Col1a2tm1.1Mcbr) and oim (Col1a2oim), were bred and maintained on the C57BL/6J congenic background by repeatedly backcrossing into wildtype C57BL/6J mice (stock number 000664, Jackson Laboratory, Bar Harbor, ME, USA) to minimize potential modifier gene effects. The mice were genotyped as previously described32,30 and had ad libitum access to water and food (Purina 5008 Formulab Diet; Purina Mills Inc., St Louis, MO). The animals were housed in an AAALAC accredited facility at the University of Missouri and the protocols used for this study performed under an approved University of Missouri Animal Care and Use Protocol.
Drug Administration
Male and female WT, +/G610C, and oim/oim mice were each randomly distributed into two groups and treated bi-weekly with either vehicle [tris-buffered saline (TBS)] as control or 10mg/kg sActRIIB-mFc in TBS (RAP-031, Acceleron Pharma. Inc, Cambridge, MA) by intraperitoneal injection beginning at 2 months of age and continuing to 4 months of age. The 8–16 week treatment interval encompasses pubertal growth through skeletal maturity and was chosen to potentially maximize the impact of sActRIIB-mFc treatment on musculoskeletal development.33 The 10mg/kg dose of sActRIIB-mFc was previously found to significantly increase muscle mass, which was not further increased by a 30mg/kg dose.28 Animals were not randomized into treatment groups and the investigators were not blinded as to the identity of the animals. At 4 months of age, mice were anesthetized to evaluate hindlimb skeletal muscle contractility in situ, and then euthanized, and the hindlimb skeletal muscles were harvested for histologic evaluation, cross sectional area measurement, and citrate synthase activity.
Contractile Properties
Contractile properties of the soleus (Sol), gastrocnemius (Gast), and tibialis anterior (TA) muscles in male and female WT, +/G610C, and oim/oim mice treated with either vehicle or sActRIIB-mFc were determined as previously described.12 Briefly, mice were anesthetized and the left Sol, Gast, and TA muscles were surgically exposed at their distal insertions. The distal tendon of each muscle was attached to the Grass force transducer and sequentially tested. The distal tendon was adjusted in length so that passive tension was zero grams. The sciatic nerve was isolated and placed on a stimulating electrode and a twitch was obtained as previously described.12 At optimal length, a peak tetanic contraction (Po) was elicited by pulses delivered at 150Hz, 300-ms duration, and an intensity of 6V for each type muscle.34 All data were collected using Power Lab® (ADinstruments, Colorado Springs, CO), and analyzed blind to genotype, sex, and treatment.
Tissue Harvest
Following the contractile function testing, the left (stimulated) and right Sol, plantaris (Plant), Gast, TA, and quadriceps (Quad) muscles were removed, cleaned of extraneous tissue, blotted, and weighed. Left Sol, Gast, and TA muscles were then placed in 4% paraformaldehyde for 24 hours and then transferred to 70% ethanol for future staining with hematoxylin and eosin for morphologic evaluation. The right side Gast was snap frozen in liquid nitrogen and stored at −80°C for future quantitation of citrate synthase activity.
Histologic Evaluation and Cross-Sectional Myofiber Measurements
Left-sided paraformaldehyde and ethanol prepared muscles were transversely sectioned at the middle of the muscle belly, embedded in paraffin and then sectioned at 5µm and stained with hematoxylin and eosin (H&E).12 An average of 300 myofibers was evaluated for morphology and evidence of damage or inflammation, and their myofiber cross-sectional areas were determined using Image J Software (NIH).12 Analyses were performed blind to genotype, sex, and treatment
Citrate Synthase Activity
Citrate synthase was determined in mixed gastrocnemius muscle as a measure of mitochondrial content/function using the methods of Srere et al. as previously described35,36.
Locomotor Activity
Locomotor activity of WT and oim/oim mice was monitored using Med Associates Open Field Test Environment monitors (Georgia, VT) at 1, 2, 3 and 4 months of age as described previously.18 The Med Associates’ Open Field Activity Software was used to evaluate the number of recorded sensor breaks and converting the data into distance traveled (cm). Mice were placed individually into the chamber for 60 minutes per day for five consecutive days. The mice were acclimated on first 2 days and the data from days 3–5 was summated and used in the statistical analyses.
Statistical Analyses
Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC). Data were analyzed as a 3×2×2 factorial [3 genotypes, 2 sexes and 2 treatments (vehicle and sActRIIB-mFc treated)] using Fisher’s Protected Least Significant Difference.37,38 Locomotor activity data was analyzed as a 2×2×4 factorial [2 genotypes, 2 sexes, and 4 time points]. Genotype and sex interactions and the genotype and sex main effects were evaluated. Body, muscle and relative muscle weights (Gast, TA, and Sol), muscle contractile function (Gast and Sol), muscle myofiber cross sectional area (Gast and Sol), and activity levels exhibited a main genotype effect, allowing pooling of genotype-matched male and female values in the absence of genotype and sex interactions. The main genotype effect values for these parameters are presented as the LSmeans±SE. Genotype and sex interactions were present only for the TA myofiber cross-sectional area and contractile generating force. Supplement Tables 1–4 contain the body, muscle and relative muscle weights, muscle contractile function, and muscle myofiber cross sectional area means ± SE separated by sex, treatment, and genotype. If heterogeneous variations were present, a log transformation was used to stabilize the variation. If the log transformation failed to stabilize the variation, a nonparametric ranked analysis was performed according to Conover et al.39 Differences were considered to be significant at p≤ 0.05.
Results
Body Weights
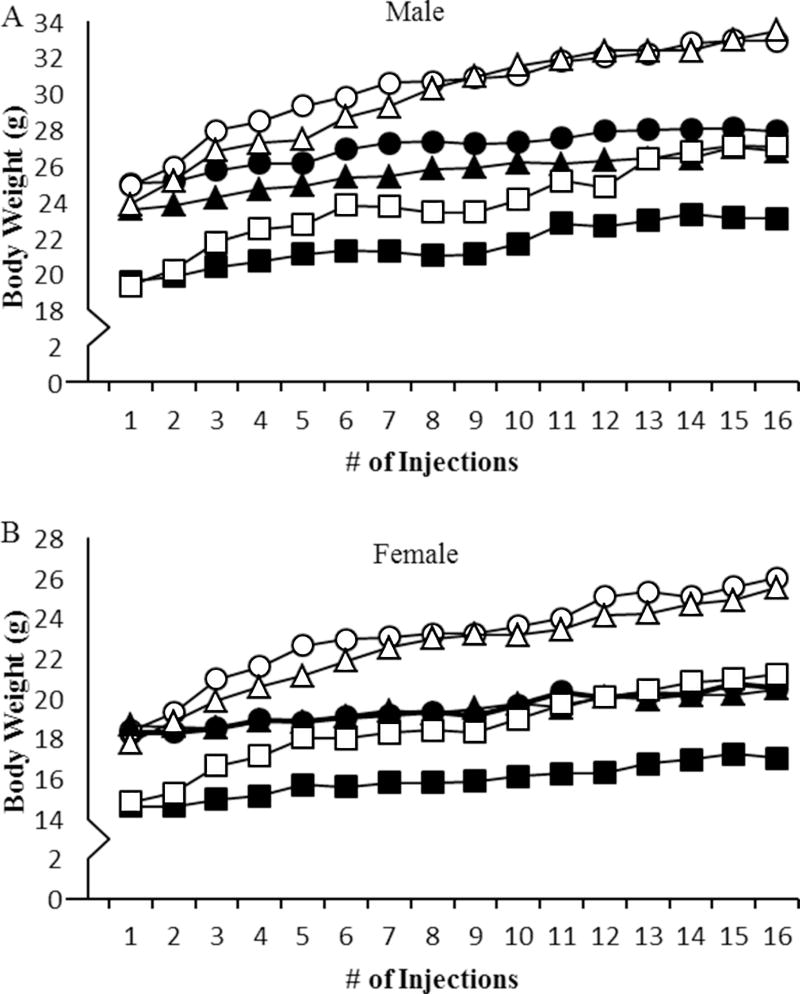
Vehicle treated oim/oim mice had lower body weights as compared to both WT and +/G610C counterparts, while sex- and treatment-matched +/G610C and WT mice were equivalent (Figure 1). Mice treated with sActRIIB-mFc exhibited differences in body weights after 1-week of treatment (after the 2nd injection) as compared to vehicle treated control mice regardless of genotype and sex (Figure 1). At 4 months of age, sActRIIB-mFc treated male and female mice had 20% and 24% increases in body weight, respectively, as compared to their vehicle treated counterparts regardless of genotype (Figure 1).
Figure 1.
sActRIIB-mFc treated mice (open symbols) had increased body weight throughout the study as compared to vehicle treated counterparts (solid symbols) regardless of sex and genotype. Differences between sActRIIB-mFc and vehicle treatments were observed by the 3rd injection regardless of genotype and sex. A) Male and B) female WT (circle), +/G610C (triangle), and oim/oim (square). (n=8–14 per groups)
Muscle Weights
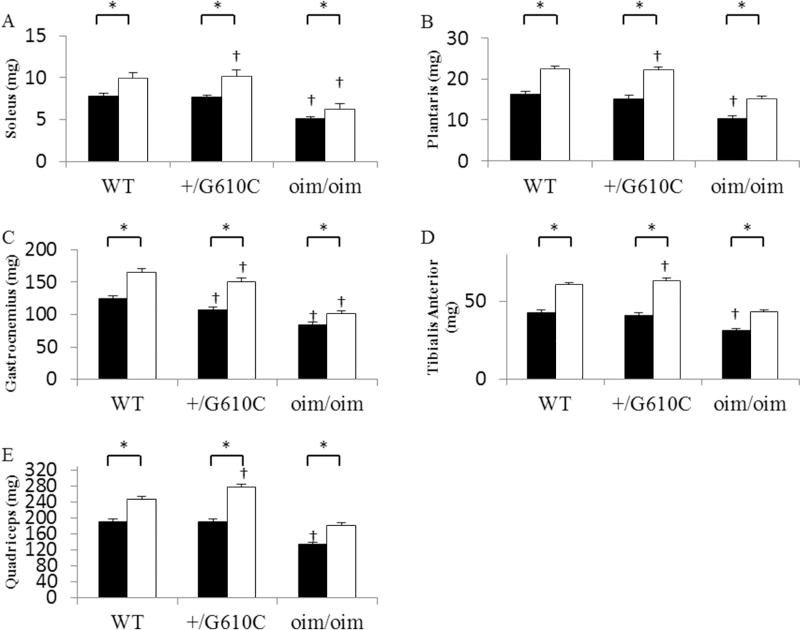
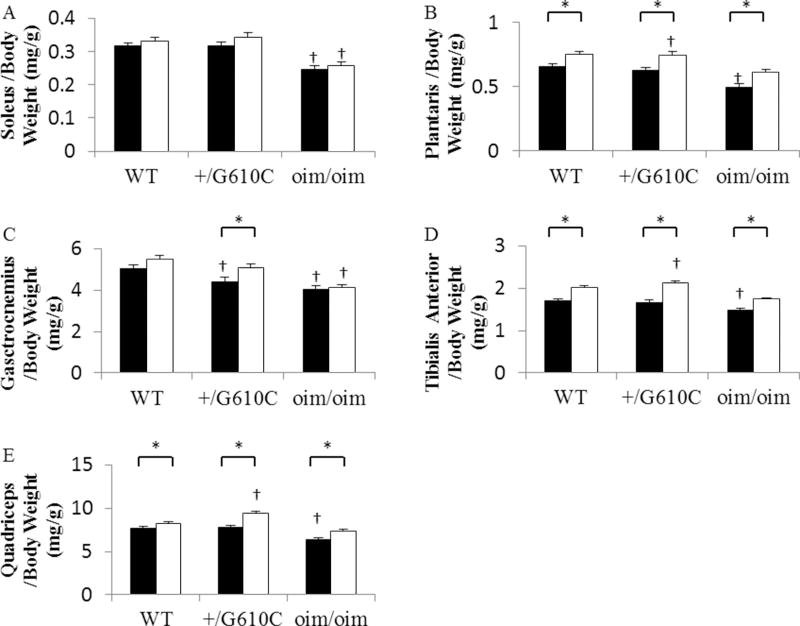
The hindlimb skeletal muscles [soleus (Sol), plantaris (Plant), gastrocnemius (Gast), tibialis anterior (TA), and quadriceps (Quad)] of the vehicle treated oim/oim mice exhibited reduced muscle wet weights as compared to both WT and +/G610C counterparts, while there were no differences in the hindlimb muscle weights of WT and +/G610C mice (Figure 2, Supplement Table 1&2). Mice treated with sActRIIB-mFc exhibited increases in the muscle wet weights of all five hindlimb skeletal muscles as compared to their vehicle treated counterparts regardless of genotype (Figure 2). The effects of genotype and sActRIIB-mFc treatment were still evident when muscle masses were normalized to their body weights [relative muscle weights (muscle wet weight/body weight)], except for sActRIIB-mFc treated Sol muscles for the three genotypes and Gast muscle for WT and oim/oim mice (Figure 3, Supplement Table 1&2). When muscle masses were normalized to their femur length, the sActRIIB-mFc treatment effects were still evident in all genotypes regardless of muscle groups, suggesting the increase in body weight is due to increases in muscle mass (data not shown).
Figure 2.
sActRIIB-mFc treated mice (open bars) had increased hindlimb skeletal muscle weights as compared to vehicle treated counterparts (solid bars: control) regardless of genotype. (A) Soleus, (B) Plantaris, (C) Gastrocnemius, (D) Tibialis Anterior, and (E) Quadriceps muscle wet weights of 4 months old WT, +/G610C, and oim/oim (n=16–25 per groups). The genotype main effect was evaluated. Values are the LSmeans±SE of the combined genotype values regardless of sex. *p<0.05; †p<0.05 vs. WT+vehicle treated mice.
Figure 3.
sActRIIB-mFc treated mice (open bars) had increased relative skeletal muscle weights as compared to vehicle treated counterparts (solid bars: control) regardless of genotypes. (A) Soleus/Body Weight, (B) Plantaris/Body Weight, (C) Gastrocnemius/Body Weight, (D) Tibialis Anterior/Body Weight, and (E) Quadriceps/Body Weight of 4 months old WT, +/G610C, and oim/oim (n=16–25 per groups). The genotype main effect was evaluated. Values are the LSmeans±SE of the combined genotype values regardless of sex. *p<0.05; †p<0.05 vs. WT+vehicle treated mice.
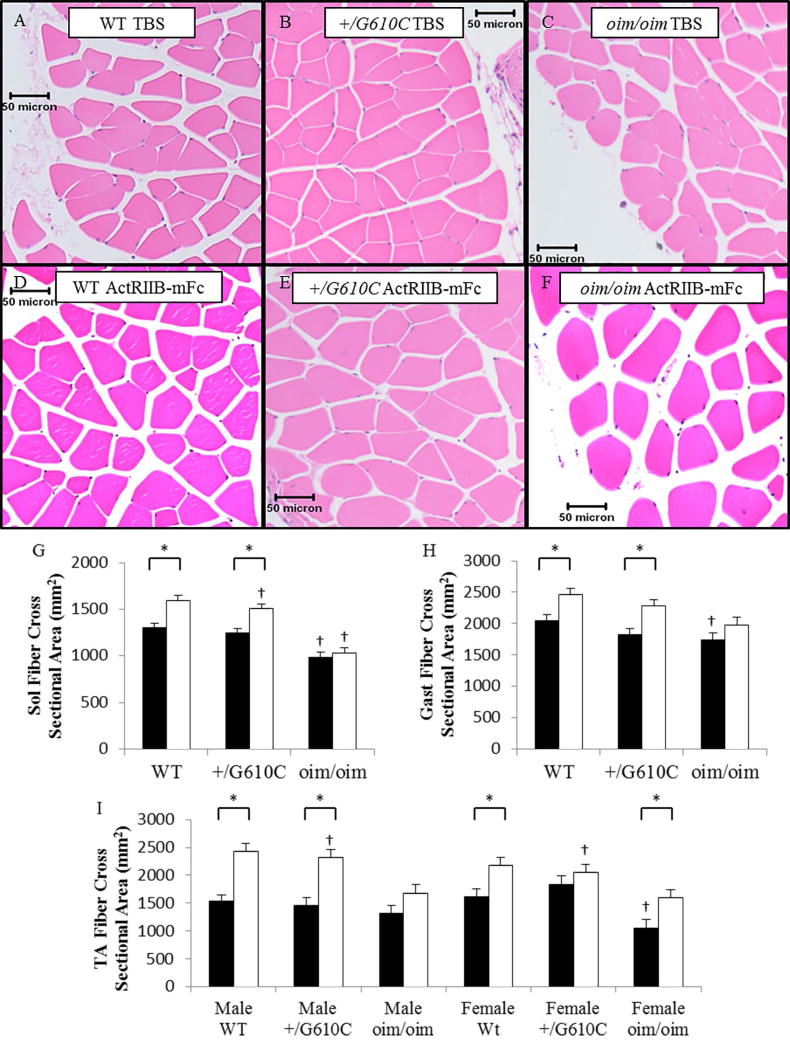
Muscle Histology and Myofiber Cross-Sectional Area
Histological evaluation found no evidence of necrosis, degeneration, or regeneration in vehicle or sActRIIB-mFc treated WT, +/G610C, and oim/oim mouse skeletal muscle morphology (Figure 4A–F, Supplement Table 3&4). sActRIIB-mFc treated WT and +/G610C mice myofiber cross-sectional areas (CSA) were increased in the Sol, Gast and TA muscles as compared to their vehicle treated counterparts ([Sol WT: 22% and +/G610C: 21%] & [Gast WT: 20% and +/G610C: 26%] & [TA Male WT: 59%, Male +/G610C: 59%, Female WT: 35%, and Female +/G610C: 12%]) (Figure 4G–I). For the oim/oim mice, only the female oim/oim TA muscle exhibited increased myofiber CSA in response to sActRIIB-mFc treatment (Figure 4I).
Figure 4.
Hematoxylin and eosin (H&E) stained muscle myofiber cross-sections of the gastrocnemius (Gast) muscle from 4 month old female mice (A–F). There was no evidence of degeneration, regeneration, or multiple centralized nuclei, 10× magnification. sActRIIB-mFc treated mouse myofiber cross-sectional areas (CSA) (open bars) were larger than vehicle treated counterparts (solid bars) (G–I). oim/oim mice exhibited reduced average muscle myofiber CSA as compared to WT and +/G610C counterparts. sActRIIB-mFc treated WT and +/G610C mice exhibited increased muscle myofiber CSA as compared to vehicle treated counterparts. (G) Sol myofiber CSA, (H) Gast myofiber CSA, (I) TA myofiber CSA (n=9–12 per groups; TA muscle groups are n=4–7). The genotype main effect was evaluated. Gast and Sol values are the LSmeans±SE of the combined genotype values regardless of sex, and the TA exhibited a genotype × sex interaction, and the values are LSmeans±SE separated by sex and genotype. *p<0.05; †p<0.05 vs. WT+vehicle treated mice.
Muscle Contractile Generating Capacity
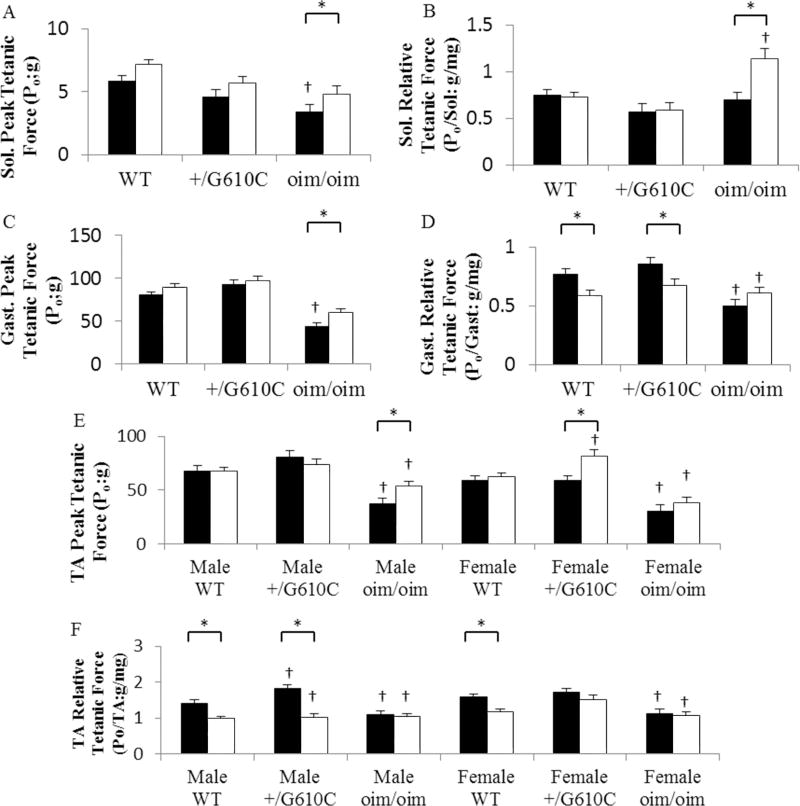
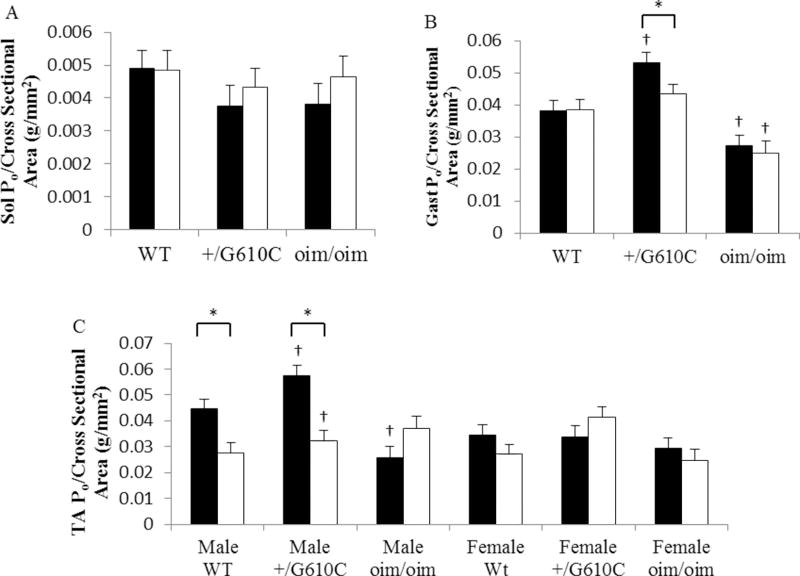
The Sol, Gast, and TA muscles of +/G610C mice exhibited equivalent levels of absolute whole muscle contractile generating capacity [peak tetanic force (Po;g)], relative contractile generating capacity [relative Po; peak tetanic force (Po;g)/muscle weight (mg)], and specific contractile generating capacity [specific Po; peak tetanic force (Po;g)/muscle myofiber cross sectional area (µm2)] as compared to their WT counterparts regardless of sActRIIB-mFc treatment except for the male relative and specific Po of TA muscle and pooled specific Po of Gast muscle (Figure 5 and 6, Supplement Table 3&4). Vehicle treated oim/oim mice had reduced peak tetanic force in Sol, Gast, and TA muscles, and reduced relative Po, and specific Po in Gast, and TA muscles as compared to their WT counterparts (Figure 5 and 6). sActRIIB-mFc treated WT and +/G610C mice had 22% and 24% increase in Sol Po and 11% and 5% increase in Gast Po, respectively, although they did not reach significance. sActRIIB-mFc treated oim/oim mice exhibited 41% (Sol muscle), 35% (Gast muscle), 43% (male TA muscle) and 27% (female TA muscle) increase in peak tetanic force as compared to vehicle treated counterparts (Figure 5). When normalized to muscle size the relative Po of the Sol muscle in the oim/oim mouse was still greater with sActRIIB-mFc treatment (Figure 5). Interestingly, the relative Po of the Gast and TA muscles were decreased in sActRIIB-mFc treated WT and +/G610C mice as compared to their vehicle treated counterparts (Figure 5). The specific Po of the TA muscles were decreased in male WT and +/G610C mice, and the specific Po in Gast muscle was decreased in +/G610C mice with sActRIIB-mFc treatment as compared to their vehicle treated counterparts (Figure 6).
Figure 5.
oim/oim mice had reduced absolute and relative peak tetanic force (Po) as compared to WT and +/G610C counterparts (A–F). sActRIIB-mFc treated oim/oim mice exhibited increased peak Po as compared vehicle treated counterparts (A, C and E). sActRIIB-mFc treated WT and +/G610C mice exhibited reduced relative peak tetanic force as compared to vehicle treated counterparts (B, D, and F). (A) Sol. Peak tetanic force (Po), (B) Sol. relative Po, (C) Gast Peak Po, (D) Gast. Relative Po, (E) TA Peak Po, and (F) TA relative Po of 4 months old WT, +/G610C, and oim/oim vehicle (solid bars: control) and sActRIIB-mFc (open bars) treated mice. (n=10–30 per groups; TA Peak Po n=6–16 per groups). The genotype main effect was evaluated. Gast and Sol values are the LSmeans±SE of the combined genotype values regardless of sex, and the TA exhibited a genotype × sex interaction, and the values are LSmeans±SE separated by sex and genotype. *p<0.05; †p<0.05 vs. WT+vehicle treated mice.
Figure 6.
sActRIIB-mFc treated +/G610C mice Gast muscle had reduced specific tetanic force [specific Po (Peak Po/Muscle myofiber CSA)] as compared to vehicle treated counterparts (B). sActRIIB-mFc treated male WT and +/G610C mice TA muscle had reduced specific Po as compared to vehicle treated counterparts (C). (A) Sol Specific Po, (B) Gast Specific Po, and (C) TA Specific Po of 4 months old WT, +/G610C, and oim/oim vehicle (solid bars: control) and sActRIIB-mFc (open bars) treated mice. (n=9–12 per groups; TA muscle groups are n=4–7). The genotype main effect was evaluated. Gast and Sol values are the LSmeans±SE of the combined genotype values regardless of sex, and the TA exhibited a genotype × sex interaction, and the values are LSmeans±SE separated by sex and genotype. *p<0.05; †p<0.05 vs. WT+vehicle treated mice.
Citrate Synthase Activity
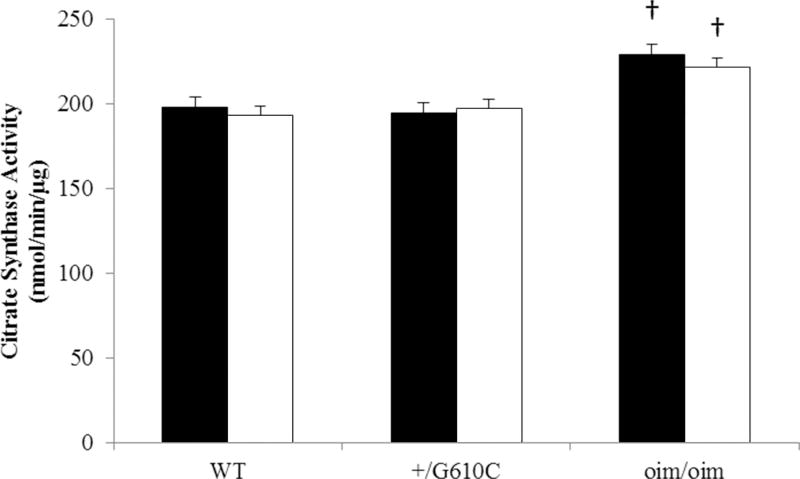
Oim/oim Gast muscle exhibited a 16% increase in citrate synthase activity as compared to WT counterparts, where the levels of +/G610C Gast synthase activity were equivalent to their WT counterparts (Figure 7). There was no change in citrate synthase activity with sActRIIB-mFc treatment regardless of genotype.
Figure 7.
oim/oim mice Gast muscle had increased citrate synthase activity as compared to WT mice. (n=10–12 per groups). There was no difference in citrate synthase activity between vehicle (solid bars: control) and sActRIIB-mFc (open bars) treated mice regardless of genotype. The genotype main effect was evaluated. Values are LSmeans±SE of the combined genotype values regardless of sex. p<0.05; †p<0.05 vs. WT+vehicle treated mice.
Locomotor Activity Levels
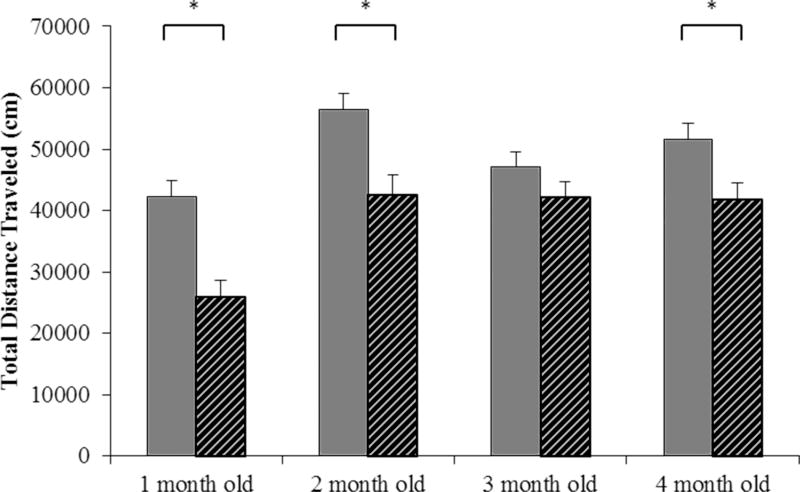
Oim/oim mice had reduced locomotor activity levels at 1, 2, and 4 months old as compared to WT mice (Figure 8).
Figure 8.
oim/oim mice (grey stripe) traveled a reduced distance (cm) over a 1 hour period on 3 consecutive days (3 hours of total travel time) as compared to WT (grey bars) littermates at 1, 2, and 4 months old. (n= 16–25 per groups). The genotype main effect was evaluated. Values are LSmeans±SE of the combined genotype values regardless of sex. *p<0.05
Discussion
Consistent with the previous studies, oim/oim mice exhibited reduced body weights, muscle mass and contractile function compared to WT littermates, whereas the +/G610C mice did not exhibit any muscle pathology (Figure 1, 2, and 5).12,18 Similar to our findings in OI mouse models, several clinical studies of muscle function found that individuals with type I, III, and IV OI exhibited decreased ambulation with reduced muscle strength, and exercise capacity.14–17,40,41 Brizola et al. suggested that the level of ambulation and muscle function in OI were inversely correlated with the severity of bone deformity regardless of OI types.17 Palomo et al. reported reduced transverse forearm muscle cross-sectional area in OI type I and III patients, but not in type IV patients; however there was no functional data.41 Interestingly, +/G610C, which models type IV human OI, did not exhibit a muscle pathology or differences in activity levels relative to WT mice.18 This could reflect species differences and/or mutation specific effects.
Our findings demonstrate an increase in body size and hindlimb skeletal muscle weights with systemic administration of sActRIIB-mFc in WT, +/G610C, and oim/oim mice, potentially due to increased myofiber CSA, suggesting that inhibition of myostatin via sActRIIB-mFc is effective in increasing muscle mass regardless of the presence or absence of OI muscle pathology. Increased muscle mass was associated with increased absolute muscle contractile function without altered relative and specific muscle contractile function in sActRIIB-mFc treated oim/oim mice. Similarly, DiGirolamo and colleagues showed increased muscle mass with 4 weeks administration of soluble activin receptor IIB in oim/oim mice, although the increases in limb muscle weights did not always reach significance and were not as great as the impact on pectoralis muscle.22 We demonstrated increases in all five hindlimb skeletal muscles weights with sActRIIB-mFc treatment. The difference in magnitude of the response likely reflects the duration and frequency of treatment (once per week for 4 weeks vs. bi-weekly for 8 weeks) and the age of treatment initiation (12 weeks vs. 8 weeks of age). 12 week old mice are adult mice near skeletal maturity, whereas 8 week old mice are still within a more rapid growth period and may be more responsive to sActRIIB-mFc treatment. Additionally, older oim/oim mice may have experienced fractures (possibly an accumulation of fractures) as compared to younger oim/oim mice, with these fractures potentially decreasing mobility and impacting muscle mass and function.
Increased muscle mass with sActRIIB-mFc treatment was also concomitant with increased myofiber CSA; with sActRIIB-mFc treated mice exhibiting increased Sol, Gast, and TA myofiber CSA as compared to vehicle treated mice regardless of sex and genotype, although oim/oim mice did not reach significance (Figure 4). Previous studies in dystrophic muscle mouse models have also consistently reported increases in muscle mass and myofiber CSA.23–26,28 Interestingly, MacDonald et al. reported that muscle atrophy in mice due to surgical denervation was not protected by soluble ActRIIB treatment, suggesting increased muscle mass and force via myostatin inhibition may not be effective when there is deterioration of the neuro-muscular junction.42 Muscle atrophy and/or weakness in OI has generally been attributed to immobilization, secondary impacts of neurological impairment or orthopedic casting, and prolonged bedrest due to fractures.43
To determine if the increase in muscle mass and myofiber CSA were associated with improved muscle function, we evaluated the contractile generating force of the Sol, Gast, and TA muscles. Surprisingly, sActRIIB-mFc treated oim/oim mice exhibited increased absolute whole muscle contractile force in Sol, Gast, and TA muscles as compared to vehicle treated counterparts, while WT and +/G610C showed only increasing trends (Figure 5). Although other studies of mouse models with muscle atrophy have shown soluble ActRIIB treatment increased absolute muscle contractile function of compromised muscle,23,24,28,44 there were also reports of earlier muscle fatigability,26,45 decreased gene expression of components involved in mitochondrial function and oxidative phosphorylation,46,47 as well as reduced myofiber specific function in mdx mice (mouse model of Duchenne muscular dystrophy) mice with treatment of higher concentrations of soluble ActRIIB.25 Consistent with these studies, myostatin knock-out (MSTN−/−) mice exhibit centralized nuclei, a sign of muscle degenerations, and reduced relative muscle contractile function as compared to WT and heterozygous myostatin deficient (MSTN+/−) littermates,48 suggesting the complete absence of myostatin is detrimental to muscle myofiber function.
Our results demonstrate sActRIIB-mFc treated oim/oim mice were able to produce higher or equivalent levels of relative and specific muscle contractile forces as compared to vehicle treated counterparts, while WT and +/G610C mice exhibited decreases in relative and specific muscle contractile forces in their Gast and TA muscles. The percent decrease of the relative contractile force of sActRIIB-mFc treated WT and +/G610C Gast and TA muscles [Male WT: −23% (Gast) and −30% (TA); Male +/G610C: −36% (Gast) and −43% (TA) (Supplement Table 3)] were greater relative to age-matched 4 month old heterozygous MSTN+/− mice [Male: −18% (Gast) and −30% (TA)],48 but not as robust as myostatin knockout (MSTN−/−) mice [Male: −39% (Gast) and −39%(TA)].48 These data suggest that sActRIIB-mFc treatment (bi-weekly, 10mg/kg) may reduce available myostatin levels to below that present in MSTN+/− mice. Unlike MSTN−/− mice, heterozygous MSTN+/− had increased muscle mass with mild lesions without exhibiting a dramatic decrease in relative contractile function.48 Consistent with the reported MSTN+/− findings, whippet dogs heterozygous for a myostatin mutation exhibit better racing performance than normal whippet dogs or whippet dogs homozygous for the MSTN defect.49 These data suggest that the magnitude of myostatin inhibition is critical to muscle function and exercise performance.
The observed improvement of oim/oim muscle contractile function with ActRIIB-mFc treatment, not seen in WT or +/G610C muscle may be due to inherent differences in the basal levels of muscle function. Children with OI can experience multiple fractures that can hamper their daily activity leading to hypoactivity and muscle atrophy. Van Brussel et al. reported greater degrees of improvement in muscle force and aerobic capacity in OI type I and IV children with well-tailored physical training as compared to healthy individuals.40 Analogous to human OI individuals, hypoactivity in oim/oim mice can contribute to muscle weakness. The lower basal level muscle strength of oim/oim mice may have greater capacity to respond to myostatin inhibition as reflected by the increase in muscle contractile function with ActRIIB-mFc treatment relative to WT and +/G610C mice.
Physical exercise contributes to bone health either directly or indirectly via muscle loads during prepubertal/pubertal growth in healthy children; a 6-year of exercise program in healthy children demonstrated improved bone geometry with increased bone mass.50 However, OI children often experience reduced physical activity levels and exhibit decreased exercise tolerance as compared to healthy age-matched individuals.15,51 This has been postulated to be associated with pain and/or behavioral choices due to concerns of fracture.52,40 Similarly, oim/oim mice exhibit reduced activity levels, which in addition to muscle weakness may reflect complications due to skeletal deformity or activity-associated pain. Although we have not addressed the impact of pain in oim/oim mice, in another OI mouse model, the Col1a1Jrt/+ mice report hypersensitivity to mechanical, heat and cold stimuli, reduced locomotor activity, and increased limping.53
Treatment with sActRIIB-mFc resulting in improved muscle function may also facilitate motor function of oim/oim mice, which may then allow development of tailored exercise regimens to further improve both the muscle and bone. The mdx mouse is characterized by reduced aerobic metabolism and muscle wasting. The combination of exercise and administration of sActRIIB-mFc to the mdx mice was more effective in reconditioning the skeletal muscle gene expression profile to reflect healthy WT mouse muscle than either exercise or sActRIIB-mFc treatment alone.54 In addition, further improvement in muscle and bone properties has been shown with a combination of myostatin deficiency and exercise in MSTN−/− mice.55 In a separate study, neutralizing myostatin antibody- treated 24 month old mice undergoing treadmill exercise had demonstrated improved exercise performance with increased habitual ambulatory activity relative to either antibody treatment or treadmill exercise alone.56
Several studies raise concerns about impairment of mitochondrial function with myostatin inhibition. A limited group of studies in MSTN−/− mice have demonstrated increased force production that was not proportional to their muscle hypertrophy, which may reflect decreased mitochondrial function,45–47,57 muscle fiber type switching,57,58 deficits in oxidative metabolism that can lead to decreased endurance capacity,57–60 and/or decreased capillary density in muscle that alters the aerobic metabolism.58 Unlike MSTN−/− mice, Bechir and colleagues have shown energy status and function of glycolytic muscles were not disturbed during 6 min fatigue exercise with ActRIIB blockade in both WT and mdx mice.44 We examined citrate synthase activity, a measure of mitochondrial content and function, in the mixed Gast muscle and found that citrate synthase activity was not altered by sActRIIB-mFc treatment for all genotypes. This suggests blockade of myostatin via sActRIIB-mFc does not alter this marker of mitochondrial function in Gast muscles. Interestingly, oim/oim Gast muscle had greater citrate synthase activity than WT or +/G610C Gast muscle (+16%), which may reflect compensatory up-regulation of oim/oim mitochondrial function to offset its compromised muscle function.
In summary, muscle weakness is often observed in individuals with OI and clinicians encourage physical exercise to strengthen both the muscle and bone properties. Depending on clinical severity, individuals with OI may be limited in their options for exercise. Our study demonstrates that sActRIIB-mFc treated oim/oim mice, which model the moderately severe type III OI in humans, exhibited increases in muscle mass with improved contractile function without any apparent adverse effects, while the WT and +/G610C mice treated with sActRIIB-mFc exhibited compromised muscle function. Further investigation of the underlying mechanisms responsible for the improved muscle function in oim/oim mice undergoing sActRIIB-mFc treatment and the differential response of WT and +/G610C mice remains to be evaluated. This study provides new insight and potential clues to design new approaches to improve muscle and/or bone properties in OI.
Supplementary Material
Acknowledgments
Funding
The authors would like to thank Mark R. Ellersieck, PhD. for statistical evaluation of this study. This work was supported by the NIH/NIAMS RO1 AR055907, Leda J. Sears Trust, Kansas City Area Life Sciences Institute Patton Trust Research, Inc., and partially supported by VA Grant VHA-CDA2 IK2BX001299. A portion of this work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
Dr. R. Scott Pearsall is an employee of Acceleron Pharma Inc. and graciously provided sActRIIB-mFc and recommended dosage for this study. However, no financial support was provided by Acceleron Pharma Inc. for this study and Acceleron Pharma Inc. had no input in research design, generation of data, or interpretation and analysis of the results.
Abbreviations
- OI
Osteogenesis Imperfecta
- WT
Wildtype
- oim
Osteogenesis imperfecta murine
- vehicle [TBS]
Tris-Buffered Saline
- sActRIIB-mFc
Soluble activin receptor type IIB
- IP
Intraperitoneal
- Sol
Soleus
- Plant
Plantrais
- Gast
Gastrocnemius
- TA
Tibialis Anterior
- Quad
Quadriceps
- CSA
Cross sectional area
- peak tetanic force, Po
Absolute whole muscle contractile generating capacity
- relative Po
Relative contractile generating capacity
- specific Po
Specific contractile generating capacity
Footnotes
Disclosure of Conflicts of Interest
The remaining authors have no conflict of interests.
Portions of this work were presented at the 2014 American Society of Bone and Mineral Research (ASBMR) Annual Meeting, Houston, Texas, September 12, 2014.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Authorship/Credit
Youngjae Jeong participated in research design, data acquisition and analyses, and drafting and revising of the article, Salah A. Daghlas, Alp S. Kahveci, R. Scott Rector, Daniel Salamango, and Marybeth Brown participated in data acquisition and analyses, Bettina A. Gentry and R. Scott Rector participated in data analyses, R. Scott Pearsall participated in research design, and Charlotte L. Phillips participated in research design, data analyses, and drafting and revising of the article. All authors have approved the final article and agreed to be accountable for all aspects of the work.
References
- 1.Tarantino U, Piccirilli E, Fantini M, Baldi J, Gasbarra E, Bei R. Sarcopenia and fragility fractures: molecular and clinical evidence of the bone-muscle interaction. The Journal of bone and joint surgery American volume. 2015;97(5):429–437. doi: 10.2106/JBJS.N.00648. [DOI] [PubMed] [Google Scholar]
- 2.Frost HM. Bone's mechanostat: a 2003 update. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;275(2):1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 3.Go SW, Cha YH, Lee JA, Park HS. Association between Sarcopenia, Bone Density, and Health-Related Quality of Life in Korean Men. Korean journal of family medicine. 2013;34(4):281–288. doi: 10.4082/kjfm.2013.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima RM, Bezerra LM, Rabelo HT, Silva MA, Silva AJ, Bottaro M, de Oliveira RJ. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2009;12(1):35–41. doi: 10.1016/j.jocd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Miyakoshi N, Hongo M, Mizutani Y, Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. Journal of bone and mineral metabolism. 2013;31(5):556–561. doi: 10.1007/s00774-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 6.Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75(2):175–180. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Verschueren S, Gielen E, O'Neill TW, Pye SR, Adams JE, Ward KA, Wu FC, Szulc P, Laurent M, Claessens F, Vanderschueren D, Boonen S. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Song YM, Sung J, Lee K, Kim YS, Kim T, Cho SI. The association between fat and lean mass and bone mineral density: the Healthy Twin Study. Bone. 2012;50(4):1006–1011. doi: 10.1016/j.bone.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(7):1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 10.Waning DL, Guise TA. Cancer-associated muscle weakness: What's bone got to do with it? BoneKEy reports. 2015;4:691. doi: 10.1038/bonekey.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi ML, Mazzanti A, Galbiati E, Saraifoger S, Dubini A, Cornelio F, Morandi L. Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(9):761–767. doi: 10.1007/s00198-003-1443-y. [DOI] [PubMed] [Google Scholar]
- 12.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix biology : journal of the International Society for Matrix Biology. 2010;29(7):638–644. doi: 10.1016/j.matbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2015 doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pouliot-Laforte A, Veilleux LN, Rauch F, Lemay M. Physical activity in youth with osteogenesis imperfecta type I. Journal of musculoskeletal & neuronal interactions. 2015;15(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- 15.Veilleux LN, Lemay M, Pouliot-Laforte A, Cheung MS, Glorieux FH, Rauch F. Muscle anatomy and dynamic muscle function in osteogenesis imperfecta type I. The Journal of clinical endocrinology and metabolism. 2014;99(2):E356–362. doi: 10.1210/jc.2013-3209. [DOI] [PubMed] [Google Scholar]
- 16.Veilleux LN, Pouliot-Laforte A, Lemay M, Cheung MS, Glorieux FH, Rauch F. The functional muscle-bone unit in patients with osteogenesis imperfecta type I. Bone. 2015;79:52–57. doi: 10.1016/j.bone.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Brizola E, Staub AL, Felix TM. Muscle strength, joint range of motion, and gait in children and adolescents with osteogenesis imperfecta. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2014;26(2):245–252. doi: 10.1097/PEP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 18.Jeong Y, Carleton SM, Gentry BA, Yao X, Ferreira JA, Salamango DJ, Weis M, Oestreich AK, Williams AM, McCray MG, Eyre DR, Brown M, Wang Y, Phillips CL. Hindlimb Skeletal Muscle Function and Skeletal Quality and Strength in +/G610C Mice With and Without Weight-Bearing Exercise. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(10):1874–1886. doi: 10.1002/jbmr.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 21.Arounleut P, Bialek P, Liang LF, Upadhyay S, Fulzele S, Johnson M, Elsalanty M, Isales CM, Hamrick MW. A myostatin inhibitor (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength. Experimental gerontology. 2013;48(9):898–904. doi: 10.1016/j.exger.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGirolamo DJ, Singhal V, Chang X, Lee SJ, Germain-Lee EL. Administration of soluble activin receptor 2B increases bone and muscle mass in a mouse model of osteogenesis imperfecta. Bone research. 2015;3:14042. doi: 10.1038/boneres.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison BM, Lachey JL, Warsing LC, Ting BL, Pullen AE, Underwood KW, Kumar R, Sako D, Grinberg A, Wong V, Colantuoni E, Seehra JS, Wagner KR. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Experimental neurology. 2009;217(2):258–268. doi: 10.1016/j.expneurol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Pistilli EE, Bogdanovich S, Mosqueira M, Lachey J, Seehra J, Khurana TS. Pretreatment with a soluble activin type IIB receptor/Fc fusion protein improves hypoxia-induced muscle dysfunction. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(1):R96–R103. doi: 10.1152/ajpregu.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistilli EE, Bogdanovich S, Goncalves MD, Ahima RS, Lachey J, Seehra J, Khurana T. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. The American journal of pathology. 2011;178(3):1287–1297. doi: 10.1016/j.ajpath.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Relizani K, Mouisel E, Giannesini B, Hourde C, Patel K, Morales Gonzalez S, Julich K, Vignaud A, Pietri-Rouxel F, Fortin D, Garcia L, Blot S, Ritvos O, Bendahan D, Ferry A, Ventura-Clapier R, Schuelke M, Amthor H. Blockade of ActRIIB signaling triggers muscle fatigability and metabolic myopathy. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22(8):1423–1433. doi: 10.1038/mt.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Chiu CS, Peekhaus N, Weber H, Adamski S, Murray EM, Zhang HZ, Zhao JZ, Ernst R, Lineberger J, Huang L, Hampton R, Arnold BA, Vitelli S, Hamuro L, Wang WR, Wei N, Dillon GM, Miao J, Alves SE, Glantschnig H, Wang F, Wilkinson HA. Increased muscle force production and bone mineral density in ActRIIB-Fc-treated mature rodents. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(10):1181–1192. doi: 10.1093/gerona/glt030. [DOI] [PubMed] [Google Scholar]
- 29.Chipman SD, Sweet HO, McBride DJ, Jr, Davisson MT, Marks SC, Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(5):1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daley E, Streeten EA, Sorkin JD, Kuznetsova N, Shapses SA, Carleton SM, Shuldiner AR, Marini JC, Phillips CL, Goldstein SA, Leikin S, McBride DJ., Jr Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 25(2):247–261. doi: 10.1359/jbmr.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daley E, Streeten EA, Sorkin JD, Kuznetsova N, Shapses SA, Carleton SM, Shuldiner AR, Marini JC, Phillips CL, Goldstein SA, Leikin S, McBride DJ., Jr Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(2):247–261. doi: 10.1359/jbmr.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips CL, Bradley DA, Schlotzhauer CL, Bergfeld M, Libreros-Minotta C, Gawenis LR, Morris JS, Clarke LL, Hillman LS. Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone. 2000;27(2):219–226. doi: 10.1016/s8756-3282(00)00311-2. [DOI] [PubMed] [Google Scholar]
- 33.Isaksson H, Tolvanen V, Finnila MA, Iivarinen J, Tuukkanen J, Seppanen K, Arokoski JP, Brama PA, Jurvelin JS, Helminen HJ. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcified tissue international. 2009;85(3):247–256. doi: 10.1007/s00223-009-9273-3. [DOI] [PubMed] [Google Scholar]
- 34.Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. American journal of physiology Endocrinology and metabolism. 2009;296(4):E854–861. doi: 10.1152/ajpendo.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srere PA. Methods in Enzymology. Vol. 13. Elsevier Inc; 1969. Citrate Synthase; pp. 3–11. [Google Scholar]
- 36.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 37.Carmer SGSM. An Evaluation of Ten Pairwise Multiple Comparison Procedures by Monte Carlo Methods. Journal of the American Statistical Association. 1973;68(341):66–74. [Google Scholar]
- 38.DJ S. Multiple Comparison Procedures: The Practical Solution. The American Statistician. 1990;44(2):174–180. [Google Scholar]
- 39.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38(3):715–724. [PubMed] [Google Scholar]
- 40.Van Brussel M, Takken T, Uiterwaal CS, Pruijs HJ, Van der Net J, Helders PJ, Engelbert RH. Physical training in children with osteogenesis imperfecta. The Journal of pediatrics. 2008;152(1):111–116. doi: 10.1016/j.jpeds.2007.06.029. 116 e111. [DOI] [PubMed] [Google Scholar]
- 41.Palomo T, Glorieux FH, Schoenau E, Rauch F. Body Composition in Children and Adolescents with Osteogenesis Imperfecta. The Journal of pediatrics. 2016;169:232–237. doi: 10.1016/j.jpeds.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald EM, Andres-Mateos E, Mejias R, Simmers JL, Mi R, Park JS, Ying S, Hoke A, Lee SJ, Cohn RD. Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Disease models & mechanisms. 2014;7(4):471–481. doi: 10.1242/dmm.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marini J, Smith SM. Osteogenesis Imperfecta. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA): 2000. [Google Scholar]
- 44.Bechir N, Pecchi E, Vilmen C, Le Fur Y, Amthor H, Bernard M, Bendahan D, Giannesini B. ActRIIB blockade increases force-generating capacity and preserves energy supply in exercising mdx mouse muscle in vivo. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(10):3551–3562. doi: 10.1096/fj.201600271RR. [DOI] [PubMed] [Google Scholar]
- 45.Bechir N, Pecchi E, Relizani K, Vilmen C, Le Fur Y, Bernard M, Amthor H, Bendahan D, Giannesini B. Mitochondrial impairment induced by postnatal ActRIIB blockade does not alter function and energy status in exercising mouse glycolytic muscle in vivo. American journal of physiology Endocrinology and metabolism. 2016;310(7):E539–549. doi: 10.1152/ajpendo.00370.2015. [DOI] [PubMed] [Google Scholar]
- 46.Rahimov F, King OD, Warsing LC, Powell RE, Emerson CP, Jr, Kunkel LM, Wagner KR. Gene expression profiling of skeletal muscles treated with a soluble activin type IIB receptor. Physiological genomics. 2011;43(8):398–407. doi: 10.1152/physiolgenomics.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao B, Li EJ, Wall RJ, Yang J. Coordinated patterns of gene expressions for adult muscle build-up in transgenic mice expressing myostatin propeptide. BMC genomics. 2009;10:305. doi: 10.1186/1471-2164-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gentry BA, Ferreira JA, Phillips CL, Brown M. Hindlimb skeletal muscle function in myostatin-deficient mice. Muscle & nerve. 2011;43(1):49–57. doi: 10.1002/mus.21796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS genetics. 2007;3(5):e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Detter F, Rosengren BE, Dencker M, Lorentzon M, Nilsson JA, Karlsson MK. A 6-year exercise program improves skeletal traits without affecting fracture risk: a prospective controlled study in 2621 children. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(6):1325–1336. doi: 10.1002/jbmr.2168. [DOI] [PubMed] [Google Scholar]
- 51.Takken T, Terlingen HC, Helders PJ, Pruijs H, Van der Ent CK, Engelbert RH. Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. The Journal of pediatrics. 2004;145(6):813–818. doi: 10.1016/j.jpeds.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Suskauer SJ, Cintas HL, Marini JC, Gerber LH. Temperament and physical performance in children with osteogenesis imperfecta. Pediatrics. 2003;111(2):E153–161. doi: 10.1542/peds.111.2.e153. [DOI] [PubMed] [Google Scholar]
- 53.Abdelaziz DM, Abdullah S, Magnussen C, Ribeiro-da-Silva A, Komarova SV, Rauch F, Stone LS. Behavioral signs of pain and functional impairment in a mouse model of osteogenesis imperfecta. Bone. 2015;81:400–406. doi: 10.1016/j.bone.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Kainulainen H, Papaioannou KG, Silvennoinen M, Autio R, Saarela J, Oliveira BM, Nyqvist M, Pasternack A, t Hoen PA, Kujala UM, Ritvos O, Hulmi JJ. Myostatin/activin blocking combined with exercise reconditions skeletal muscle expression profile of mdx mice. Molecular and cellular endocrinology. 2015;399:131–142. doi: 10.1016/j.mce.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(3):477–483. doi: 10.1359/JBMR.051203. [DOI] [PubMed] [Google Scholar]
- 56.LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(9):940–948. doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- 57.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(6):1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsakas A, Macharia R, Otto A, Elashry MI, Mouisel E, Romanello V, Sartori R, Amthor H, Sandri M, Narkar V, Patel K. Exercise training attenuates the hypermuscular phenotype and restores skeletal muscle function in the myostatin null mouse. Experimental physiology. 2012;97(1):125–140. doi: 10.1113/expphysiol.2011.063008. [DOI] [PubMed] [Google Scholar]
- 59.Mouisel E, Relizani K, Mille-Hamard L, Denis R, Hourde C, Agbulut O, Patel K, Arandel L, Morales-Gonzalez S, Vignaud A, Garcia L, Ferry A, Luquet S, Billat V, Ventura-Clapier R, Schuelke M, Amthor H. Myostatin is a key mediator between energy metabolism and endurance capacity of skeletal muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2014;307(4):R444–454. doi: 10.1152/ajpregu.00377.2013. [DOI] [PubMed] [Google Scholar]
- 60.Savage KJ, McPherron AC. Endurance exercise training in myostatin null mice. Muscle & nerve. 2010;42(3):355–362. doi: 10.1002/mus.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.