Abstract
Escherichia coli have the genetic potential to use chitin as a carbon source in the absence of glucose, importing it via the chitin-uptake channel EcChiP for processing by the glucosamine catabolic pathway. The chip gene is usually not expressed when E. coli are grown on glucose-enriched nutrients, providing a general regulatory mechanism for the pathway. EcChiP is unusual in that it is homologous to porins and monomeric instead of trimeric, the typical form of sugar-specific channels, making it unclear how this channel operates. We recently reported that EcChiP could form a stable channel in lipid membranes and that the channel is specific for chitooligosaccharides. This report describes the biophysical nature of sugar-channel interactions and the kinetics of sugar association and dissociation. Titrating EcChiP with chitohexaose resulted in protein fluorescence enhancement in a concentration-dependent manner, yielding a binding constant of 2.9 × 105 m−1, consistent with the value of 2.5 × 105 m−1 obtained from isothermal titration microcalorimetry. Analysis of the integrated heat change suggested that the binding process was endothermic and driven by entropy. Single-channel recordings confirmed the voltage dependence of the penetration of chitohexaose molecules into and their release from EcChiP. Once inside the pore, the sugar release rate (koff) from the affinity site increased with elevated voltage, regardless of the side of sugar addition. Our findings revealed distinct thermodynamic and kinetic features of the activity of sugar-specific EcChiP and advance our knowledge of the physiological possibility of chitin utilization by non-chitinolytic bacteria.
Introduction
Escherichia coli are heterotrophic bacteria that typically grow on various polysaccharides such as starch, cellulose, and hemicelluloses (1) but not on chitin, because the genes responsible for chitin degradation and uptake by E. coli are usually repressed during growth on glucose-enriched media (2, 3). Although the chitin catabolic pathway is well-preserved in non-chitinolytic bacteria (4, 5), the chip gene, encoding the chitin oligosaccharide transporter chitoporin (so-called ChiP), is usually quiescent in the absence of chitooligosaccharide inducers (6, 7), because the expression of this gene is repressed by non-coding small RNAs (6–10). Recently we identified the chip gene, which encodes chitoporin (denoted EcChiP), in the genome of E. coli. Based on its extensive amino acid sequence identity with the amino acid transporter OccD (formerly OprD) from Pseudomonas aeruginosa (11), EcChiP has been classified as a member of the OccD family of porins. The chip gene was cloned into the pET23d(+) vector, and the recombinant EcChiP was then expressed in the cell wall of the Omp-deficient E. coli BL21(DE3) Omp8 Rosetta strain (12). The purified EcChiP was shown to form a fully opening channel in artificial planar lipid membranes, displaying an average single-channel conductance of 0.5 ± 0.05 nS. This conductance value is about one-third of the average conductance of trimeric VhChiP from Vibrio harveyi (1.8 ± 0.3 nS) (13), and our previous biochemical work (12) on native EcChiP showed that the functional channel comprises only one subunit. To date, EcChiP is the first monomeric sugar-specific channel to have been reported, whereas other sugar-specific channels typically comprise three identical subunits (14–19). When compared with other putative ChiPs from different bacteria, EcChiP has the highest sequence identity with an uncharacterized ChiP from Salmonella typhimurium (Q7CQY4) (90%) (7), followed by ChiP from Serratia marcescens (A0A0G8BBQ7) (70%) (20), both of which have also been suggested to be OccD-like porins. EcChiP has a particularly low sequence identity (<14%) with the unrelated OmpC-like ChiPs, such as those from V. harveyi ChiP (VhChiP) and Vibrio furnissii ChiP (VfChiP) (21, 22). In this study, we evaluated the thermodynamic and kinetic characteristics of chitooligosaccharide-EcChiP interactions, using a combination of biophysical approaches including isothermal titration calorimetry (ITC),3 protein fluorescence spectroscopy, and single molecule electrophysiology. The data obtained from this study provide the mechanistic details of chitin transport across the outer membrane of non-chitinolytic bacteria.
Results
Chitohexaose-induced enhancement of EcChiP fluorescence
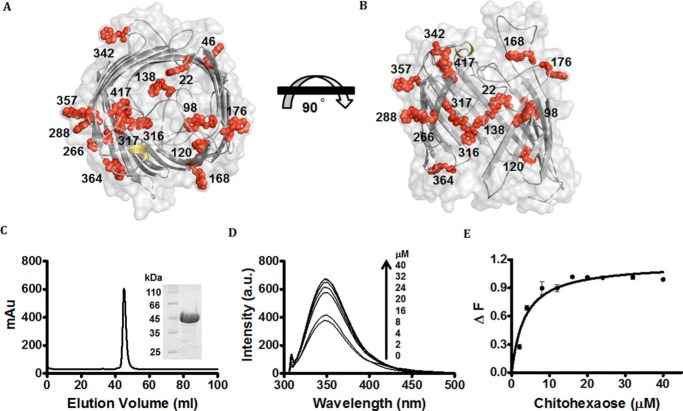
Inspection of the amino acid sequence of EcChiP shows a large number of Trp residues. Fig. 1 shows the amino acid sequence of EcChiP, containing a total of 15 Trp residues, (indicated in red). Fig. 2 (A and B) shows the modeled 3D structure of EcChiP, with the precise locations of these Trp residues within the molecule. Residues Trp-46, Trp-168, Trp-176, Trp-266, Trp-288, Trp-342, and Trp-357 are all located within the transmembrane segments that form the outer surface of the protein barrel, whereas Trp-98 (in L2), Trp-138 (in L3), Trp-176 (in L4), Trp-364 (in L8), and Trp-417 (in L9) are in the extracellular loops (denoted Ln, where n indicates the loop number). These loops are presumed to be highly flexible and could protrude into the protein lumen and serve as part of the sugar-conducting pathway. For the assay of chitohexaose binding by fluorescence enhancement, we first prepared highly purified EcChiP. As shown in Fig. 2C, recombinant EcChiP expressed in the Omp-deficient E. coli strain was purified to homogeneity in two steps: detergent extraction, followed by gel filtration chromatography. Fig. 2C (inset) shows the single band of EcChiP obtained in the gel filtration step. The protein migrated on SDS-PAGE with an apparent molecular mass slightly above 45 kDa, which agrees well with the predicted value of 52,780 Da, and with the value for native, monomeric EcChiP determined previously (12). To quantify sugar-channel interactions, we measured changes in protein fluorescence intensity when EcChiP was titrated with chitohexaose in the concentration range of 0–40 μm. Fig. 2D shows fluorescence emission spectra (300–450 nm) of EcChiP, excited at 295 nm. The maximum fluorescence emission intensity (Fmax) was observed at 345 nm, in the absence and presence of sugar. When the protein was titrated with chitohexaose, the fluorescence was enhanced in a concentration-dependent manner. Plotting the fluorescence intensity change (ΔF) against sugar concentration yielded a typical hyperbolic binding curve (Fig. 2E), which approached saturation with a sugar concentration of >8 μm. No observable blue or red shift in the maximal intensity was induced by sugar addition. Fitting the fluorescence intensity data with a non-linear regression function, assuming single-site-binding model, yielded the Kd value of 3.4 μm, equivalent to a binding constant (K) of 2.9 × 105 m−1.
Figure 1.

EcChiP sequence analysis for fluorescence spectroscopy. The amino acid sequence of EcChiP (P75733) was retrieved from the Uniprot database. The Trp residues that are putatively important for fluorescence spectroscopy are indicated in red. The secondary structure of E. coli was constructed by ESPript 3.0, according to the structure of P. aeruginosa OprD (Protein Data Bank code 2odj). β-Strands are marked with gray arrows, and α-helices are marked with gray spirals.
Figure 2.
Protein–ligand binding studied by fluorescence spectroscopy. A and B, surface and cartoon representation of top view of model EcChiP (A) and surface and cartoon representation (B) of EcChiP, showing a top view and a 90° rotation representing a cross-section of the side view. All Trp residues in the EcChiP pore are represented as red stick-and-ball models. β-Barrels, smooth loops, and the surface of EcChiP are shown in gray, and α-helices are shown in yellow. Trp-22, Trp-120, Trp-138, Trp-316, and Trp-317 are positioned inside the pore, whereas Trp-98 (in L2), Trp-176 (in L4), Trp-364 (in L8), and Tyr-417 (in L9) can protrude into the channel from extracellular side. Trp-342 is part of a periplasmic turn. Other tryptophan residues (positions 46, 168, 266, 288, and 357) are located at different positions around the outer surface of the barrel. C, chromatographic profile of EcChiP purification with a HiPrep 16/60 Sephacryl S-200 high-resolution column connected to an ÄKTA Prime plus FPLC system. Bound proteins were eluted with a 20 mm phosphate buffer, pH 7.4, in the presence of 0.05% LDAO. SDS-PAGE analysis of purified EcChiP is shown in an inset (EcChiP samples were heated). D, effects of chitohexaose on the intrinsic fluorescence of EcChiP. Aliquots of chitohexaose were added to 720 ng/ml (final concentration) of EcChiP as shown. E, binding curves for chitohexaose. Binding curves were evaluated with a non-linear regression function available in Prism version 5.0 (GraphPad Software) using a model based on a single binding site. Experiments were performed in triplicate, and the results shown are averages.
Thermodynamic evaluation of the interaction of EcChiP with dissolved chitosugars and non-chitin homologs
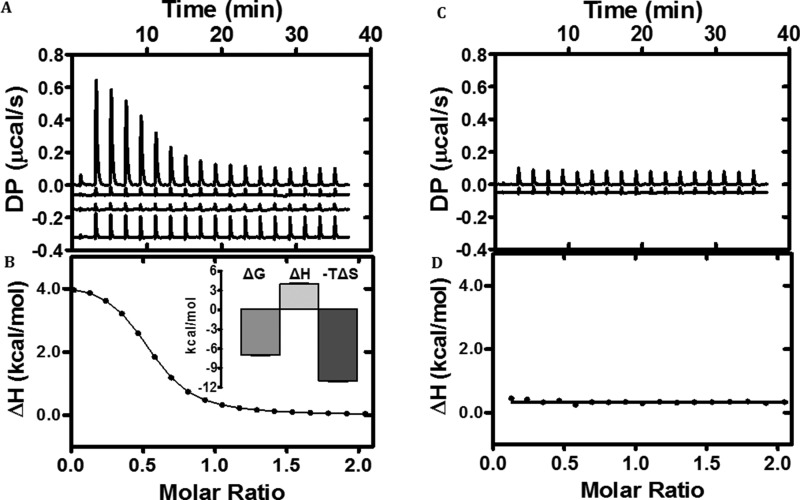
We employed ITC to assess the binding affinity and the thermodynamic characteristics of sugar-channel interactions. In these experiments, purified EcChiP in solution was titrated with chitohexaose, generating the thermogram shown in Fig. 3A (top trace). Other traces are the control thermograms derived from titrating sugar into buffer (second trace from top), buffer into buffer (third trace from top), and buffer into EcChiP (bottom trace). These control traces were used for background subtraction to obtain the normalized integrated heat change of the sugar-channel complex shown in Fig. 3B. Fitting the integrated heat to the ligand–protein ratio using a one-site binding model yielded an ideal hyperbolic curve, with an average binding constant (K) of 2.5 × 105 m−1, a Gibb's free energy change for binding (ΔGbinding) of −7.0 ± 0.07 kcal mol−1, an enthalpy change (ΔH) of +4.0 ± 0.11 kcal mol−1, and an entropy change (−TΔS) of −11.0 ± 0.15 kcal mol−1. In contrast, zero integrated heat change was obtained when the channel was titrated with chitosan hexamer or maltohexaose, showing that the channel did not interact with these structurally related sugars (Fig. 3, C and D).
Figure 3.
Calorimetric titration of EcChiP with chitohexaose and maltohexaose. A, ITC profile of the binding of chitohexaose to EcChiP, with three different controls (control traces are shown by offsetting −0.06, −0.15, and −0.32 μcal·s−1 for ligand to buffer, buffer to buffer, and buffer to EcChiP, respectively). B, integrated heat of binding of chitohexaose obtained from raw data, after subtracting the controls. The solid line represents the best fit to the experimental data using a one-site model from Microcal PEAQ-ITC. The inset is the signature plot for chitohexaose binding to EcChiP with error bars for three independent experiments. C, ITC profile of the binding of maltohexaose to EcChiP, with control trace maltohexaose to buffer offset by −0.05 μcal·s−1. D, integrated heat of binding of maltohexaose obtained from raw data, indicating no detectable binding.
Single molecule electrophysiology of the interaction of EcChiP with dissolved chitosugars and non-chitin homologs
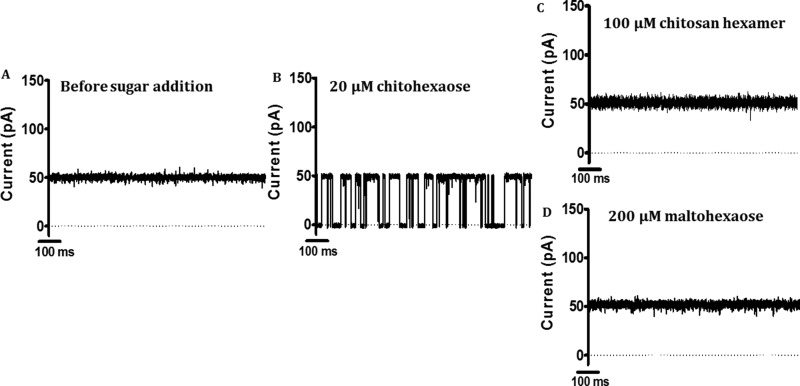
Sugar-channel affinity was further confirmed by single molecule electrophysiology. Explicitly, ion conductance, sugar specificity, and the kinetics of EcChiP binding to chitin substrates were determined using the black lipid membrane (BLM) reconstitution technique and time-resolved current measurements. In our trials, we found that single molecules of EcChiP could readily insert into phospholipid bilayers when stimulated with high potentials, i.e. ±150 to ±199 mV. Once inserted, a channel remained fully open for the entire period of data acquisition and did not exhibit gating under a wide range of applied potentials, although gating did occasionally occur when ±199 mV was applied. The average conductance of EcChiP in 1 KCl electrolyte was found to be about 0.5 nS (Fig. 4A); this value was consistent throughout this study. To test sugar specificity, chitohexaose was added to either the cis or the trans side of the chamber, and the kinetics of channel blockade by the sugar molecules were examined. The trace in Fig. 4B shows the transient drops in ion current, I(t), in a single EcChiP channel, caused by channel occlusion with chitohexaose. Clearly, the addition of chitohexaose at the low concentration of 20 μm triggered frequent, complete blockages of ion current, whereas the I(t) trace without sugar showed no change in ion current (Fig. 4A). In contrast, two sugar analogs of equivalent chain length, chitosan hexamer (Fig. 4C) and maltohexaose (Fig. 4D), did not block the ion flow at all, even at concentrations up to 200 μm. This result suggested that the EcChiP was highly selective for chitohexaose.
Figure 4.
Channel activity of purified EcChiP in an artificial lipid bilayer. Lipid bilayers were formed across a 70-μm aperture by the lowering and raising technique, using 5 mg ml−1 1,2-diphytanoyl-sn-glycero-3-phosphatidylcholine in n-pentane and 1 m KCl in 20 mm HEPES, pH 7.4, on both sides of the chamber. The protein was added on the cis side. Ion current fluctuations were monitored for 120 s at applied potentials of ±100 mV with sugars added on either the cis or the trans side. Here, only current traces for 1,000 ms at +100 mV, trans are presented. A, fully open state of EcChiP before sugar addition. B, 20 μm chitohexaose (GlcNAc6). C, 100 μm chitosan hexamer. D, recording with maltohexaose at a final concentration of 200 μm.
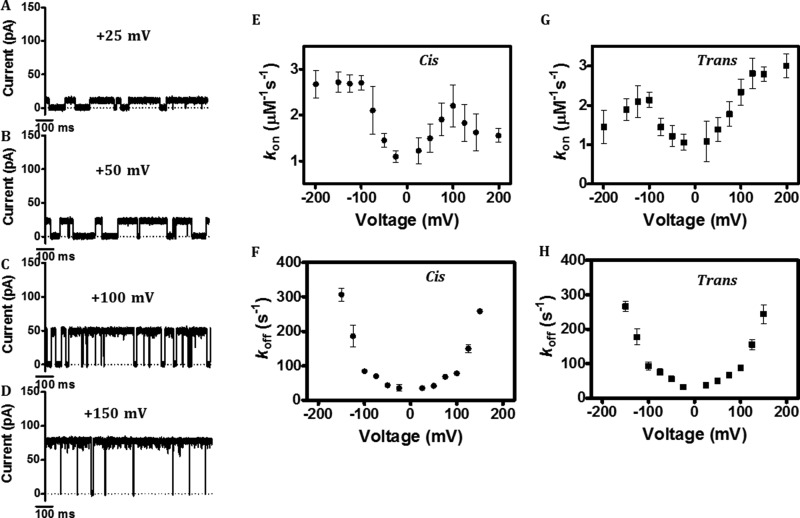
Next we examined the voltage dependence of EcChiP sugar uptake and release behavior through measurements of the ionic channel current in the presence of chitohexaose and at a set of different transmembrane potentials. The on (kon) and off rate (koff) values were determined at applied voltages of ±25 to ±200 mV. Fig. 5 (A–D) shows the I(t) traces at +25, +50, +100, and +150 mV, respectively. The ionic current, as well as the number of sugar blocking events, increased with increasing applied potential, whereas the residence time (τc), which reflects the duration of sugar binding inside the channel, decreased with increasing voltage. Plots of the on-rate constant (kon) for the sugar addition on the cis side versus applied voltage (Fig. 5E) showed that kon increased with increasing applied voltage up to ±100 mV and then remained unchanged at negative voltages of −125 to −200 mV, whereas the value dropped at high positive voltages. Similar results were observed with trans sugar addition (Fig. 5G), but the behavior of the on-rate fluctuations was the converse of those with cis addition. In the case of off-rate (koff) analysis (Fig. 5F for cis addition and Fig. 5H for trans addition), plots of koff versus applied voltage showed a steady increase in the value of koff as the applied potential was raised discretely from ±25 to ±100 mV, and then the value was sharply elevated at >±100 mV. The same results were obtained with sugar addition on both the cis and the trans sides.
Figure 5.
Typical ion current recordings through a single EcChiP channel at different voltages. Ion current fluctuations were monitored for 120 s at applied potentials of ±100 mV with sugars on either the cis or the trans side in 1 m KCl in 20 mm HEPES, pH 7.4. Here, only current traces for 1,000 ms at cis are presented. A–D, ion current fluctuations in a single EcChiP channel in the presence of 5 μm chitohexaose at +25, +50, +100, and +150 mV, respectively. E, association rates on cis with potentials ranging from ±25 to ±199 mV. F, dissociation rates on cis with potentials ranging from ±25 to ±199 mV. G, association rates on trans with potentials ranging from ±25 to ±199 mV in the presence of 5 μm chitohexaose. H, dissociation rates on trans with potentials ranging from ±25 to ±199 mV in the presence of 5 μm chitohexaose.
Kinetic assessment of chitosugar interaction with EcChiP in black lipid membranes
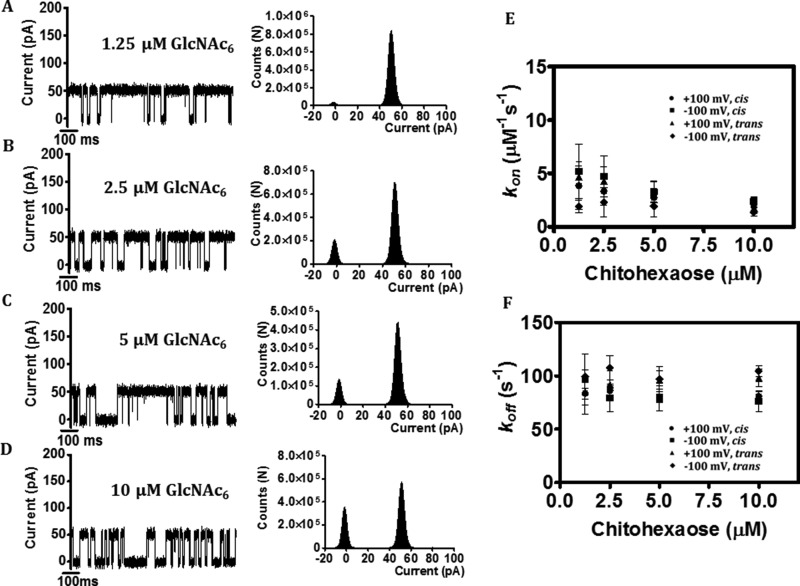
To obtain further information on the kinetics of sugar-channel interactions, we titrated the membrane-inserted EcChiP channel with 2-fold increments in the concentration of chitohexaose (GlcNAc6). Fig. 6 (A–D, left panel) shows I(t) recordings and shows frequent current fluctuations with the cumulative addition of chitohexaose on the trans side and an applied potential of +100 mV. Histogram analyses indicate progressive decreases in the ratio of the open-to-closed states of the channel, correlated with increases in the sugar concentration from 1.25 to 10 μm (Fig. 6, A–D, right panels). Subsequent sugar titration experiments allowed evaluation of the kinetic parameters for the sugar-channel interactions. The kon value, representing the number of sugar blocking events per unit time, is given by Equation 2, whereas the koff value, representing the reciprocal of the residence time (1/τc), is given by Equation 3. Fig. 6E shows the nearly constant kon values calculated from the increasing number of blocking events per unit time with increasing concentrations of chitohexaose. At higher concentrations of chitohexaose, however, the calculated value of kon was found to decrease. This may occur because of the resolution limit of detection of our BLM setup, very frequent entry of sugar molecules into one side of the channel leading to frequent, unresolved blocking events and, as a result, the underestimation of the kon values. On the other hand, the calculated values of koff were essentially constant over the entire range of chitohexaose concentration (Fig. 6F).
Figure 6.
Reduction of single EcChiP channel conductance by increasing concentrations of chitohaxaose. Ion current fluctuations were monitored for 120 s at applied potentials of ±100 mV with sugar addition on either the cis or the trans side. Here only current traces for 1,000 ms are presented (A–D, left panels) for four different chitohexaose concentrations at trans/+100 mV, with the corresponding histograms (A–D, right panels). E, dependence of association rates (kon) on chitohexaose concentration. F, dependence of dissociation rates (koff) on chitohexaose concentration.
The respective values of kon, koff, and K for the three oligosaccharides tested are summarized in Table 1. Both on- and off-rate constants were found to be dependent on the length of the chitooligosaccharide, under all the conditions investigated. With sugar addition on cis side and an applied potential of +100 mV (hereafter referred as cis/+100 mV), the average time for sugar molecules residing inside the EcChiP channel (defined as the residence time, τc) increased from 0.4 ± 0.2 ms for chitotetraose to 3.5 ± 0.9 ms for chitopentaose and 12.0 ± 0.7 ms for chitohexaose, yielding a 10-fold decrease in the off-rate constant (koff, 1/τc) from chitotetraose (2,550 s−1) to chitopentaose (250 s−1) and a 32-fold decrease to chitohexaose (80 s−1), respectively. The on-rate constant (kon) for the same series of sugars increased respectively from (1.0 ± 0.3) × 106 to (2 ± 1.5) × 106 and (4 ± 2) × 106 m−1 s−1. Hence, the correlated binding constant (K, m−1) increased with increasing length of sugar chain from chitotetraose (400 m−1) to chitopentaose (8,000 m−1) and chitohexaose (50,000 m−1), reflecting an increased affinity in sugar-channel interactions. Comparable results were observed, with reduced effects on conductance, when BLM data were acquired under trans/−100 mV conditions.
Table 1.
Substrate specificity of EcChiP
The on rate (kon) is given by (number of blocking events per second)/[sugar concentration]. In each case sugar concentration = 1.25 μm. The off rate (koff) was obtained from the relationship koff = 1/τc, where τc is the average residence (dwell) time (s) of the sugar molecule in the channel. The equilibrium binding constant is given by the relationship K = kon/koff. N.D. indicates that there were no well-resolved blocking events, so that the residence time could not be evaluated with confidence.
| Substrate |
cis side addition |
trans side addition |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +100 mV |
−100 mV |
+100 mV |
−100 mV |
|||||||||
| kon | koff | K | kon | koff | K | kon | koff | K | kon | koff | K | |
| 106m−1 s−1 | s−1 | m−1 | 106m−1 s−1 | s−1 | m−1 | 106m−1 s−1 | s−1 | m−1 | 106m−1 s−1 | s−1 | m−1 | |
| GlcNAc2 | N.D. | N.D. | N.D. | N.D. | ||||||||
| GlcNAc3 | N.D. | N.D. | N.D. | N.D. | ||||||||
| GlcNAc4 | 1 | 2,550 ± 320 | 390 ± 10 | 1.7 | 2,350 ± 255 | 700 ± 70 | 3 | 1,608 ± 25 | 1,900 ± 350 | 1 | 2,642 ± 136 | 380 ± 50 |
| GlcNAc5 | 2 | 250 ± 19 | 8,000 ± 3,550 | 4 | 280 ± 12 | 14,300 ± 8,300 | 3 | 255 ± 3 | 11,000 ± 1,880 | 2 | 385 ± 6 | 5,200 ± 570 |
| GlcNAc6 | 4 | 80 ± 1 | 50,000 ± 28,700 | 5 | 78 ± 1 | 64,000 ± 39,200 | 5 | 93 ± 2 | 54,000 ± 5,100 | 2 | 98 ± 1 | 20,400 ± 200 |
Discussion
The discovery of chitin-uptake channels in non-chitinolytic bacteria suggests that these bacteria have an adaptive strategy to cope with environmental changes to thrive and survive under high-stress conditions, such as a sudden shortage of glucose nutrients. E. coli are non-chitinolytic bacteria that maintain a silent chip gene in their chromosome, to allow them to utilize chitin as an emergency source of energy. We recently identified the chip gene, encoding a chitin-uptake channel (so-called EcChiP), in the genome of E. coli. This gene is usually unexpressed when the bacteria are grown in glucose-enriched medium. However, it has been reported that expression of the chip gene could be induced when the bacteria were grown on chitin oligosaccharides (GlcNAc2 or GlcNAc3) (7, 10) and the chitoporin inactive mutant of E. coli failed to grow in minimal medium containing GlcNAc3 (23). These results indicated that the chitin-utilization pathway of E. coli was positively regulated by the availability of chitin nutrients. In a previous study, we cloned and expressed EcChiP in the Omp-deficient E. coli host (12). The recombinant EcChiP was shown to be a 50-kDa protein that formed a monomeric channel in planar lipid membranes. Liposome swelling assays showed the bulk permeation of chitosugars, the greatest permeation rate being observed with chitohexaose.
Detailed thermodynamic and kinetic analysis of EcChiP was used to elucidate the mechanistic features of EcChiP-mediated passage of chitin oligosaccharides through the bacterial outer membrane. We first investigated sugar-channel interactions using protein fluorescence spectroscopy. Inspection of the amino acid sequence of EcChiP showed 15 Trp residues, seven of which, Trp-46, Trp-168, Trp-176, Trp-266, Trp-288, Trp-342, and Trp-357, are distributed throughout the outer wall of the protein barrel and presumed to interact with lipid membranes at the lipid-protein interface. Other Trp residues are part of the extracellular loops, which could also protrude into the protein channel. Among these, Trp-22, Trp-98, Trp-138, Trp-316, Trp-317, and Trp-417 reside on the wall of the channel interior (Fig. 2B) and are likely to play crucial roles in sugar binding and translocation. Trp residues are innate fluorophores that are typically excited at a wavelength (λEX) of ∼295 nm, with an emission wavelength (λEM) of ∼340 nm (24). From our observations, we expected that the Trp residues located in the inner wall of the EcChiP pore would be highly susceptible to sugar titration and as such would function as specific reporter groups for ligand binding. In our study, titrating EcChiP with chitohexaose caused enhancement of the intrinsic fluorescence in a concentration-dependent manner, reflecting changes in the hydrophobicity of the environment. Fluorescence enhancement usually indicates that the Trp residues are covered, partially or fully, through their binding to a sugar molecule, and as a result are screened from quenching by the hydrophilic environment (25, 26). We also observed that the emission wavelength of maximum intensity (Fmax) in the emission spectra was always 345 nm and remained unchanged in the presence of chitohexaose. This result suggested that no overall protein conformation change was induced by sugar binding. Non-linear curve fitting of the sugar titration curve yielded the binding constant (K) of 2.9 × 105 m−1, a value that agreed well with the values obtained from ITC experiments and from single channel recordings. Fluorescence enhancement derived from sugar-Trp interactions had been observed for other carbohydrate-binding proteins. For instance, when a sheep secretory glycoprotein (SPS-40) was titrated with chitosugars, Trp fluorescence intensity was enhanced in a concentration-dependent manner, yielding the best binding constant value for chitohexaose (25). Likewise, fluorescence study was used to demonstrate a major contribution of Trp residues of GH-18 chitinases and a chitinase-like protein in chitooligosaccharide binding (27–29). In another study, the same approach was also used to probe sugar specificity of E. coli lactose transporter (30).
We employed ITC to further demonstrate the tight binding of chitooligosaccharide to EcChiP. The data obtained from this study allowed us to assess the thermodynamic characteristics of sugar-channel interactions. Titration of EcChiP with chitohexaose generated the endothermic binding isotherm, and a theoretical fit of the integrated heat change yielded an average binding constant (K) of 2.5 × 105 m−1. Although the formation of a chitohexaose–EcChiP complex was an endothermic reaction, the signature plot (Fig. 3B, inset) gave an overall negative ΔG value, which was derived from the sum of a small positive ΔH and a large negative value of −TΔS, suggesting that the binding process occurred spontaneously and was entropy-driven. The entropy gain in the binding process suggested that hydrophobic interactions play a predominant role in chitohexaose-EcChiP binding (31, 32). This assumption was supported by the Trp fluorescence binding assay, in that the hyperbolic binding isotherm was essentially derived from hydrophobic interactions between the indole side chains of Trp residues and the occupying sugar molecule.
The highly specific chitosugar interaction with EcChiP was also marked in all of our electrophysiology BLM experiments. In the course of high time-resolution recordings of ion flow (current) through membrane-reconstituted EcChiP, the presence of chitosugar in one of the two BLM chambers reproducibly induced bursts of signal blockage, the duration of the transient current depressions being dependent on the length of the chitooligosaccharide. In control trials with chitosan hexaose, the deacetylation product of chitohexaose, and with maltohexaose, the preferred substrate of maltoporin, the channel currents were not disturbed, because these oligosaccharides did not enter the channel. Obviously only oligosaccharide molecules with N-acetyl functionalities at carbon C2 of the sugar ring arrangement could enter the EcChiP channel, and admission was effectively prohibited for structurally different ones. This distinct chitosugar affinity of EcChiP is comparable with the adaptation of VhChiP, from the marine microorganism V. harveyi, for efficient chitooligosaccharide uptake (13, 21). Apparently, the evolutionary distribution of the gene for ChiP expression from chitinolytic microbes surviving in harsh marine habitats to bacteria active the mammalian body occurred with retention of the substrate specificity. The demonstration of the generic affinity of chitoporins for the molecular building blocks of chitin is a central conclusion of this study with EcChiP.
Usually, the major path for nutritional internalization across the E. coli outer membrane is primarily mediated by maltodextrin-specific porin (so-called maltoporin or LamB) (34, 35). To reflect the physiological competency of EcChiP, which serves as a standby route for nutrient uptake by the bacteria, the kinetic constants for EcChiP against chitohexaose is compared with the values for maltoporin against maltohexaose (14, 16, 17). The on and off rates obtained from single channel analysis were 5 × 106 m−1 s−1 and 78 s−1 for EcChiP binding to chitohexaose and 17.7 × 106 m−1 s−1 and 1,000 s−1 for maltoporin binding to maltohexaose (36). The resultant binding constant (K) estimated for the EcChiP/chitohexaose was 64,000 m−1, which is 3-fold greater than the value for maltoporin/maltohexaose (20,000 m−1). Crystal structures revealed that the tubular lumen of maltoporin has a highly asymmetrical feature. One side of the channel interior is well-aligned by a few polar residues that form a “polar track.” On the other hand, the opposite side comprises six aromatic residues that form a “greasy slide” (33). Both the polar tract and the greasy slide were proved to play a key role in facilitating sugar transport through maltoporin (34, 35). EcChiP and maltoporin have a particularly low sequence identity (<10%), and the structural model of EcChiP did not suggest a clear polar track and a greasy slide as seen in maltoporin. Therefore, the sugar passage of EcChiP is presumably arranged by a dissimilar setting. The BLM data that showed a complete loss of the binding affinity when the EcChiP channel was exposed to the sugar analogs: chitosan oligosaccharide (Fig. 4B) and maltohexaose (Fig. 4C) clearly indicated that spatial arrangements of the pore-lining amino acids surrounding the N-acetamido groups of the transporting chitoologosaccharide chain would rather play a crucial role in the substrate specificity of the EcChiP channel. Nevertheless, the structural details will further provide an insight into the molecular basis of the chitin–EcChiP interactions.
Inspection of the voltage dependence of EcChiP channel blocking by chitohexaose revealed increases in both channel association and dissociation rates with increased transmembrane potentials. As suggested in a recent BLM study of the blockage of E. coli OMPF by norfloxacin, in which comparable plots of kon and koff against Vm were reported (37), the dipolar character of chitohexaose and related electrical field effects on molecule arrangement and electrostatic attraction may be the main reason for the effects of the cis/trans electrode polarity. Furthermore, the cis and trans plots of kon and koff against Vm appeared as approximately mirror images of each other, suggesting channel symmetry with respect to chitosugar uptake and release by EcChiP. For mechanistic understanding of bacterial nutrition, it is important to distinguish between the transmembrane translocation of trapped chitosugar through EcChiP and its release back to the side of entry. For charged porin substrates and porins with noticeable asymmetry of channel activity, the forward and backward movements of trapped molecules have recently been predicted from molecular dynamic modeling and analysis of the voltage dependence of the association and dissociate rate constants (38–40). Theoretical and experimental investigation of the interaction of EcChiP with and translocation of chitosugars is ongoing and will be the subject of future reports.
Concluding remarks
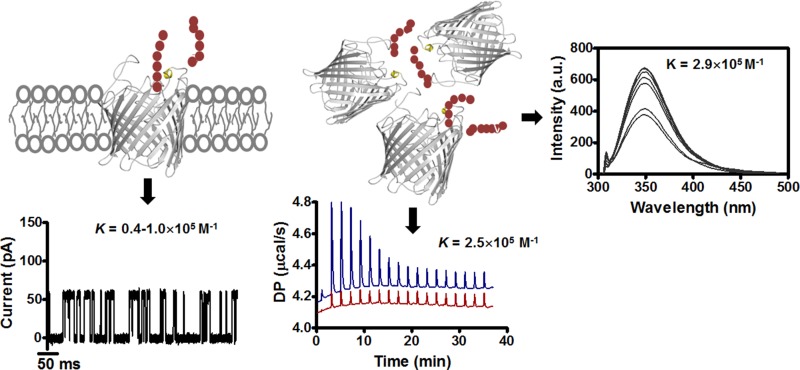
In this report, we employed biochemical and electrophysiological approaches to demonstrate some important aspects of the molecular basis of chitin transport by the usually non-chitinolytic bacterium E. coli. Data from thermal and spectroscopic affinity analysis confirmed the occurrence of highly specific sugar-channel interactions in solution, with the extracted binding constants supporting the values obtained by single channel electrophysiology (Fig. 7). The ITC trials provided the first thermodynamic insights into the nature of substrate binding by the EcChiP channel, which apparently is an endothermic, entropy-driven process. In summary, the combination of kinetic and thermodynamic data of this study revealed important mechanistic details of chitin uptake by usually non-chitinolytic bacteria.
Figure 7.
Summary of the data obtained from ITC, fluorescence spectroscopy, and electrophysiology experiments, comparing binding constants of chitohexaose with EcChiP in solution and at the single molecule level.
Experimental procedures
Structural prediction and sequence analysis
The amino acid sequence of the EcChiP (UniProtKB entry P75733) was submitted to Swiss-Model (http://swissmodel.expasy.org/)4 for tertiary structure prediction using the 3D structure of P. aeruginosa OprD (Protein Data Bank code 2odj) as a structural template (11). The annotated structures were edited and displayed in PyMOL, and the secondary structure of the E. coli ChiP was constructed by ESPript 3.0 (41).
Purification of EcChiP for ITC, fluorescence titrations, and electrophysiology
EcChiP was expressed and purified as described previously (12). As a final purification step after ion exchange chromatography, EcChiP was subjected to size-exclusion chromatography using a HiPrep 16/60 Sephacryl S-200 high-resolution column, run in 20 mm phosphate buffer, pH 7.4, in the presence of 0.05% N,N-dimethyldodecylamine N-oxide (LDAO). Pooled high purity EcChiP was dialyzed overnight in 20 mm phosphate buffer, pH 7.4, in the presence of 0.05% LDAO before use in ITC experiments. The protein concentration was determined from A280 using an extinction coefficient for EcChiP of 131,670 m−1 cm−1, derived from its amino acid content (Expasy protparam tool).
Isothermal titration calorimetry
ITC measurements were performed using a Microcal PEAQ-ITC (Malvern Instruments Limited, Malvern, Worcestershire, UK) thermostat at 10 °C, with EcChiP in 20 mm phosphate buffer, pH 7.4, in the presence of 0.05% LDAO. EcChiP (300 μl) was in the sample cell at a concentration of 100 μm, with 40 μl of 1 mm ligand (prepared in dialysis buffer) in the syringe. To ensure proper mixing after each injection, a constant stirring speed of 600 rpm was maintained during the experiments. Control experiments were performed by injecting ligand solution into the buffer, buffer into the protein solution, and buffer into the buffer in an identical manner; the resulting heat changes were subtracted from the measured heat of binding using the manufacturer's Microcal PEAQ-ITC analysis software, and binding thermodynamics were analyzed with a “one set of sites” fitting model. The experiments were performed in triplicate, and the results shown are averages.
Binding studies using fluorescence measurements
Changes in intrinsic tryptophan fluorescence intensity on chitooligosaccharide binding were monitored in a LS-50 fluorescence spectrometer (PerkinElmer Life Sciences). The excitation wavelength was set to 295 nm, and emission spectra were collected over the range 300–500 nm, with excitation and emission slit widths of 5 and 10 nm, respectively. Purified EcChiP (final concentration 720 ng/ml in 20 mm phosphate buffer, pH 7.4, and 0.05% LDAO) was titrated with chitohexaose (2–40 μm) at 25 °C. In all cases, spectra of buffer blanks were subtracted from experimental spectra. Experiments were performed in triplicate, and the results given are averages. Binding curves were evaluated with a non-linear regression function available in Prism version 5.0 (GraphPad Software) using a model based on a single binding site. To estimate the dissociation constant, normalized relative fluorescence ΔF = (F − F0) was plotted as a function of sugar concentration, yielding a rectangular hyperbolic binding curve, which allowed the calculation of the dissociation constant for the chitooligosaccharides using a single-site binding model, according to Equation 1 (25, 27, 29).
| (Eq. 1) |
Here F and F0 refer to the fluorescence intensity in the presence and absence of ligand, respectively, Fb is the maximum fluorescence signal of the EcChiP-ligand complex at saturation, L0 is the initial ligand concentration, and Kd is the equilibrium dissociation constant.
Electrophysiological studies of chitooligosaccharide permeation through EcChiP
BLM reconstitution was carried out in electrolyte containing 1 m KCl in 20 mm HEPES, pH 7.4, at room temperature (23 °C). Solvent-free bilayer (Montal–Mueller type) formation was performed using 1,2-diphytanoyl-sn-glycero-3-phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL), 5 mg/ml in n-pentane. First, a 25-μm-thick Teflon film with an aperture of 40–70 μm was sandwiched between the two chambers of a Teflon cuvette, and the aperture was prepainted with a few microliters of 1% (v/v) hexadecane in hexane; then a planar bilayer was formed across the aperture by lowering and raising the liquid level (42). Ionic currents were detected using Ag/AgCl electrodes (World Precision Instruments, Sarasota, FL), one connected to the cis side of the membrane (ground) and the other connected to the head-stage of the Axopatch 200B amplifier (Axon Instruments, Molecular Devices, Sunnywale, CA). Single channel measurements were performed with an Axopatch 200B amplifier (Axon Instruments, Molecular Devices) in the voltage clamp mode and digitized using the Axon Digidata 1440 digitizer, whereas data acquisition was performed using Clampex software (Axon Instruments). The traces obtained were filtered at 10 kHz, using a low-pass Bessel filter with a sampling frequency of 50 kHz. Single channel analyses were performed using Clampfit software (all from Molecular Devices). Single protein channels were reconstituted in lipid bilayers, with EcChiP always being added to the cis side of the cuvette.
The equilibrium binding constant for chitooligosaccharides K (m−1) was calculated by single channel analysis using clampfit v10 (38, 43). The rate of association (kon, m−1 s−1) is given by Equation 2.
| (Eq. 2) |
where N is number of blocking events per second and [c] is the molar concentration of chitosugar added during titration.
The off rate (koff, s−1) was obtained from Equation 3, where τc is the average residence (dwell) time (s) of the sugar molecule in the channel, which can be calculated from the exponential fit of the dwell-time histogram.
| (Eq. 3) |
The equilibrium binding constant (K) is given as
| (Eq. 4) |
Author contributions
H. S. M. S. designed, performed, and analyzed all the experiments and co-wrote the paper. A. S. interpreted data and co-wrote the paper. W. S. was the grant holder; conceived, designed, and coordinated the study; and co-wrote and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We acknowledge the Biochemistry Laboratory of the Center for Scientific and Technological Equipment at Suranaree University of Technology for providing all research facilities. We greatly appreciate a critical proofreading of this manuscript by Dr. David Apps (Centre for Integrative Physiology, School of Biomedical Sciences, University of Edinburgh, Edinburgh, UK). We thank the group of Prof. Mathias Winterhalter at Jacobs University (Bremen, Germany) for generously providing access to high time resolution BLM equipment and supplies. We also thank Dr. Satya P. Bhamidimarri for providing us with an introduction to single channel analysis.
This work was supported by Suranaree University of Technology and the Office of the Higher Education Commission under the National Research University (NRU) project of Thailand. The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- ITC
- isothermal microcalorimetry
- BLM
- black lipid membrane.
References
- 1. Kim B. H., and Gadd G. M. (2008) Bacterial physiology and metabolism, Cambridge University Press, Cambridge [Google Scholar]
- 2. Francetic O., Belin D., Badaut C., and Pugsley A. P. (2000) Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 19, 6697–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Francetic O., Badaut C., Rimsky S., and Pugsley A. P. (2000) The ChiA (YheB) protein of Escherichia coli K-12 is an endochitinase whose gene is negatively controlled by the nucleoid-structuring protein H-NS. Mol. Microbiol. 35, 1506–1517 [DOI] [PubMed] [Google Scholar]
- 4. Peri K. G., Goldie H., and Waygood E. B. (1990) Cloning and characterization of the N-acetylglucosamine operon of Escherichia coli. Biochem. Cell Biol. 68, 123–137 [DOI] [PubMed] [Google Scholar]
- 5. Yang C., Rodionov D. A., Li X., Laikova O. N., Gelfand M. S., Zagnitko O. P., Romine M. F., Obraztsova A. Y., Nealson K. H., and Osterman A. L. (2006) Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J. Biol. Chem. 281, 29872–29885 [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen A. A., Johansen J., Nielsen J. S., Overgaard M., Kallipolitis B., and Valentin-Hansen P. (2009) A conserved small RNA promotes silencing of the outer membrane protein YbfM. Mol. Microbiol. 72, 566–577 [DOI] [PubMed] [Google Scholar]
- 7. Figueroa-Bossi N., Valentini M., Malleret L., Fiorini F., and Bossi L. (2009) Caught at its own game: regulatory small RNA inactivated by an inducible transcript mimicking its target. Genes Dev. 23, 2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valentin-Hansen P., Johansen J., and Rasmussen A. A. (2007) Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 10, 152–155 [DOI] [PubMed] [Google Scholar]
- 9. Vogel J., and Papenfort K. (2006) Small non-coding RNAs and the bacterial outer membrane. Curr. Opin. Microbiol. 9, 605–611 [DOI] [PubMed] [Google Scholar]
- 10. Plumbridge J., Bossi L., Oberto J., Wade J. T., and Figueroa-Bossi N. (2014) Interplay of transcriptional and small RNA-dependent control mechanisms regulates chitosugar uptake in Escherichia coli and Salmonella. Mol Microbiol 92, 648–658 [DOI] [PubMed] [Google Scholar]
- 11. Biswas S., Mohammad M. M., Patel D. R., Movileanu L., and van den Berg B. (2007) Structural insight into OprD substrate specificity. Nat. Struct. Mol. Biol. 14, 1108–1109 [DOI] [PubMed] [Google Scholar]
- 12. Soysa H. S., and Suginta W. (2016) Identification and functional characterization of a novel OprD-like chitin uptake channel in non-chitinolytic bacteria. J. Biol. Chem. 291, 13622–13633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suginta W., Chumjan W., Mahendran K. R., Janning P., Schulte A., and Winterhalter M. (2013) Molecular uptake of chitooligosaccharides through chitoporin from the marine bacterium Vibrio harveyi. PLoS One 8, e55126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Danelon C., Brando T., and Winterhalter M. (2003) Probing the orientation of reconstituted maltoporin channels at the single-protein level. J. Biol. Chem. 278, 35542–35551 [DOI] [PubMed] [Google Scholar]
- 15. Andersen C., Cseh R., Schülein K., and Benz R. (1998) Study of sugar binding to the sucrose-specific ScrY channel of enteric bacteria using current noise analysis. J. Membr. Biol. 164, 263–274 [DOI] [PubMed] [Google Scholar]
- 16. Andersen C., Jordy M., and Benz R. (1995) Evaluation of the rate constants of sugar transport through maltoporin (LamB) of Escherichia coli from the sugar-induced current noise. J. Gen. Physiol. 105, 385–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bezrukov S. M., Kullman L., and Winterhalter M. (2000) Probing sugar translocation through maltoporin at the single channel level. FEBS Lett. 476, 224–228 [DOI] [PubMed] [Google Scholar]
- 18. Suginta W., Winterhalter M., and Smith M. F. (2016) Correlated trapping of sugar molecules by the trimeric protein channel chitoporin. Biochim. Biophys. Acta 1858, 3032–3040 [DOI] [PubMed] [Google Scholar]
- 19. Forst D., Welte W., Wacker T., and Diederichs K. (1998) Structure of the sucrose-specific porin ScrY from Salmonella typhimurium and its complex with sucrose. Nat Struct Biol 5, 37–46 [DOI] [PubMed] [Google Scholar]
- 20. Takanao S., Honma S., Miura T., Ogawa C., Sugimoto H., Suzuki K., and Watanabe T. (2014) Construction and basic characterization of deletion mutants of the genes involved in chitin utilization by Serratia marcescens 2170. Biosci. Biotechnol. Biochem. 78, 524–532 [DOI] [PubMed] [Google Scholar]
- 21. Suginta W., Chumjan W., Mahendran K. R., Schulte A., and Winterhalter M. (2013) Chitoporin from Vibrio harveyi, a channel with exceptional sugar specificity. J. Biol. Chem. 288, 11038–11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keyhani N. O., Li X. B., and Roseman S. (2000) Chitin catabolism in the marine bacterium Vibrio furnissii: identification and molecular cloning of a chitoporin. J. Biol. Chem. 275, 33068–33076 [DOI] [PubMed] [Google Scholar]
- 23. Verma S. C., and Mahadevan S. (2012) The chbG gene of the chitobiose (chb) operon of Escherichia coli encodes a chitooligosaccharide deacetylase. J. Bacteriol. 194, 4959–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghisaidoobe A. B., and Chung S. J. (2014) Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on forster resonance energy transfer techniques. Int. J. Mol. Sci. 15, 22518–22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srivastava D. B., Ethayathulla A. S., Kumar J., Singh N., Sharma S., Das U., Srinivasan A., and Singh T. P. (2006) Crystal structure of a secretory signalling glycoprotein from sheep at 2.0 Å resolution. J. Struct. Biol. 156, 505–516 [DOI] [PubMed] [Google Scholar]
- 26. Eftink M. R. (1997) Fluorescence methods for studying equilibrium macromolecule-ligand interactions. Methods Enzymol. 278, 221–257 [DOI] [PubMed] [Google Scholar]
- 27. Songsiriritthigul C., Pantoom S., Aguda A. H., Robinson R. C., and Suginta W. (2008) Crystal structures of Vibrio harveyi chitinase A complexed with chitooligosaccharides: implications for the catalytic mechanism. J. Struct. Biol. 162, 491–499 [DOI] [PubMed] [Google Scholar]
- 28. Ranok A., Wongsantichon J., Robinson R. C., and Suginta W. (2015) Structural and thermodynamic insights into chitooligosaccharide binding to human cartilage chitinase 3-like protein 2 (CHI3L2 or YKL-39). J. Biol. Chem. 290, 2617–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schimpl M., Rush C. L., Betou M., Eggleston I. M., Recklies A. D., and van Aalten D. M. (2012) Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem. J. 446, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vázquez-Ibar J. L., Guan L., Svrakic M., and Kaback H. R. (2003) Exploiting luminescence spectroscopy to elucidate the interaction between sugar and a tryptophan residue in the lactose permease of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 100, 12706–12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abraham T., Lewis R. N., Hodges R. S., and McElhaney R. N. (2005) Isothermal titration calorimetry studies of the binding of the antimicrobial peptide gramicidin S to phospholipid bilayer membranes. Biochemistry 44, 11279–11285 [DOI] [PubMed] [Google Scholar]
- 32. Anbazhagan V., Sankhala R. S., Singh B. P., and Swamy M. J. (2011) Isothermal titration calorimetric studies on the interaction of the major bovine seminal plasma protein, PDC-109 with phospholipid membranes. PLoS One 6, e25993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dutzler R., Wang Y. F., Rizkallah P., Rosenbusch J. P., and Schirmer T. (1996) Crystal structures of various maltooligosaccharides bound to maltoporin reveal a specific sugar translocation pathway. Structure 4, 127–134 [DOI] [PubMed] [Google Scholar]
- 34. Schirmer T., Keller T. A., Wang Y. F., and Rosenbusch J. P. (1995) Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science 267, 512–514 [DOI] [PubMed] [Google Scholar]
- 35. Van Gelder P., Dumas F., Bartoldus I., Saint N., Prilipov A., Winterhalter M., Wang Y., Philippsen A., Rosenbusch J. P., and Schirmer T. (2002) Sugar transport through maltoporin of Escherichia coli: role of the greasy slide. J. Bacteriol. 184, 2994–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hilty C., and Winterhalter M. (2001) Facilitated substrate transport through membrane proteins. Phys. Rev. Lett. 86, 5624–5627 [DOI] [PubMed] [Google Scholar]
- 37. Bajaj H., Acosta Gutierrez S., Bodrenko I., Malloci G., Scorciapino M. A., Winterhalter M., and Ceccarelli M. (2017) Bacterial outer membrane porins as electrostatic nanosieves: exploring transport rules of small polar molecules. ACS Nano 11, 5465–5473 [DOI] [PubMed] [Google Scholar]
- 38. Bhamidimarri S. P., Prajapati J. D., van den Berg B., Winterhalter M., and Kleinekathöfer U. (2016) Role of electroosmosis in the permeation of neutral molecules: CymA and cyclodextrin as an example. Biophys. J. 110, 600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh P. R., Bárcena-Uribarri I., Modi N., Kleinekathöfer U., Benz R., Winterhalter M., and Mahendran K. R. (2012) Pulling peptides across nanochannels: resolving peptide binding and translocation through the hetero-oligomeric channel from Nocardia farcinica. ACS Nano 6, 10699–10707 [DOI] [PubMed] [Google Scholar]
- 40. Piguet F., Discala F., Breton M. F., Pelta J., Bacri L., and Oukhaled A. (2014) Electroosmosis through α-hemolysin that depends on alkali cation type. J. Phys. Chem. Lett. 5, 4362–4367 [DOI] [PubMed] [Google Scholar]
- 41. Robert X., and Gouet P. (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Montal M., and Mueller P. (1972) Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U.S.A. 69, 3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwarz G., Danelon C., and Winterhalter M. (2003) On translocation through a membrane channel via an internal binding site: kinetics and voltage dependence. Biophys. J. 84, 2990–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]