Abstract
Droplets slip and bounce on superhydrophobic surfaces, enabling remarkable functions in biology and technology. These surfaces often contain microscopic irregularities in surface texture and chemical composition, which may affect or even govern macroscopic wetting phenomena. However, effective ways to quantify and map microscopic variations of wettability are still missing, because existing contact angle and force-based methods lack sensitivity and spatial resolution. Here, we introduce wetting maps that visualize local variations in wetting through droplet adhesion forces, which correlate with wettability. We develop scanning droplet adhesion microscopy, a technique to obtain wetting maps with spatial resolution down to 10 µm and three orders of magnitude better force sensitivity than current tensiometers. The microscope allows characterization of challenging non-flat surfaces, like the butterfly wing, previously difficult to characterize by contact angle method due to obscured view. Furthermore, the technique reveals wetting heterogeneity of micropillared model surfaces previously assumed to be uniform.
Real-world surfaces exhibit spatially varying wettability, which affects water repellency and droplet behaviour on such surfaces. Here, the authors use scanning droplet adhesion microscopy to create wetting maps that visualize variations in wettability with a spatial resolution down to 10 μm.
Introduction
Superhydrophobic surfaces enable exceptional functions in biology and technology1–5. Understanding how water-repellency emerges from the microscale and nanoscale features1 is critical to advance the development of these surfaces6–8. Biological superhydrophobic surfaces often contain irregular surface texture and details9, 10, such as creases or veins, and synthetic surfaces are prone to fabrication defects. Such irregularities in surface texture and chemical composition lead to spot-to-spot variation of wetting properties11, 12, which may affect or even govern droplet mobility13, 14, icing15, and condensation16. Even though these variations have been considered in theory1, so far they have not been probed experimentally, partly because existing contact angle and force-based methods lack sensitivity and spatial resolution17–19. The contact angle method, describing a surface by a single pair of apparent advancing and receding contact angle values, is still viewed as the gold standard in hydrophobic surface characterization. As the measurement is based on observing a moving contact line, it is inherently unsuitable for precise spatial mapping. Moreover, as an optical method, contact angle measurements become increasingly inaccurate for contact angles beyond 150°17, 18 due to resolution limit of the optical system, and often suffer from obscured view of the contact line on curvy surfaces9. Wetting properties have also been characterized by droplet friction forces, i.e., resistance to lateral motion20–22 and by droplet adhesion forces, i.e., resistance to detaching a droplet in the normal direction8, 19, 23, 24. It has been experimentally verified that snap-in (first droplet contact) and pull-off (droplet separation) adhesion forces on hydrophobic surfaces are related to, respectively, the advancing contact angle (θadv) and the receding contact angle (θrec)19. Smaller forces correspond to larger contact angles. However, current tensiometers are not sensitive enough to detect droplet adhesion forces below 1 µN. On the other hand, scanning probe microscopy techniques, e.g., atomic force microscopy (AFM), can have nN or even better force resolution, though so far have not been used to map spatial wetting variations. To study wetting in ambient air, the water droplet must be large enough so that evaporation during a single measurement becomes insignificant, and nN sensitive AFM cantilevers cannot support such large droplets in air. Droplet–surface interactions have therefore previously been measured by AFM not in ambient air but instead in liquid–liquid and bubble-liquid systems25, or by using the AFM tip itself as the surface under study24, hence greatly limiting the scope of AFM to study wetting of surfaces.
Here, we introduce scanning droplet adhesion microscopy, a technique for obtaining wetting maps of surfaces based on high-precision force measurements. The technique enables us to map in extraordinary detail the wetting forces, even on challenging, non-flat, biological surfaces. Since butterfly wings and their eyespots have sparked tremendous scientific interest26, 27, we select the wing of the striped blue crow butterfly (Euploea mulciber) as a model surface. Wetting maps obtained on an eyespot of the wing show correlation between wetting and visual appearance. We also show that on micropillared model surfaces, the wetting forces can vary pillar-to-pillar.
Results
Measurement concept
The apparatus we built comprises a vertically mounted force sensor with a liquid droplet probe (e.g., water) and a multi-axis sample stage (Fig. 1a and Supplementary Fig. 1) for measuring normal forces point-by-point in a fully automated manner (Supplementary Movie 1). By scanning an eyespot area (Fig. 1b) on the striped blue crow butterfly wing (Fig. 1c), we obtain snap-in (Fig. 1d) and pull-off (Fig. 1e) force maps. Force measurement on a single point starts with moving up the sample surface to approach the droplet, followed by first contact (snap-in) with the droplet, then the sample surface moves down until it separates from the droplet (pull-off) (Fig. 1f). A typical force curve with nanonewton resolution, recorded at 100 Hz sampling rate, is shown in Fig. 1g. The droplet volume is refilled to 1.5 µl before every measurement; the effect of evaporation is not significant as only ~1% of the volume evaporates during a typical single measurement lasting around 20 s.
Fig. 1.
Concept of scanning droplet adhesion microscopy to construct wetting maps. a Schematic diagram of the microscope (not to scale). b Optical micrograph of scanned eyespot area on the wing of c, striped blue crow butterfly (typical wing span of adult specimen 80–90 mm; image by Frederic Moore, PD-1923); with corresponding d snap-in and e pull-off force maps. Dots on d, e denote the measurement points with 200 µm spacing. Colours in the maps denote measured force values, linearly interpolated between the data points. f Snapshots of individual measurement on a single hydrophobic 5 µm radius pillar (white arrows indicate direction of sample surface movement, inset scale bar 70 µm), and g corresponding typical force curve. Roman numerals in f, g indicate corresponding moments in the measurement
Accuracy and repeatability
To verify accuracy and repeatability of the microscope, we measured snap-in and pull-off forces on single silicon pillars (Fig. 2a–c and Supplementary Fig. 2) of varying radius, one pillar at a time. For each radius, the measurement is carried out on ten different pillars, with ten repetitions on each pillar. The mean snap-in force for ten 10 µm radius pillars is 125.9 nN with pooled standard deviation of 6.6 nN (Fig. 2b), and the mean pull-off force is 4606.4 nN with pooled standard deviation of 5.3 nN (Fig. 2c). Analysis of variance indicates that pillar-to-pillar variation of snap-in force is six times larger than within-pillar variation and nine times larger in case of pull-off force. For the larger pillars, this difference is even clearer (Supplementary Table 1). We are thus capable of measuring tiny but significant differences in adhesion force from one pillar to another, likely caused by tiny structural variations on the pillar tops (see Fig. 2a). The 6.6 nN standard deviation in within-pillar measurements is predominantly attributed to sensor noise of the microscope (measured as 5 nN), verifying excellent repeatability of the method. We measured snap-in and pull-off forces for pillar radii between 10 and 400 µm. The results (Fig. 2d, e) show high consistency for each radius and agree well with numerical simulation. The simulation uses Young–Laplace equation and boundary conditions to solve the shape of the droplet, which can be used to compute the forces18. A detailed discussion on modelling and simulation can be found in Supplementary Note 1.
Fig. 2.
Accuracy and repeatability of droplet adhesion force measurements. Silicon micropillars with undercut tops were used for the accuracy and repeatability measurements. a SEM micrographs of four different 10 µm radius pillars showing tiny structural variations on the pillar tops. b, c Snap-in and pull-off force data from ten different 10 µm radius pillars. Data for 20–50 µm radius pillars are in Supplementary Fig. 2. d, e Snap-in and pull-off forces as function of pillar radius, the number of data points was 100 (10–50 µm radius pillars) or three (100–400 µm radius pillars). Solid lines show numerical simulation results. Error bars denote standard deviations in force
Wetting forces on superhydrophobic surfaces
We also compared snap-in and pull-off forces to optically measured contact angles for various hydrophobic and superhydrophobic surfaces. The results are shown in Supplementary Table 2 and Supplementary Figs. 3 and 4. The smallest measured snap-in force, 7.8 ± 1.9 nN, was obtained on Hydrobead-coated silicon wafer with optically measured contact angles θadv/θrec = 169°/168°. The smallest pull-off force, 218.5 ± 9.7 nN, was measured on a silicon nanograss surface coated with fluoropolymer, where the optically measured contact angles were θadv/θrec = 175°/169°. In contrast to a recent study19, there seems to be no contact angle limit beyond which the snap-in or pull-off force becomes zero. Therefore, using force sensors with enhanced sensitivity should allow characterization of even higher contact angle superhydrophobic surfaces.
Micropillar arrays are common model superhydrophobic substrates1–3, 28, 29, and water droplets have been imaged to advance and recede step-wise, respectively, by pinning and depinning from pillar to pillar29, 30. Droplets rolling off such micropillar surfaces may oscillate due to the pinning and depinning steps31, 32. Here we quantify, for the first time, the tiny forces during pinning and depinning in great detail (Fig. 3). While a water droplet advances, in total 16 pinning steps were detected with forces ranging from 20 to 60 nN. Eight depinning steps were detected during droplet receding, with forces ranging from hundreds of nN to more than 10 µN. The butterfly wing exhibits similar pinning/depinning steps (Supplementary Fig. 6). The number of pinning steps in Fig. 3 is much higher than the number of depinning steps, because depinning from multiple pillars are combined into larger steps. It should be noted that the magnitude of pinning steps shown in Fig. 3c corresponds to snap-in force on an individual pillar, shown in Fig. 1g. On the other hand, the dynamics of collective depinning involves lateral deformation of the meniscus, so the magnitude of depinning steps in normal direction (Fig. 3c) is smaller than pull-off on individual pillars (Fig. 1g), except for the final depinning step. It should also be noted that during a long measurement such as the one presented in Fig. 3, the volume loss due to evaporation of the droplet may affect the magnitude of the pinning and depinning steps (see Supplementary Note 1 for further theoretical discussion on the volume dependency).
Fig. 3.
Multiple wetting steps during droplet advancing and receding. a Optical micrograph with SEM insets of the pillar surface (5 µm pillar radius, 70 µm spacing). b While a water droplet advances (sample moving up) 16 consecutive pinning steps (P1–P16) were detected, and 8 depinning steps (DP1–DP8) while droplet recedes (droplet evaporating, sample not moving). c Force values of individual pinning/depinning steps. d Selected details of droplet adhesion force curve and corresponding side view of droplet contact area. Details of all the steps are shown in Supplementary Fig. 5 and in Supplementary Movie 2
Wetting force mapping
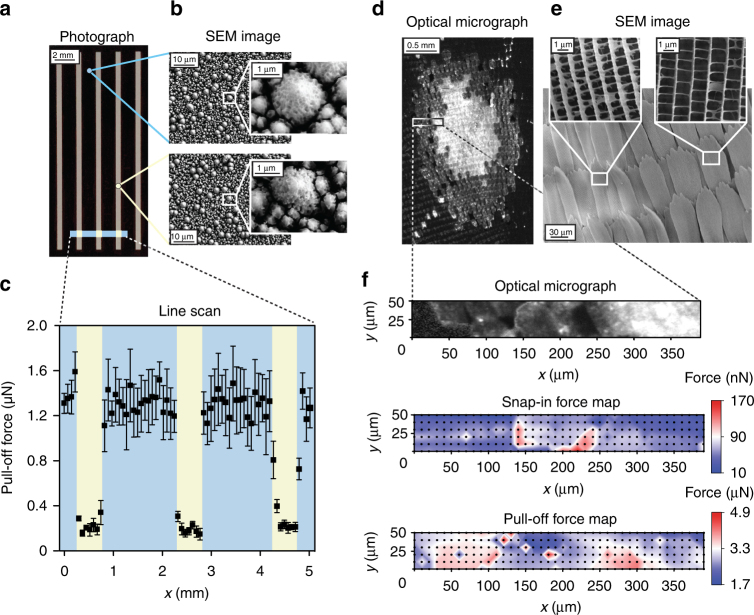
To challenge the sensitivity of the scanning droplet adhesion microscope, we measured snap-in and pull-off forces on a superhydrophobic-superhydrophobic patterned surface consisting of parallel stripes (θadv/θrec = 175°/174°) on background (θadv/θrec = 173°/170°) (Fig. 4a, b). θadv and θrec are all beyond 170°, and thus difficult to determine accurately with contact angle measurement18. We performed a line scan across the stripes, recording snap-in and pull-off forces every 75 µm. Whereas the snap-in force is too small to be detected for this surface, the pull-off force shows a clear difference between the stripes and the background (Fig. 4c).
Fig. 4.
Detecting spatial variation of small (nN to µN) wetting forces. a–c Line scan at 75 µm resolution over superhydrophobic-superhydrophobic patterns of silicon nanograss with slightly different surface topography shows consistent difference in pull-off force. The error bars in c denote standard deviation of three measurements. d–f Area scan at 10 µm resolution over striped blue crow butterfly wing eyespot, where optically and topographically different surface structure is reflected in the corresponding wetting force maps
While contact angle measurements of biological surfaces are often impossible due to an obscured baseline (Supplementary Fig. 7), we constructed detailed wetting force maps of striped blue crow butterfly wing. We probed an area of 2.2 × 3.2 mm covering one of the eyespots on the wing, with 200 µm spacing between measurement points, resulting in snap-in and pull-off force maps (Fig. 1b, d, e). We probed furthermore a smaller area (400 × 50 µm) of the eyespot in greater detail, with 10 µm spacing between measurement points (Fig. 4d, e). The corresponding snap-in and pull-off force maps are shown in Fig. 4f, together with an optical micrograph of the area. The wetting force maps reveal variations in snap-in and pull-off force when moving across the edge of the eyespot. The wetting variations correspond to variations in structural colour, and are thus due to subtle structural differences of the wing surface (scanning electron microscopy (SEM) micrographs of the probed area in Fig. 4e).
Discussion
Microscale spatial heterogeneity is generally acknowledged as a major source of contact angle hysteresis and droplet friction1, though how wetting on model surfaces and especially on irregular surfaces is related to the microscopic surface features is still not completely understood. Scanning droplet adhesion microscopy provides the sensitivity and the resolution that is critical to create wetting maps, a concept for hydrophobic surface characterization to study this relation in both biology and materials science. Using a sensor probe with increased sensitivity in pN range, measurement could become possible of superhydrophobic surfaces exhibiting even lower adhesion forces. Combination of scanning droplet adhesion microscopy with optical microscopy techniques would open the way to multimodal characterization of pinning and depinning events towards comprehensive understanding of wetting. Whereas this study focused on water, arguably the most relevant probe liquid, the scanning droplet adhesion microscopy could in principle be extended to study wetting of other liquids, including organic liquids on oleophobic surfaces.
Methods
Silicon micropillars
Silicon micropillars were fabricated by deep reactive ion etching. First, a plasma-enhanced chemical vapour deposited (PECVD) oxide (Oxford PlasmaLab 80+, 300 °C, 8.5 sccm SiH4, 1000 mTorr, 20 W) was deposited on a 4-inch silicon wafer (<100>, p-type doping 1–20 ohm-cm). The thickness of the oxide was 750 nm (12 min deposition time). The oxide was patterned by UV lithography and reactive ion etching (Oxford PlasmaLab 80+, 25 sccm Ar, 25 sccm CHF3, 200 W, 30 mTorr, 21 min etching time). The pillar radius was 5 µm. The silicon pillars were then etched by cryogenic deep reactive ion etching (Oxford PlasmaLab System 100, −110 °C, 40 sccm SF6, 6 sccm O2, 1050 W ICP power, 3 W platen power, 8 mTorr). The etch depth was 20 µm. The oxide mask was then removed by hydrofluoric acid. Finally, a thin hydrophobic fluoropolymer coating was deposited on top of the pillars by PECVD (Oxford PlasmaLab 80+, 100 sccm CHF3, 50 W, 30 mTorr, 5 min).
Silicon undercut micropillars
Silicon undercut micropillars with a silicon dioxide top were fabricated for the accuracy and repeatability experiments. Undercut pillars with radius 10 µm - 50 µm were fabricated with the following process: a thermally oxidized (oxide thickness 1.2 µm) silicon wafer was used as a substrate. The oxide was patterned by UV lithography and reactive ion etching (Oxford PlasmaLab 80+, 25 sccm Ar, 25 sccm CHF3, 200 W, 30 mTorr, 38 min). The samples were then etched anisotropically by cryogenic deep reactive ion etching (Oxford PlasmaLab System 100, −110 °C, 40 sccm SF6, 6 sccm O2, 1050 W ICP power, 3 W platen power, 8 mTorr, 10 min) to the depth of 22 µm. Finally, an isotropic silicon etching step was performed to create the undercut (Oxford PlasmaLab 80+, 100 sccm SF6, 100 W, 100 mTorr, 18 min). Undercut pillars with radius 75 µm - 200 µm were fabricated by a process reported before33.
Silicon nanograss coated with fluoropolymer
The substrate was a 4-inch silicon wafer (<100>, p-type doping 1–20 ohm-cm). Silicon nanograss was created by maskless cryogenic deep reactive ion etching (the so-called black silicon process) (Oxford PlasmaLab System 100, −110 °C, 40 sccm SF6, 18 sccm O2, 1000 W ICP power, 6 W platen power, 10 mTorr, 7 min etching time). The nanograss was made superhydrophobic by a PECVD deposition of a fluoropolymer thin film (Oxford PlasmaLab 80+, 100 sccm CHF3, 50 W, 30 mTorr, 5 min deposition time).
Fluoropolymer on Si wafer
Fluoropolymer coated silicon surface was fabricated by the same PECVD process as with the fluoropolymer coated silicon nanograss. The substrate was an unprocessed 4 inch silicon wafer (<100>, p-type doping 1–20 ohm-cm).
Silicon nanograss patterned surface for line scan
To pattern the nanograss, a 500 nm thick silicon dioxide film was deposited (Oxford PlasmaLab 80+, 300 °C, 8.5 sccm SiH4, 1000 mTorr, 20 W, 8 min) as a hard mask. The oxide mask was patterned by UV lithography and reactive ion etching (Oxford PlasmaLab 80+, 25 sccm Ar, 25 sccm CHF3, 200 W, 30 mTorr, 14 min). To modify the nanograss topography, a silicon etching step (Oxford PlasmaLab 80+, 100 sccm SF6, 100 W, 100 mTorr, 3 min) was performed. After this, the oxide mask was stripped in hydrofluoric acid and a second 3-min plasma etching step was performed with the same recipe (without a mask). Finally, the patterned nanograss was coated with the same PECVD fluoropolymer as the plain nanograss (previous section).
Glaco on Si wafer
Test-grade silicon (100) wafers were cleaned with an alkaline solvent (Deconex 11 Universal, VWR), rinsed thoroughly with Milli-Q water, and dried under nitrogen flow. Commercial Glaco Mirror Coat Zero (Soft99 Co.) was coated on the wafer by spraying followed by drying for 1 h.
Hydrobead on Si wafer
Test-grade silicon (100) wafers were cleaned with an alkaline solvent (Deconex 11 Universal, VWR), rinsed thoroughly with Milli-Q water, and dried under nitrogen flow. Afterwards, commercial Hydrobead Standard was applied on Si wafer and dried for 10 min.
Silicone nanofilaments on Si wafer
Surface was prepared as described in the literature34. Silicon (100) wafer was cleaned by ultrasonication in alkaline solvent (Deconex 11 Universal, VWR), rinsed thoroughly by Milli-Q water, and dried under nitrogen flow. Synthesis of the nanofilaments was done by chemical vapour deposition in an in-house built gas-phase reactor operating at atmospheric pressure. The reactor was purged with dry argon followed by pre-humidified argon until the relative humidity reached ca. 30% followed by sealing the reactor and injecting methyltrichlorosilane (99%, Aldrich) through a silicone septum. Methyltrichlorosilane evaporated and nanofilaments were grown onto the surface.
Copper (II) hydroxide nanowires
Copper (II) hydroxide nanowires were prepared as described in literature35. (NH4)2S2O8 (98%, Aldrich), NaOH (Fluka), HCl (Aldrich), and lauric acid (Fluka) were used as obtained. Mechanically polished copper substrate was immersed in HCl aqueous solution for 1 min, followed by washing with Milli-Q water and drying with nitrogen flow. Subsequently, the substrate was immersed into a mixed solution of 2.5 M NaOH and 0.13 M (NH4)2S2O8 for 20 min at room temperature and washed afterwards with Milli-Q water and dried under nitrogen flow. Chemical modification was performed by immersing substrate into 5 mM lauric acid (ethanol solution) for 20 min, followed by rinsing with Milli-Q water and drying under ambient conditions for a few minutes.
PDMS replica of silicon nanograss
Polydimethylsiloxane (PDMS) replica of silicon nanograss was fabricated as described in literature36. Cryogenic deep reactive ion etching was used to fabricate the silicon nanograss master on a 100 mm silicon wafer. PDMS mixture with base-to-curing ratio of 10:1 was prepared and poured over the silicon nanograss master, and then baked in an oven at 75 °C for 90 min. The cured PDMS was peeled off the master and functionalized with (1H,-1H,-2H,-2H-perfluorooctyl)-trichlorosilane inside a vacuum desiccator at room temperature. The silanization time was 12 h.
Silicon nanograss coated with parylene
Parylene-C coated silicon nanograss was prepared on a 4-inch silicon <100> wafer. Silicon nanograss was created by cryogenic deep reactive ion etching (Oxford Plasmalab System 100, −110 °C, 40 sccm SF6, 18 sccm O2, 1000 W ICP power, 6 W platen power, 10 m Torr, 7 min etching time). In the next step, approximately 250 nm of parylene-C was deposited on the silicon nanograss using a parylene deposition system (SCS Labcoter 2 PDS 2010). Finally, the surface was fluorinated in a reactive ion etcher (Oxford PlasmaLab 80+, 100 sccm SF6, 100 W, 20 mTorr, 1 min), resulting in 200 nm parylene-C thickness.
Fluoroalkyl self-assembled monolayer on Si wafer
A silicon wafer was cleaned and activated with oxygen plasma treatment (PVA Tepla, 1000 W, 500 ml min−1 O2, 1 min). The sample was then put on a hotplate at 75 °C in a closed container together with 0.5 ml of (1H,-1H,-2H,-2H-perfluorooctyl)-trichlorosilane. The silanization time was 2.5 h.
Butterfly wings
Golden birdwing (Troides aeacus) and striped blue crow (Euploea mulciber) butterfly wings were purchased from Beijing Jiaying Store of Arts and Insects on taobao.com.
Measurement setup
The measurement setup (Supplementary Fig. 1) consists of a microforce sensing probe (FT-S100, range ± 100 µN and FT-S1000, range ± 1000 µN; FemtoTools AG, Switzerland) and a data acquisition board (NI USB-6351, National Instruments Inc., USA) that was used to collect data at 100 Hz with custom-built software; motorized high-precision positioning stages for x-direction (M-404.8PD, 0.50 µm precision, Physik Instrumente GmbH, Germany), y-direction (M-122.2DD, 0.15 µm precision, Physik Instrumente GmbH, Germany), and z-direction (M-111.1DG, 0.10 µm precision, Physik Instrumente GmbH, Germany); a piezoelectric microdispenser (PicPIP, 100 pl minimum droplet size, GeSiM GmbH, Germany); a high-speed camera sideview (Phantom Miro LC310, Vision Research Inc., USA) with a 1–5× macro lens (MP-E 65, Canon Inc., Japan) and another CCD camera sideview (IGV B1320M, Imperx Inc., USA) with a 1–6× microscope lens (VZM 600i, Edmund Optics Inc., USA). Measurements were carried out in room temperature (~22 °C) and in ~40% relative humidity.
Sensor probe preparation
1 mm diameter SU-8 discs were used for holding the probe droplet. The SU-8 discs were fabricated by UV lithography. First, 150 nm of aluminium was sputtered on top of a silicon wafer. The wafer was then baked overnight in an oven at 120 °C. Next, a 80 µm thick layer of SU-8 50 was applied on the wafer by spin coating (1500 rpm, 30 s), baked for 15 min at 95 °C exposed for 20 s (Karl Suss MA6 mask aligner) and baked for 15 min at 95 °C. The probes were then released by heating the wafer to 200 °C and cooling to room temperature, which causes thermal expansion mismatch stress that releases the SU-8 from the aluminium. The SU-8 discs were then sputtered with gold on both sides and glued at the end of the force sensor tip using UV-curable glue (Byllux 5118, Byla GmbH, Germany). A droplet is deposited on the SU-8 disc by shooting sub-nl droplets using the piezoelectric microdispenser. The small droplets bounce off a superhydrophobic surface onto the disc, accumulating into a larger droplet used in the measurements. The volume of the droplet is measured through the force given by the sensor.
Procedure for measuring individual points
A single measurement starts with the sample surface brought close to a water droplet pinned on the sensor probe. The sample stage starts moving up towards the droplet at a constant speed (2 µm s−1 for accuracy and repeatability experiments, 5 µm s−1 for all other experiments) until the droplet touches the surface (snap-in), i.e., a step down is detected in the force. The stage keeps moving up either for a fixed time after snap-in, or until the force reaches a predetermined value. For any set of experiments, either the fixed time delay or the fixed force limit was used to minimize variation between measurements. The maximum force depends on the distance moved up during the time delay, or is directly the preset force limit. The stage is then retracted down at 10 µm s−1 until the droplet separates from the surface (pull-off), i.e., a step up is detected in the force. The volume of water droplet used in the measurements is 1.5 µl. After each measurement, the droplet is refilled to 1.5 µl using the microdispenser. The above-mentioned low approach speeds (2 µm s−1 or 5 µm s−1), and moderate retraction speed (10 µm s−1) were chosen to avoid dynamic effects while keeping the measurement duration reasonable.
Approach/retraction speed characterization
The effect of approach speed on snap-in force, and the effect of retraction speed on pull-off force, was characterized for five different speeds. Measurements were carried out on a fluoropolymer-coated silicon nanograss surface (slightly less superhydrophobic than the one listed in Supplementary Table 2). A force vs. speed comparison is shown in Supplementary Fig. 8.
Automated scanning
The measurements were fully automated for accuracy and repeatability testing on micropillars, line scanning, and area scanning of wetting maps. The force readout of the probe is used as feedback to detect contact when probing a surface, and to monitor the droplet volume when refilling the droplet after every measurement. Measurement locations are generated beforehand, but after that hundreds of measurements can be carried out automatically without human intervention.
Data analysis
Snap-in and pull-off events are extracted from individual force recordings using a step detection algorithm based on Student’s t-test metric:
| 1 |
where, for each force value in the signal, is the mean of the N previous values, is the mean of the N subsequent values, is the variance of the N previous values, and is the variance of the N subsequent values. The threshold t is adjusted based on the magnitude of measured forces and the sensor noise. In practice t varied from 20 to 80. The value of N depends on the force data sampling rate and was set to 8 for the 100 Hz used in the measurements. After detection of snap-in event in the force recording (time of snap-in), polynomial curves are fitted to the preceding and succeeding values, so that magnitude of the step (snap-in force) can be extracted from the difference between the fitted curves at the time of the event. Pull-off force is determined by the difference in the force after the detected pull-off event (time of pull-off) and the minimum force in the recording.
Numerical simulation
For detailed discussion on modelling and simulation, see Supplementary Note 1.
Contact angle measurements
Contact angles were measured using sessile drop method by Attension Theta optical tensiometer with automated liquid pumping system. Advancing contact angles were measured by placing a 0.2 µl droplet on the surface and increasing its volume to 40 µl, at a rate of 0.10 µl s−1. Receding contact angles were measured by decreasing droplet volume at a rate of 0.10 µl s−1, starting with a drop volume of 40 µl.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the European Research Council ERC-2016-CoG (725513-SuperRepel), Academy of Finland (Centres of Excellence Programme (2014-2019) and projects #295006, #296250, #266820, #256206, #263560, #268685, #292477, #297360, and #299087), the AScI/ELEC Thematic Research Programme, Aalto ELEC Doctoral School, Finnish Society of Automation, and Finnish Cultural Foundation. This work made use of the Aalto University Nanomicroscopy Centre premises and the cleanroom facilities of Micronova, Centre for Micro and Nanotechnology.
Author contributions
R.H.A.R. conceived the research. Q.Z. designed the apparatus. Q.Z., R.H.A.R., V.L. and M.V. designed the sensor probe. V.L. and M.V. constructed the apparatus. V.L. and Q.Z. designed the measurement algorithms. R.H.A.R., Q.Z., M.V., V.L. and V.J. designed the experiments and analysed the results. M.V., V.L. and M.J.H. performed experiments. V.J. fabricated the sensor probe disc and microstructured Si samples. V.S. carried out modelling and numerical simulations. All authors discussed and co-wrote the paper.
Competing interests
The authors have filed a patent application based on the content of the manuscript.
Footnotes
Ville Liimatainen and Maja Vuckovac contributed equally to this work.
A correction to this article is available online at https://doi.org/10.1038/s41467-018-03042-0.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-01510-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Quan Zhou, Email: quan.zhou@aalto.fi.
Robin H. A. Ras, Email: robin.ras@aalto.fi
References
- 1.Quéré D. Wetting and roughness. Annu. Rev. Mater. Res. 2008;38:71–99. doi: 10.1146/annurev.matsci.38.060407.132434. [DOI] [Google Scholar]
- 2.Tian Y, Su B, Jiang L. Interfacial material system exhibiting superwettability. Adv. Mater. 2014;26:6872–6897. doi: 10.1002/adma.201400883. [DOI] [PubMed] [Google Scholar]
- 3.Bhushan B, Jung YC. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011;56:1–108. doi: 10.1016/j.pmatsci.2010.04.003. [DOI] [Google Scholar]
- 4.Schutzius TM, et al. Spontaneous droplet trampolining on rigid superhydrophobic surfaces. Nature. 2015;527:82–85. doi: 10.1038/nature15738. [DOI] [PubMed] [Google Scholar]
- 5.Bird JC, Dhiman R, Kwon H-M, Varanasi KK. Reducing the contact time of a bouncing drop. Nature. 2013;503:385–388. doi: 10.1038/nature12740. [DOI] [PubMed] [Google Scholar]
- 6.Wong T-S, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature. 2011;477:443–447. doi: 10.1038/nature10447. [DOI] [PubMed] [Google Scholar]
- 7.Liu TL, Kim C-JC. Turning a surface superrepellent even to completely wetting liquids. Science. 2014;346:1096–1100. doi: 10.1126/science.1254787. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Wang S, Wei Z, Song Y, Jiang L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 2009;21:665–669. doi: 10.1002/adma.200801782. [DOI] [Google Scholar]
- 9.Ensikat HJ, Ditsche-Kuru P, Neinhuis C, Barthlott W. Superhydrophobicity in perfection: the outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011;2:152–161. doi: 10.3762/bjnano.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, et al. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature. 2016;532:85–89. doi: 10.1038/nature17189. [DOI] [PubMed] [Google Scholar]
- 11.Marmur A. Solid-surface characterization by wetting. Annu. Rev. Mater. Res. 2009;39:473–489. doi: 10.1146/annurev.matsci.38.060407.132425. [DOI] [Google Scholar]
- 12.Lai Y, et al. Bioinspired patterning with extreme wettability contrast on TiO2 nanotube array surface: a versatile platform for biomedical applications. Small. 2013;9:2945–2953. doi: 10.1002/smll.201300187. [DOI] [PubMed] [Google Scholar]
- 13.’t Mannetje D, et al. Trapping of drops by wetting defects. Nat. Commun. 2014;5:3559. doi: 10.1038/ncomms4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verho T, et al. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011;23:673–678. doi: 10.1002/adma.201003129. [DOI] [PubMed] [Google Scholar]
- 15.Amirfazli, A. & Antonini, C. in Non-Wettable Surfaces: Theory, Preparation and Applications (eds Ras, R. H. A. & Marmur, A.) Ch. 11 (Royal Society of Chemistry, Cambridge, UK, 2016).
- 16.Rykaczewski K, et al. How nanorough is rough enough to make a surface superhydrophobic during water condensation? Soft Matter. 2012;8:8786. doi: 10.1039/c2sm25502b. [DOI] [Google Scholar]
- 17.Srinivasan S, McKinley GH, Cohen RE. Assessing the accuracy of contact angle measurements for sessile drops on liquid-repellent surfaces. Langmuir. 2011;27:13582–13589. doi: 10.1021/la2031208. [DOI] [PubMed] [Google Scholar]
- 18.Butt H-J, et al. Characterization of super liquid-repellent surfaces. Curr. Opin. Colloid Interface Sci. 2014;19:343–354. doi: 10.1016/j.cocis.2014.04.009. [DOI] [Google Scholar]
- 19.Samuel B, Zhao H, Law KY. Study of wetting and adhesion interactions between water and various polymer and superhydrophobic surfaces. J. Phys. Chem. C. 2011;115:14852–14861. doi: 10.1021/jp2032466. [DOI] [Google Scholar]
- 20.Pilat DW, et al. Dynamic measurement of the force required to move a liquid drop on a solid surface. Langmuir. 2012;28:16812–16820. doi: 10.1021/la3041067. [DOI] [PubMed] [Google Scholar]
- 21.Tadmor R, et al. Measurement of lateral adhesion forces at the interface between a liquid drop and a substrate. Phys. Rev. Lett. 2009;103:266101. doi: 10.1103/PhysRevLett.103.266101. [DOI] [PubMed] [Google Scholar]
- 22.Timonen JVI, Latikka M, Ikkala O, Ras RHA. Free-decay and resonant methods for investigating the fundamental limit of superhydrophobicity. Nat. Commun. 2013;4:2398. doi: 10.1038/ncomms3398. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, et al. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009;21:3799–3803. doi: 10.1002/adma.200900686. [DOI] [Google Scholar]
- 24.Delmas M, Monthioux M, Ondarçuhu T. Contact angle hysteresis at the nanometer scale. Phys. Rev. Lett. 2011;106:136102. doi: 10.1103/PhysRevLett.106.136102. [DOI] [PubMed] [Google Scholar]
- 25.Tabor RF, Grieser F, Dagastine RR, Chan DYC. Measurement and analysis of forces in bubble and droplet systems using AFM. J. Colloid Interface Sci. 2012;371:1–14. doi: 10.1016/j.jcis.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 26.Brakefield PM, et al. Development, plasticity and evolution of butterfly eyespot patterns. Nature. 1996;384:236–242. doi: 10.1038/384236a0. [DOI] [PubMed] [Google Scholar]
- 27.Nadeau NJ, et al. The gene cortex controls mimicry and crypsis in butterflies and moths. Nature. 2016;534:106–110. doi: 10.1038/nature17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enright R, Miljkovic N, Al-Obeidi A, Thompson CV, Wang EN. Condensation on superhydrophobic surfaces: the role of local energy barriers and structure length scale. Langmuir. 2012;28:14424–14432. doi: 10.1021/la302599n. [DOI] [PubMed] [Google Scholar]
- 29.Paxson AT, Varanasi KK. Self-similarity of contact line depinning from textured surfaces. Nat. Commun. 2013;4:1492. doi: 10.1038/ncomms2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schellenberger F, Encinas N, Vollmer D, Butt H-J. How water advances on superhydrophobic surfaces. Phys. Rev. Lett. 2016;116:96101. doi: 10.1103/PhysRevLett.116.096101. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhury MK, Goohpattader PS. Activated drops: self-excited oscillation, critical speeding and noisy transport. Eur. Phys. J. E. 2013;36:15. doi: 10.1140/epje/i2013-13015-2. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhury MK, Chakrabarti A, Daniel S. Generation of motion of drops with interfacial contact. Langmuir. 2015;31:9266–9281. doi: 10.1021/la504925u. [DOI] [PubMed] [Google Scholar]
- 33.Liimatainen V, Sariola V, Zhou Q. Controlling liquid spreading using microfabricated undercut edges. Adv. Mater. 2013;25:2275–2278. doi: 10.1002/adma.201204696. [DOI] [PubMed] [Google Scholar]
- 34.Korhonen JT, Huhtamäki T, Verho T, Ras RHA. Hollow polysiloxane nanostructures based on pressure-induced film expansion. Surf. Innovations. 2014;2:116–126. doi: 10.1680/si.13.00047. [DOI] [Google Scholar]
- 35.Zhang F, et al. Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013;25:4192–4198. doi: 10.1002/adma.201301480. [DOI] [PubMed] [Google Scholar]
- 36.Chang B, et al. Capillary self-alignment of microchips on soft substrates. Micromachines. 2016;7:41. doi: 10.3390/mi7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.