Abstract
The skin of mammalian organisms is home for a myriad of microbes. Many of these commensals are thought to have beneficial effects on the host by critically contributing to immune homeostasis. Consequently, dysbiosis can have detrimental effects for the host that may manifest with inflammatory diseases at the barrier tissue. Besides bacteria, fungi make an important contribution to the microbiota and among these, the yeast Malassezia widely dominates in most areas of the skin in healthy individuals. There is accumulating evidence that Malassezia spp. are involved in a variety of skin disorders in humans ranging from non- or mildly inflammatory conditions such as dandruff and pityriasis versicolor to more severe inflammatory skin diseases like seborrheic eczema and atopic dermatitis. In addition, Malassezia is strongly linked to the development of dermatitis and otitis externa in dogs. However, the association of Malassezia spp. with such diseases remains poorly characterized. Until now, studies on the fungus–host interaction remain sparse and they are mostly limited to experiments with isolated host cells in vitro. They suggest a multifaceted crosstalk of Malassezia spp. with the skin by direct activation of the host via conserved pattern recognition receptors and indirectly via the release of fungus-derived metabolites that can modulate the function of hematopoietic and/or non-hematopoietic cells in the barrier tissue. In this review, we discuss our current understanding of the host response to Malassezia spp. in the mammalian skin.
Keywords: Malassezia, commensalism, opportunistic pathogenic fungi, skin disorders, innate immunity, adaptive immunity, allergic response, indoles
Introduction
Malassezia spp. are lipophilic yeasts, which are part of the skin microbiota of many mammals and birds. In fact, the genus Malassezia is by far the most abundant eukaryotic member of the microbial flora of the skin in these organisms (1). Most Malassezia spp. have a predilection for seborrheic skin sites such as the scalp and the trunk. They rely on exogenous fatty acid sources for their nutritive requirements because of their lack of genes encoding for the fatty acid synthase and genes involved in carbohydrate metabolism (2–4). In agreement, the cell wall of Malassezia spp. is particularly rich in lipids (5).
The genus Malassezia currently comprises 17 species, three of which have only recently been proposed (6–8). Malassezia globosa, Malassezia restricta, and Malassezia sympodialis are most frequently isolated from the healthy human skin with distinct relative frequencies at specific body sites (1, 9). The age of the host and geographic factors also influence their distribution (10). Malassezia pachydermatis, Malassezia nana, and Malassezia caprae are found predominantly in non-human hosts (6). Surprisingly, the microbial communities of the skin are astonishingly stable and maintained over time, despite the skin’s exposure to the external environment (11). It is currently unknown whether Malassezia spp. play a mutualistic role and may thus contribute to immune homeostasis of the host.
Apart from their commensal nature, Malassezia spp. are also associated with common skin disorders such as pityriasis versicolor and seborrheic dermatitis as well as more severe inflammatory skin pathologies including atopic eczema and atopic dermatitis in humans (10) and dermatitis and otitis externa in animals, most frequently in dogs (12). The composition of the skin mycobiome can vary under pathological conditions and some species of Malassezia such as M. sympodialis and Malassezia furfur are found to be enriched in certain skin disorders (10). To date, a causative link between Malassezia and disease development has only been made for Pityriasis versicolor, while the role of the yeast in other pathologies remains correlative (10, 13, 14). Changes in the degree of colonization in diseased compared to healthy skin have been documented in dogs (15) but remain uncertain in humans (16).
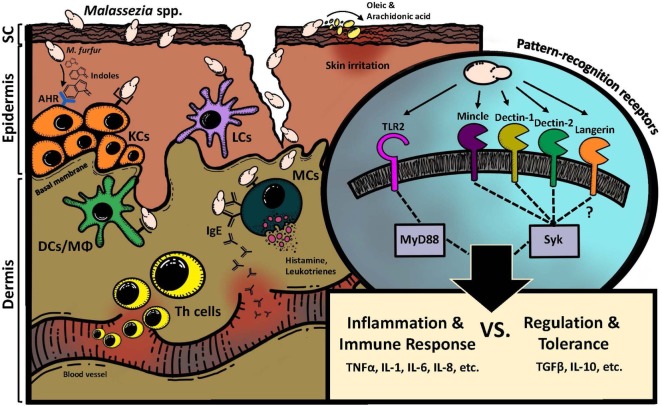
The pathophysiology of Malassezia-associated skin conditions is largely unknown. The lack of knowledge on the cellular and molecular interactions between Malassezia spp. and the host preclude a better understanding of the factors determining commensalism versus disease. Herein, we review the current knowledge with regard to how the host recognizes Malassezia spp. and responds to it (Figure 1).
Figure 1.
Interaction of Malassezia spp. with the mammalian skin. Direct interactions involve various PRRs, which recognize fungal cell wall constituents and are distinctly expressed on the surface of non-hematopoietic (i.e., keratinocytes) and hematopoietic cells (i.e., Langerhans cells, mast cells) of the skin. Spatial and temporal signal integration of different PRR signals results in the induction of inflammation and immunity or, alternatively, in the regulation and tolerance of the host toward Malassezia spp. Indirect interactions of Malassezia spp. with the skin include fungus-derived metabolites such as irritant fatty acids on the one hand and indoles that are potent agonists for the AhR, which is expressed by various skin cells, on the other hand. KCs, keratinocytes; LCs, Langerhans cells; DCs, dendritic cells; Mϕ, macrophages; MCs, mast cells; SC, stratum corneum; AhR, aryl hydrocarbon receptor.
Sensing of Malassezia spp. by the Host
Through their localization in the skin, Malassezia spp. interact primarily with keratinocytes, tissue-resident dendritic cells (DCs), and macrophages, as well as with myeloid cells that are recruited to the skin under inflammatory conditions. Activation of DCs is key for induction of adaptive immunity and memory formation. The fungus is recognized by the host either directly through interaction of fungal cell wall components with membrane bound pattern recognition receptors (PRRs) or indirectly through soluble metabolites that are released by Malassezia spp. The set of receptors expressed by the hematopoietic and the non-hematopoietic compartment are largely distinct.
Direct Recognition of Malassezia spp. by Surface-Bound Receptors
The fungal cell wall is rich in carbohydrates and glycoproteins that are recognized by PRRs of the family of Syk-coupled C-type lectin receptor (CLR), which are expressed primarily by myeloid cells (17, 18). Binding to these receptors results in ligand internalization and activation of multiple signaling pathways, including the MAPK, NF-κB, and NFAT pathways as well as the inflammasome.
The polysaccharides of the Malassezia cell wall are organized differently than in other fungal species analyzed to date (19, 20). Moreover, the cell wall is surrounded by a lipid-rich outer layer (21). Several CLRs have been shown to respond to Malassezia spp. in vitro. The two FcRγ-associated receptors Dectin-2 and Mincle sense Malassezia spp., albeit through recognition of distinct ligands (22). While Mincle binds to two distinct glycolipids in Malassezia, Dectin-2 recognizes the fungus through α-1,2-linked mannose. High-mannose binding is a general feature of Dectin-2, which is reported to recognize a variety of fungi, including Candida albicans, Saccharomyces cerevisiae, Blastomyces dermatitidis, Aspergillus fumigatus, Cryptococcus neoformans, and Fonsecaea pedrosoi (23). In contrast, Malassezia spp. were initially found to be unique agonists of Mincle when a large panel of 50 different fungi was tested in a glycoconjugate microarray (24). More recently, other fungi such as Pneumocystis carinii, F. pedrosoi, and Fonsecaea monomorpha were also reported to engage Mincle (25–27), in addition to bacterial ligands (28–32), mammalian alarmins released from damaged cells (33, 34) and even cholesterol crystals (35, 36). Mincle is thus a highly pleiotropic receptor, which can bind chemically and structurally distinct ligands through at least two complementary binding sites (37–40). The β-glucan receptor Dectin-1, which was the first member of the family of Syk-coupled CLRs to be identified (41), was also found to sense Malassezia and was linked to the activation of the NLRP3 inflammasome (42). Finally, Langerin was suggested to act as a receptor for Malassezia in the skin due to its prominent expression by epidermal Langerhans cells and by a subset of dermal DCs. Direct binding of the fungus to recombinant Langerin was indeed observed (43, 44).
Activation of myeloid cells by Malassezia spp. via these different CLRs was shown to induce the secretion of proinflammatory cytokines. However, the relative contribution of individual receptors to fungal control in vivo during commensalism and in infectious settings remains to be determined. At least partial redundancy of receptors that signal via the same pathway may occur, similarly to what was found for other fungi (45, 46). Dissecting the role of Mincle in the context of Malassezia spp. in more detail will also be interesting in light of its reported antagonizing activity, e.g., in response to Fonsacaea spp. (27), and thus this receptor may also mediate regulatory or inhibitory responses to Malassezia spp.
In addition to CLRs, Toll-like receptors (TLRs), and in particular TLR2, also contribute to fungal recognition by the host. TLR2 was implicated in sensing of Malassezia spp. and inducing a proinflammatory response characterized by the release of cytokines, chemokines and antimicrobial peptides by keratinocytes (47–50).
The proinflammatory response is generally enhanced by lipid removal from the yeast to enhance exposure of fungal cell wall carbohydrates (51, 52). In contrast, thymic stromal lymphopoietin secretion from keratinocytes was found to be induced specifically by the lipid layer components of M. restricta and M. globosa but not by yeasts that were depleted of lipids (53).
Indirect Interaction
Specific products of Malassezia metabolic pathways are thought to act as virulence factors promoting inflammation and pathology, while others downregulate the production of inflammatory mediators and thereby contribute to immune regulation. Fungal strains with altered production of such factors have been linked to Malassezia-associated skin disorders (54–56).
Malassezia-derived lipases and phospholipases, which are required to assimilate host-derived lipids, can initiate an inflammatory response in the skin by releasing unsaturated free fatty acids from the sebum lipids (57–60). Oleic acid has irritant and desquamative effects on keratinocytes (61–63), whereas arachidonic acid produces proinflammatory eicosanoids and leads to inflammation and damage to the stratum corneum, thereby contributing to the disruption of the epithelial barrier function and induction of abnormal keratinization (64).
Malassezia furfur is able to convert tryptophan into a variety of indole alkaloids. This pathway is mainly active if tryptophan is the sole source of nitrogen (65). M. furfur-derived indoles including malassezin, indirubin, and indolo [3,2-b] carbazole (ICZ) serve as potent ligands for the host aryl hydrocarbon receptor (AhR) and thereby potentially modify the function of all cells in the epidermis expressing this receptor (54, 55, 66, 67). For example, some tryptophan metabolites can promote apoptosis of melanocytes (68) or inhibit the respiratory burst in neutrophils (69). Given the broad spectrum of biological responses that are influenced by AhR activity, M. furfur may engage this pathway to modulate inflammation and/or promote skin immune homeostasis (70) but may also promote skin pathology (71) or even contribute to carcinogenesis (72). The significance of yeast-derived indoles in each of these contexts remains to be demonstrated in vivo.
Innate Immunity to Malassezia spp.
The majority of what is currently known about the host response to Malassezia spp. is based on in vitro studies with isolated myeloid cells or keratinocyte cell lines. Stimulation of these cells with Malassezia yeast leads to the induction of mainly proinflammatory cytokines, chemokines, and antimicrobial peptides (22, 24, 47–52, 73–76). In line with an inflammatory character of the innate response to the fungus, the intraperitoneal injection of Malassezia into mice results in the recruitment of neutrophils to the peritoneum (24). Only few studies have examined regulatory cytokines such as IL-10 and TGF-β by the yeast (24, 49, 51, 74, 77), but these may be relevant with regard to the role of Malassezia spp. as a skin commensal.
Given the association of Malassezia spp. with inflammatory skin disorders and allergic responses, the fungus may also interact with mast cells. Progenitor cell-derived mast cells from atopic patients show increased release of proinflammatory cytokines upon stimulation with Malassezia (76) and are enriched in the skin of atopic eczema patients where they are positioned in the superficial dermis and can interact with the fungus (78). Mast cell activation in response to Malassezia spp. has also been reported in studies with bone-marrow-derived mast cells. These cells are directly activated by the fungus in a TLR2-dependent manner and release inflammatory mediators and cytokines (79). Moreover, the crosslinking of the high-affinity IgE receptor (FcεRI) by antigen-bound IgE can induce mast cell degranulation (79). Therefore, mast cells may contribute to further barrier disruption and thereby amplify the inflammatory response.
The access of Malassezia to immune cells in the skin may be facilitated by disruption of the epithelial barrier as it frequently occurs during chronic inflammation. Moreover, Malassezia spp. were reported to release nanovesicles/exosomes that contain immunogenic proteins and trigger increased release of cytokines by DCs (80).
Adaptive Immunity to Malassezia spp.
As a commensal, Malassezia interacts continuously with the immune system. Therefore, cellular and humoral immune memory to the fungus can be evidenced in healthy individuals (81). Although there are fewer studies related to dogs when compared with humans, dogs also develop cellular and humoral immune responses to their commensal yeast, M. pachydermatis (82–84). Generally, the adaptive immune responses are heightened and qualitatively distinct in patients with Malassezia-associated diseases.
Humoral Responses
During steady state, Malassezia-specific antibodies are predominantly of the IgG and IgM isotypes (81). In contrast, although Malassezia-specific IgE is not usually detected in healthy individuals, it is common in atopic patients (85). A positive correlation was found between the sensitization to Malassezia-specific IgE and the severity of atopic dermatitis (86, 87). Similar observations were made in atopic dogs (83, 84). However, whether the IgE response plays a pathogenic role in atopic and other Malassezia-associated inflammatory disorders or rather serves as a marker for the severity of disease remains unclear.
T Cell Responses
Patients with atopic dermatitis often show positive skin prick test and atopic patch test reactions to Malassezia (85). T cell-responsiveness to Malassezia in such patients was associated with a Th2 response (88), in line with the classical paradigm of Th2-polarized allergic T cells. GATA3+ T cells were identified in pityriasis versicolor lesions (89) and likewise Malassezia-specific T cell in allergic dogs were found to be strongly polarized toward a type 2 response (82). More recently, other T helper cell subsets such as Th17 and Th22 cells have been found enriched in allergic individuals (90, 91) as well as in non-allergic immune-mediated skin diseases such as psoriasis (92). Consistent with this notion, Malassezia-reactive skin homing T cells from Malassezia-sensitized atopic dermatitis patients comprise not only Th1 and Th2 subsets but also IL-17- and IL-22-secreting cells (93). Of note, IL-4/IL-17 coproducers have also been described in the context of atopic eczema especially in children (94). Importantly, Th17 differentiation is a hallmark of T cell responses induced by CLR signaling (46) and T cells directed against other fungi, in particular Candida spp., belong predominantly to the Th17 subset (95). Whether and how IL-17 and/or IL-22 may contribute to pathogenicity in atopic dermatitis remains to be determined. It is also unknown to which subset Malassezia-specific T cells belong in healthy individuals and to what extent T cell plasticity contributes to sensitization.
Malassezia Allergens
To date, 13 Malassezia-derived allergens have been identified from M. furfur and M. sympodialis (3, 96). Interestingly, more allergens are released from M. sympodialis when cultured at the increased pH conditions of atopic skin compared with culture at the pH of healthy skin (97). Several of the known allergens belong to a class of phylogenetically highly conserved proteins and display a high degree of homology with the corresponding mammalian proteins. Cross-reactivity between Malassezia-derived allergens and endogenous human proteins (e.g., thioredoxin, manganese-dependent superoxide dismutase) has been indeed demonstrated (93, 98, 99). Therefore, the induction of autoreactive T cells by Malassezia allergens may play a role in sustained inflammation.
Conclusion
Malassezia spp. have been implicated in various pathologies. Yet, direct evidence for a causal relationship between Malassezia spp. and the mammalian host remains elusive. For instance, it is unclear whether Malassezia actively promotes atopic dermatitis or whether the inflammatory environment in the atopic skin triggers a dysregulated immune response toward the fungus.
At the basis of this is the key question of what determines the balance between commensalism and pathogenicity of Malassezia spp. The answer likely relates to changes occurring in both the fungus (55) (e.g., variable secretion of AhR agonists) and in the host (e.g., barrier defects, changes in immune polarization) which are responsible for promoting the development of pathology. Changes in the environment such as seasonal variations in sebum production have also been linked to altered disease prevalence (100).
Inter-species variations in the skin mycobiome may further contribute as different species of Malassezia can induce variable inflammatory responses (51, 75, 101). Moreover, Malassezia spp. have been shown to display a large intra-species diversity (73) similarly to what is known for other opportunistic fungal pathogens (102), and thus the exact composition of Malassezia strains and species present in an individual at a given time may contribute to different outcomes in the interaction between the fungus and the host. The recently completed assembly and detailed annotation of the genome of M. sympodialis makes an important contribution to approach this complexity (103). Future research will help fill the important gaps in our knowledge on the pathophysiology of and the host response to Malassezia in vivo. Enhanced understanding of host-Malassezia interactions may contribute to improved diagnostic and therapeutic options for patients affected by Malassezia-associated pathologies.
Author Contributions
Both authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the University of Zürich.
References
- 1.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature (2013) 498:367–70. 10.1038/nature12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu G, Zhao H, Li C, Rajapakse MP, Wong WC, Xu J, et al. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet (2015) 11:e1005614. 10.1371/journal.pgen.1005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gioti A, Nystedt B, Li W, Xu J, Andersson A, Averette AF, et al. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. mBio (2013) 4:e00572–12. 10.1128/mBio.00572-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park M, Cho Y-J, Lee YW, Jung WH. Whole genome sequencing analysis of the cutaneous pathogenic yeast Malassezia restricta and identification of the major lipase expressed on the scalp of patients with dandruff. Mycoses (2017) 60:188–97. 10.1111/myc.12586 [DOI] [PubMed] [Google Scholar]
- 5.Thompson E, Colvin JR. Composition of the cell wall of Pityrosporum ovale (Bizzozero) Castellani and Chalmers. Can J Microbiol (1970) 16:263–5. 10.1139/m70-048 [DOI] [PubMed] [Google Scholar]
- 6.Cabanes FJ. Malassezia yeasts: how many species infect humans and animals? PLoS Pathog (2014) 10:e1003892. 10.1371/journal.ppat.1003892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honnavar P, Prasad GS, Ghosh A, Dogra S, Handa S, Rudramurthy SM. Malassezia arunalokei sp. nov., a novel yeast species isolated from seborrheic dermatitis patients and healthy individuals from India. J Clin Microbiol (2016) 54:1826–34. 10.1128/JCM.00683-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabanes FJ, Coutinho SDA, Puig L, Bragulat MR, Castella G. New lipid-dependent Malassezia species from parrots. Rev Iberoam Micol (2016) 33:92–9. 10.1016/j.riam.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 9.Jo J-H, Kennedy EA, Kong HH. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence (2017) 8:324–33. 10.1080/21505594.2016.1249093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prohic A, Jovovic Sadikovic T, Krupalija-Fazlic M, Kuskunovic-Vlahovljak S. Malassezia species in healthy skin and in dermatological conditions. Int J Dermatol (2016) 55:494–504. 10.1111/ijd.13116 [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Byrd AL, Park M, Kong HH, Segre JA. Temporal stability of the human skin microbiome. Cell (2016) 165:854–66. 10.1016/j.cell.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond R, Guillot J, Cabañes FJ. Malassezia yeasts in animal disease. In: Boekhout T, Mayser P, Guého-Kellermann E, Velegraki A, editors. Malassezia and the Skin: Science and Clinical Practice. Berlin, Heidelberg: Springer; (2010). p. 271–99. [Google Scholar]
- 13.Johansson C, Eshaghi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cells response. J Invest Dermatol (2002) 118:1044–51. 10.1046/j.1523-1747.2002.01758.x [DOI] [PubMed] [Google Scholar]
- 14.Casagrande BF, Fluckiger S, Linder MT, Johansson C, Scheynius A, Crameri R, et al. Sensitization to the yeast Malassezia sympodialis is specific for extrinsic and intrinsic atopic eczema. J Invest Dermatol (2006) 126:2414–21. 10.1038/sj.jid.5700431 [DOI] [PubMed] [Google Scholar]
- 15.Cafarchia C, Gallo S, Romito D, Capelli G, Chermette R, Guillot J, et al. Frequency, body distribution, and population size of Malassezia species in healthy dogs and in dogs with localized cutaneous lesions. J Vet Diagn Invest (2005) 17:316–22. 10.1177/104063870501700403 [DOI] [PubMed] [Google Scholar]
- 16.Sandstrom Falk MH, Tengvall Linder M, Johansson C, Bartosik J, Back O, Sarnhult T, et al. The prevalence of Malassezia yeasts in patients with atopic dermatitis, seborrhoeic dermatitis and healthy controls. Acta Derm Venereol (2005) 85:17–23. 10.1080/00015550410022276 [DOI] [PubMed] [Google Scholar]
- 17.Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity (2015) 43:845–58. 10.1016/j.immuni.2015.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol (2015) 32:21–7. 10.1016/j.coi.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stalhberger T, Simenel C, Clavaud C, Eijsink VGH, Jourdain R, Delepierre M, et al. Chemical organization of the cell wall polysaccharide core of Malassezia restricta. J Biol Chem (2014) 289:12647–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruppa MD, Lowman DW, Chen YH, Selander C, Scheynius A, Monteiro MA, et al. Identification of (1 – >6)-beta-D-glucan as the major carbohydrate component of the Malassezia sympodialis cell wall. Carbohydr Res (2009) 344:2474–9. 10.1016/j.carres.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittag H. Fine structural investigation of Malassezia furfur. II. The envelope of the yeast cells. Mycoses (1995) 38:13–21. 10.1111/j.1439-0507.1995.tb00003.x [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe (2013) 13:477–88. 10.1016/j.chom.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 23.Brown GD, Crocker PR. Lectin receptors expressed on myeloid cells. Microbiol Spectr (2016) 4(5):MCHD-0036-2016. 10.1128/microbiolspec.MCHD-0036-2016 [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A (2009) 106:1897–902. 10.1073/pnas.0805177106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kottom TJ, Hebrink DM, Jenson PE, Nandakumar V, Wuthrich M, Wang H, et al. The interaction of pneumocystis with the C-type lectin receptor Mincle exerts a significant role in host defense against infection. J Immunol (2017) 198:3515–25. 10.4049/jimmunol.1600744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa MdG., Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe (2011) 9:436–43. 10.1016/j.chom.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TBH, et al. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host Microbe (2014) 15:494–505. 10.1016/j.chom.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 28.Iborra S, Martinez-Lopez M, Cueto FJ, Conde-Garrosa R, Del Fresno C, Izquierdo HM, et al. Leishmania uses Mincle to target an inhibitory ITAM signaling pathway in dendritic cells that dampens adaptive immunity to infection. Immunity (2016) 45:788–801. 10.1016/j.immuni.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabes A, Zimmermann S, Reppe K, Lang R, Seeberger PH, Suttorp N, et al. The C-type lectin receptor Mincle binds to Streptococcus pneumoniae but plays a limited role in the anti-pneumococcal innate immune response. PLoS One (2015) 10:e0117022. 10.1371/journal.pone.0117022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devi S, Rajakumara E, Ahmed N. Induction of Mincle by Helicobacter pylori and consequent anti-inflammatory signaling denote a bacterial survival strategy. Sci Rep (2015) 5:15049. 10.1038/srep15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schick J, Etschel P, Bailo R, Ott L, Bhatt A, Lepenies B, et al. Toll-like receptor 2 and Mincle cooperatively sense corynebacterial cell wall glycolipids. Infect Immun (2017) 85:e00075–17. 10.1128/IAI.00075-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med (2009) 206:2865–8. 10.1084/jem.20092533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol (2008) 9:1179–88. 10.1038/ni.1651 [DOI] [PubMed] [Google Scholar]
- 34.Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, et al. Intracellular metabolite beta-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc Natl Acad Sci U S A (2017) 114:E3285–94. 10.1073/pnas.1618133114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S. Human Mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem (2015) 290:25322–32. 10.1074/jbc.M115.645234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostarnoy AV, Gancheva PG, Lepenies B, Tukhvatulin AI, Dzharullaeva AS, Polyakov NB, et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc Natl Acad Sci U S A (2017) 114:E2758–65. 10.1073/pnas.1611665114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinberg H, Jegouzo SAF, Rowntree TJW, Guan Y, Brash MA, Taylor ME, et al. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J Biol Chem (2013) 288:28457–65. 10.1074/jbc.M113.497149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jegouzo SAF, Harding EC, Acton O, Rex MJ, Fadden AJ, Taylor ME, et al. Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology (2014) 24:1291–300. 10.1093/glycob/cwu072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg H, Rambaruth NDS, Jegouzo SAF, Jacobsen KM, Djurhuus R, Poulsen TB, et al. Binding sites for acylated trehalose analogs of glycolipid ligands on an extended carbohydrate recognition domain of the macrophage receptor Mincle. J Biol Chem (2016) 291:21222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa A, Kamishikiryo J, Mori D, Toyonaga K, Okabe Y, Toji A, et al. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc Natl Acad Sci U S A (2013) 110:17438–43. 10.1073/pnas.1312649110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature (2001) 413:36–7. 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- 42.Kistowska M, Fenini G, Jankovic D, Feldmeyer L, Kerl K, Bosshard P, et al. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp Dermatol (2014) 23:884–9. 10.1111/exd.12552 [DOI] [PubMed] [Google Scholar]
- 43.de Jong MA, Vriend LE, Theelen B, Taylor ME, Fluitsma D, Boekhout T, et al. C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol (2010) 47:1216–25. 10.1016/j.molimm.2009.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tateno H, Ohnishi K, Yabe R, Hayatsu N, Sato T, Takeya M, et al. Dual specificity of Langerin to sulfated and mannosylated glycans via a single C-type carbohydrate recognition domain. J Biol Chem (2010) 285:6390–400. 10.1074/jbc.M109.041863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med (2009) 206:2037–51. 10.1084/jem.20082818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol (2007) 8:630–8. 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- 47.Angrisano T, Pero R, Paoletti I, Keller S, Lembo L, Baroni A, et al. Epigenetic regulation of IL-8 and beta-defensin genes in human keratinocytes in response to Malassezia furfur. J Invest Dermatol (2013) 133:2104–2104. 10.1038/jid.2013.143 [DOI] [PubMed] [Google Scholar]
- 48.Buommino E, De Filippis A, Parisi A, Nizza S, Martano M, Iovane G, et al. Innate immune response in human keratinocytes infected by a feline isolate of Malassezia pachydermatis. Vet Microbiol (2013) 163:90–6. 10.1016/j.vetmic.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 49.Donnarumma G, Perfetto B, Paoletti I, Oliviero G, Clavaud C, Del Bufalo A, et al. Analysis of the response of human keratinocytes to Malassezia globosa and restricta strains. Arch Dermatol Res (2014) 306:763–8. 10.1007/s00403-014-1479-1 [DOI] [PubMed] [Google Scholar]
- 50.Baroni A, Orlando M, Donnarumma G, Farro P, Iovene MR, Tufano MA, et al. Toll-like receptor 2 (TLR2) mediates intracellular signalling in human keratinocytes in response to Malassezia furfur. Arch Dermatol Res (2006) 297:280–8. 10.1007/s00403-005-0594-4 [DOI] [PubMed] [Google Scholar]
- 51.Thomas DS, Ingham E, Bojar RA, Holland KT. In vitro modulation of human keratinocyte pro- and anti-inflammatory cytokine production by the capsule of Malassezia species. FEMS Immunol Med Microbiol (2008) 54:203–14. 10.1111/j.1574-695X.2008.00468.x [DOI] [PubMed] [Google Scholar]
- 52.Kesavan S, Holland KT, Ingham E. The effects of lipid extraction on the immunomodulatory activity of Malassezia species in vitro. Med Mycol (2000) 38:239–47. 10.1080/mmy.38.3.239.247 [DOI] [PubMed] [Google Scholar]
- 53.Ishibashi Y, Sugawara K, Sugita T, Nishikawa A. Secretion of thymic stromal lymphopoietin from human keratinocytes in response to Malassezia yeasts. J Dermatol Sci (2011) 62:124–8. 10.1016/j.jdermsci.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 54.Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol (2013) 133:2023–30. 10.1038/jid.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaitanis G, Magiatis P, Stathopoulou K, Bassukas ID, Alexopoulos EC, Velegraki A, et al. AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J Invest Dermatol (2008) 128:1620–5. 10.1038/sj.jid.5701252 [DOI] [PubMed] [Google Scholar]
- 56.Cafarchia C, Otranto D. Association between phospholipase production by Malassezia pachydermatis and skin lesions. J Clin Microbiol (2004) 42:4868–9. 10.1128/JCM.42.10.4868-4869.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riciputo RM, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses (1996) 39:233–5. 10.1111/j.1439-0507.1996.tb00131.x [DOI] [PubMed] [Google Scholar]
- 58.Plotkin LI, Squiquera L, Mathov I, Galimberti R, Leoni J. Characterization of the lipase activity of Malassezia furfur. J Med Vet Mycol (1996) 34:43–8. 10.1080/02681219680000071 [DOI] [PubMed] [Google Scholar]
- 59.Lee YW, Lee SY, Lee Y, Jung WH. Evaluation of expression of lipases and phospholipases of Malassezia restricta in patients with seborrheic dermatitis. Ann Dermatol (2013) 25:310–4. 10.5021/ad.2013.25.3.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortiz G, Martin MC, Carrillo-Muñoz AJ, Paya MJ. [Phospholipase and proteinase production by Malassezia pachydermatis isolated in dogs with and without otitis]. Rev Iberoam Micol (2013) 30:235–8. 10.1016/j.riam.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 61.DeAngelis YM, Gemmer CM, Kaczvinsky JR, Kenneally DC, Schwartz JR, Dawson TL, Jr. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J Investig Dermatol Symp Proc (2005) 10:295–7. 10.1111/j.1087-0024.2005.10119.x [DOI] [PubMed] [Google Scholar]
- 62.Katsuta Y, Iida T, Hasegawa K, Inomata S, Denda M. Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N-methyl D-aspartate-type glutamate receptors. Br J Dermatol (2009) 160:69–74. 10.1111/j.1365-2133.2008.08860.x [DOI] [PubMed] [Google Scholar]
- 63.Katsuta Y, Iida S, Fau-Inomata T, Inomata M, Fau-Denda S, Denda M. Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. J Investig Dermatol (2005) 124:1008–13. 10.1111/j.0022-202X.2005.23682.x [DOI] [PubMed] [Google Scholar]
- 64.Plotkin LI, Mathov I, Squiquera L, Leoni J. Arachidonic acid released from epithelial cells by Malassezia furfur phospholipase A2: a potential pathophysiologic mechanism. Mycologia (1998) 90:163–9. 10.2307/3761291 [DOI] [Google Scholar]
- 65.Mayser P, Wille G, Imkampe A, Thoma W, Arnold N, Monsees T. Synthesis of fluorochromes and pigments in Malassezia furfur by use of tryptophan as the single nitrogen source. Mycoses (1998) 41:265–71. 10.1111/j.1439-0507.1998.tb00336.x [DOI] [PubMed] [Google Scholar]
- 66.Mexia N, Gaitanis G, Velegraki A, Soshilov A, Denison MS, Magiatis P. Pityriazepin and other potent AhR ligands isolated from Malassezia furfur yeast. Arch Biochem Biophys (2015) 571:16–20. 10.1016/j.abb.2015.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayser P, Schafer U, Kramer HJ, Irlinger B, Steglich W. Pityriacitrin – an ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch Dermatol Res (2002) 294:131–4. 10.1007/s00403-002-0294-2 [DOI] [PubMed] [Google Scholar]
- 68.Kramer HJ, Podobinska M, Bartsch A, Battmann A, Thoma W, Bernd A, et al. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast Malassezia furfur, induces apoptosis in primary human melanocytes. Chembiochem (2005) 6:860–5. 10.1002/cbic.200400247 [DOI] [PubMed] [Google Scholar]
- 69.Kramer HJ, Kessler D, Hipler UC, Irlinger B, Hort W, Bodeker RH, et al. Pityriarubins, novel highly selective inhibitors of respiratory burst from cultures of the yeast Malassezia furfur: comparison with the bisindolylmaleimide arcyriarubin A. Chembiochem (2005) 6:2290–7. 10.1002/cbic.200500163 [DOI] [PubMed] [Google Scholar]
- 70.Vlachos C, Schulte BM, Magiatis P, Adema GJ, Gaitanis G. Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br J Dermatol (2012) 167:496–505. 10.1111/j.1365-2133.2012.11014.x [DOI] [PubMed] [Google Scholar]
- 71.Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, et al. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol (2005) 25:9360–8. 10.1128/MCB.25.21.9360-9368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaitanis G, Velegraki A, Magiatis P, Pappas P, Bassukas ID. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med Hypotheses (2011) 77:47–51. 10.1016/j.mehy.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 73.Buommino E, Nocera FP, Parisi A, Rizzo A, Donnarumma G, Mallardo K, et al. Correlation between genetic variability and virulence factors in clinical strains of Malassezia pachydermatis of animal origin. New Microbiol (2016) 39:216–23. [PubMed] [Google Scholar]
- 74.Donnarumma G, Paoletti I, Buommino E, Orlando M, Tufano MA, Baroni A. Malassezia furfur induces the expression of beta-defensin-2 in human keratinocytes in a protein kinase C-dependent manner. Arch Dermatol Res (2004) 295:474–81. 10.1007/s00403-003-0445-0 [DOI] [PubMed] [Google Scholar]
- 75.Watanabe S, Kano R, Sato H, Nakamura Y, Hasegawa A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest Dermatol (2001) 116:769–73. 10.1046/j.1523-1747.2001.01321.x [DOI] [PubMed] [Google Scholar]
- 76.Ribbing C, Engblom C, Lappalainen J, Lindstedt K, Kovanen PT, Karlsson MA, et al. Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy (2011) 66:110–9. 10.1111/j.1398-9995.2010.02437.x [DOI] [PubMed] [Google Scholar]
- 77.Baroni A, Paoletti I, Ruocco E, Agozzino M, Tufano MA, Donnarumma G. Possible role of Malassezia furfur in psoriasis: modulation of TGF-beta1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis-affected patients. J Cutan Pathol (2004) 31:35–42. 10.1046/j.0303-6987.2004.0135.x [DOI] [PubMed] [Google Scholar]
- 78.Jarvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol (1997) 136:871–7. 10.1111/j.1365-2133.1997.tb03927.x [DOI] [PubMed] [Google Scholar]
- 79.Selander C, Engblom C, Nilsson G, Scheynius A, Andersson CL. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J Immunol (2009) 182:4208–16. 10.4049/jimmunol.0800885 [DOI] [PubMed] [Google Scholar]
- 80.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses – novel mechanisms for host-microbe interactions in atopic eczema. PLoS One (2011) 6:e21480. 10.1371/journal.pone.0021480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ashbee HR, Bond R. Malassezia species and immunity: host–pathogen interactions. In: Boekhout T, Mayser P, Guého-Kellermann E, Velegraki A, editors. Malassezia and the Skin: Science and Clinical Practice. Berlin, Heidelberg: Springer; (2010). p. 139–73. [Google Scholar]
- 82.Valli JL, Williamson A, Sharif S, Rice J, Shewen PE. In vitro cytokine responses of peripheral blood mononuclear cells from healthy dogs to distemper virus, Malassezia and Toxocara. Vet Immunol Immunopathol (2010) 134:218–29. 10.1016/j.vetimm.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 83.Kang MH, Kim HJ, Jang HJ, Park HM. Sensitization rates of causative allergens for dogs with atopic dermatitis: detection of canine allergen-specific IgE. J Vet Sci (2014) 15:545–50. 10.4142/jvs.2014.15.4.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen TA, Halliwell RE, Pemberton AD, Hill PB. Identification of major allergens of Malassezia pachydermatis in dogs with atopic dermatitis and Malassezia overgrowth. Vet Dermatol (2002) 13:141–50. 10.1046/j.1365-3164.2002.00291.x [DOI] [PubMed] [Google Scholar]
- 85.Johansson C, Sandstrom MH, Bartosik J, Sarnhult T, Christiansen J, Zargari A, et al. Atopy patch test reactions to Malassezia allergens differentiate subgroups of atopic dermatitis patients. Br J Dermatol (2003) 148:479–88. 10.1046/j.1365-2133.2003.05093.x [DOI] [PubMed] [Google Scholar]
- 86.Glatz M, Buchner M, von Bartenwerffer W, Schmid-Grendelmeier P, Worm M, Hedderich J, et al. Malassezia spp.-specific immunoglobulin E level is a marker for severity of atopic dermatitis in adults. Acta Derm Venereol (2015) 95:191–6. 10.2340/00015555-1864 [DOI] [PubMed] [Google Scholar]
- 87.Zhang E, Tanaka T, Tajima M, Tsuboi R, Kato H, Nishikawa A, et al. Anti-Malassezia-specific IgE antibodies production in Japanese patients with head and neck atopic dermatitis: relationship between the level of specific ige antibody and the colonization frequency of cutaneous Malassezia species and clinical severity. J Allergy (Cairo) (2011) 2011:645670. 10.1155/2011/645670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johansson C, Ahlborg N, Andersson A, Lundeberg L, Karlsson MA, Scheynius A, et al. Elevated peripheral allergen-specific T cell response is crucial for a positive atopy patch test reaction. Int Arch Allergy Immunol (2009) 150:51–8. 10.1159/000210380 [DOI] [PubMed] [Google Scholar]
- 89.Levy JMS, Magro C. Atrophying pityriasis versicolor as an idiosyncratic T cell-mediated response to Malassezia: a case series. J Am Acad Dermatol (2017) 76:730–5. 10.1016/j.jaad.2016.08.062 [DOI] [PubMed] [Google Scholar]
- 90.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest (2009) 119:3573–85. 10.1172/JCI40202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cavani A, Pennino D, Eyerich K. Th17 and Th22 in skin allergy. Chem Immunol Allergy (2012) 96:39–44. 10.1159/000331870 [DOI] [PubMed] [Google Scholar]
- 92.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol (2009) 129:1339–50. 10.1038/jid.2009.59 [DOI] [PubMed] [Google Scholar]
- 93.Balaji H, Heratizadeh A, Wichmann K, Niebuhr M, Crameri R, Scheynius A, et al. Malassezia sympodialis thioredoxin-specific T cells are highly cross-reactive to human thioredoxin in atopic dermatitis. J Allergy Clin Immunol (2011) 128:92–9.e4. 10.1016/j.jaci.2011.02.043 [DOI] [PubMed] [Google Scholar]
- 94.Esaki H, Brunner PM, Renert-Yuval Y, Czarnowicki T, Huynh T, Tran G, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol (2016) 138:1639–51. 10.1016/j.jaci.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 95.Sparber F, LeibundGut-Landmann S. Interleukin 17-mediated host defense against Candida albicans. Pathogens (2015) 4:606–19. 10.3390/pathogens4030606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev (2012) 25:106–41. 10.1128/CMR.00021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selander C, Zargari A, Mollby R, Rasool O, Scheynius A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy (2006) 61:1002–8. 10.1111/j.1398-9995.2006.01108.x [DOI] [PubMed] [Google Scholar]
- 98.Schmid-Grendelmeier P, Fluckiger S, Disch R, Trautmann A, Wuthrich B, Blaser K, et al. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol (2005) 115:1068–75. 10.1016/j.jaci.2005.01.065 [DOI] [PubMed] [Google Scholar]
- 99.Glaser AG, Limacher A, Fluckiger S, Scheynius A, Scapozza L, Crameri R. Analysis of the cross-reactivity and of the 1.5 A crystal structure of the Malassezia sympodialis Mala s 6 allergen, a member of the cyclophilin pan-allergen family. Biochem J (2006) 396:41–9. 10.1042/BJ20051708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Youn SW, Na JI, Choi SY, Huh CH, Park KC. Regional and seasonal variations in facial sebum secretions: a proposal for the definition of combination skin type. Skin Res Technol (2005) 11:189–95. 10.1111/j.1600-0846.2005.00119.x [DOI] [PubMed] [Google Scholar]
- 101.Kim SY, Kim SH, Kim SN, Kim AR, Kim YR, Kim MJ, et al. Isolation and identification of Malassezia species from Chinese and Korean patients with seborrheic dermatitis and in vitro studies on their bioactivity on sebaceous lipids and IL-8 production. Mycoses (2016) 59:274–80. 10.1111/myc.12456 [DOI] [PubMed] [Google Scholar]
- 102.Schonherr FA, Sparber F, Kirchner FR, Guiducci E, Trautwein-Weidner K, Gladiator A, et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol (2017) 10:1335–50. 10.1038/mi.2017.2 [DOI] [PubMed] [Google Scholar]
- 103.Zhu Y, Engstrom PG, Tellgren-Roth C, Baudo CD, Kennell JC, Sun S, et al. Proteogenomics produces comprehensive and highly accurate protein-coding gene annotation in a complete genome assembly of Malassezia sympodialis. Nucleic Acids Res (2017) 45:2629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]