Abstract
Objective
Traumatic brain injury causes tissue damage, breakdown of cerebral blood flow and metabolic regulation. This study aims to investigate the protective influence of antioxidant Ganoderma lucidum (G. lucidum) polysaccharides (GLPs) on brain injury in brain-traumatized rats.
Methods
Sprague-Dawley conducted a head-traumatized method on rats by dropping off 300 g weight from 1 m height. Groups were categorized as control, G. lucidum, trauma, trauma+ G. lucidum (20 mL/kg per day via gastric gavage). Brain tissues were dissected from anesthetized rats 7 days after injury. For biochemical analysis, malondialdehyde, glutathione and myeloperoxidase values were measured.
Results
In histopathological examination, neuronal damage in brain cortex and changes in blood brain barrier were observed. In the analysis of immunohistochemical and western blot, p38 mitogen-activated protein kinase, vascular endothelial growth factor and cluster of differentiation 68 expression levels were shown. These analyzes demonstrated the beneficial effects of GLPs on brain injury.
Conclusion
We propose that GLPs treatment after brain injury could be an alternative treatment to decraseing inflammation and edema, preventing neuronal and glial cells degeneration if given in appropriate dosage and in particular time intervals.
Keywords: Brain, Ganoderma lucidum, Oxidative stress, Rat, Trauma
Introduction
The brain is especially vulnerable to oxidative stress injury because of its high consumption of oxygen, abundant polyunsaturated fatty acids, and low levels of endogenous antioxidants.23,27) Free radicals may attack protein and polyunsaturated phospholipids in membranes, including plasma membranes and cellular organelles, leading to the disruption of these organelles. Therefore, inducing anti-oxidative effects is considered to be a promising treatment for cerebral injury.9,26) Glutamate is a primary contributor of excitotoxicity, leading to ischemic neuronal death and other cellular components of the neurovascular unit. Glutathione (GSH) is the principal antioxidant in brain.5) Some researchers figure out pharmaceutical products of Ganoderma lucidum (G. lucidum) isolated from mushrooms show curative effects on a variety of diseases such as cancers, immunologic disorders, and neurodegenerative diseases.2,36,37) G. lucidum is a mushroom coming from polyporaceae family of Basidiomycota and has been utilized as medicine for thousands of years, especially in Asian countries.12) Various bioactive chemical substances such as polysaccharides, triterpenoids, and proteins are shown to be found in the fruiting bodies, cultured mycelia, and spores of G. lucidum by many researchers.25,37) Many experimental studies point that these chemicalsobtained from G. lucidum have anti-inflammatory, antioxidant, anti-tumour, and immunomodulatory activities.14,20,22,41) According to previous studies, G. lucidum polysaccharides (GLPs), extracts derived from G. lucidum, could have neuroprotective effects and induce the cell viability of cerebral cortical neurons exposed to ischaemia/reperfusion injury in a rat model.28,40) Takin all those evidences into consideration, GLPs might be taken as advanteage of therapeutic drug and could be a promising treatment candidate for traumatic brain injury (TBI). Vascular endothelial growth factors (VEGFs) were involved in the pathophysiological responses of damaged nerve tissues.29,38) Expressions of brain VEGF ligands are increased after head trauma.30) Macrophages and microglia do not exist as immutable subsets; rather, they are sensitive to their host tissue microenvironments, with their phenotypes determined in part by their surroundings and the length of time after injury.15,18,32) The p38 mitogen-activated protein kinase (MAPK) is an important member of MAPK family. A variety of harmful factors following cerebral injury such as glutamate and inflammatory factors can activate p38 MAPK, which plays a negative damaging effect in brain injury. Regulation of MAPK family signaling has become a convergence of various signaling pathways in nervous system diseases.21,33) In this study, anti-oxidative and anti-inflammatory effects of GLPs against the secondary neuronal damage resulted from the diffuse TBI were assessed by analysis of several biochemical markers, histological, immunohistochemical (IHC) and western blotting methods.
Materials and Methods
Materials
The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by U.S. National Institutes of Health (NIH; NIH Publication no. 85-23, revised 1996). All experimental protocols were approved by the Dicle University Animal Care and Use Committee. Male Sprague-Dawley rats (300–350 g) were housed in an air-conditioned room with 12 hour light and dark cycles, where the temperature (23±2℃) and relative humidity (65–70%) kept constant. The animals were intraperitoneally anesthetized with 5 mg/kg xylazine hydrochloride (HCl; Rompun, Bayer HealthCare AG, Leverkusen, Germany) and 40 mg/kg ketamine HCl (Ketalar; Pfizer Inc., New York, NY, USA), and were allowed to breathe spontaneously. A rectal probe was inserted, and the animals were positioned on a heating pad that maintained the body temperature at 37℃. Diffuse brain injury (DBI) model described by Marmarou et al.24) was used. Briefly, a trauma device which works by dropping a constant weight from a specific height through a tube was used. A weight of 300 g was dropped from a 1 m height, which can induce mild trauma, as shown by Ucar et al.34) The G. lucidum fungus mixture (water-soluble) was provided by Shandong Si Wei Co., Ltd. (Heze, Shandong Province, China) (license No. Z200220083). The preparation of G. lucidum fungus mixture was prepared by inoculating a pure culture of G. lucidum mycelia into a solid culture medium (composed of bagasse and defatted rice bran) and cultured until just before the formation of the fruit body (for 3–4 months). The air-dried G. lucidum fruit bodies were extracted with hot water and sterilized by filtration, as described previously.6,42) G. lucidum was administrated to rats at 20 mL/kg per day viagastric gavage (the polysaccharides is 2 mg/mL).11) The 7-day treated-rats groups were administrated for their respective treatment exposure after the trauma. Rats in both the trauma and control groups were administrated water at 20 mL/kg for 7 days. Fresh drug solutions were prepared on each day of experimentation and administered within 60 minutes after preparation. Rats were randomly assigned to four groups, with sixteen rats per group. Thirty minutes after the trauma, rats were administered either saline or GLPs (20 mL/kg/day) viagastric gavage. All of the animals were sacrificed 7 days after trauma. The animals were anesthetized by an intraperitoneal injection of 5 mg/kg xylazine HCl (Rompun) and 40 mg/kg ketamine HCl (Ketalar), and were allowed to breathe spontaneously.
Malondialdehyde (MDA) and GSH assays
Tissue samples were homogenized with ice-cold 150 mM potassium chloride (KCl) for the determination of MDA and GSH levels. The MDA levels were assayed for products of lipid peroxidation, and the results were expressed as nmoL MDA/g tissue.8) GSH was determined by the spectrophotometric method, which was based on the use of Ellman's reagent, and the results were expressed as µmoL GSH/g tissue.8)
Tissue myeloperoxidase (MPO) activity
MPO in tissues was measured by a procedure similar to that described by Hillegass et al.10) MPO activity was expressed as U/g tissue.
Evans blue (EB) assay for blood-brain barrier (BBB) permeability
To evaluate the BBB integrity, EB dye was used as a marker of albumin extravasation.8) EB was expressed as µg/mg of brain tissue against a standard curve.
Brain water content
Brain edema was evaluated by the drying-weighing method based on the measurement of the water content of the brain.8) The percentage of water was calculated according to the following formula: %H2O=([wet weight-dry weight]/wet weight)×100.
Histological preparation and analysis
At the end of the experiment all animals were anesthetized via the intraperitoneal administration of ketamine HCl (0.15 mL/100 g body weight). Animals were anesthetized with an intraperitoneal injection of 5 mg/kg xylazine HCl (Rompun) and 40 mg/kg ketamine HCl (Ketalar) and allowed to breathe spontaneously. Surgical procedures were performed while animals were asleep. The brains were dissected and the frontal cortex was processed. For the histological examination, brain tissues were fixed in 10% formaldehyde solution, postfixed in 70% alcohol, and embedded in paraffin wax. The 6 µm-sections were stained with hematoxylin-eosin to visualize the general cytoarchitecture of the cerebral cortex. Microscopic scoring was done by experienced histologists who were unaware of which treatment the animal had received. Two criteria were evaluated: Measurements were made under microscope from 20 different points randomly chosen in the traumatized area. 1) degeneration of neurons (0=none, 1=1–3 cells, 2=4–6 cells, and 3=7–10 cells/area); and 2) pericellular and vascular edema and inflammatory cell infiltration (0=none, 1=slight, 2=moderate, and 3=severe).
IHC technique
Formaldehyde-fixed tissueswere embedded in paraffin wax for further IHC examination. Sections were deparaffinized in absolute alcohol. Antigen retrieval process was performed twice in citrate buffer solution (pH:6.0), first for 7 minutes, and second for 5 minutes, 90℃×3 minutes in the microwave waited. boiled in a microwave oven at 700 W. They were allowed to cool at room temperature for 30 minutes and washed twice in distilled water for 5 minutes. Endogenous peroxidase activity was blocked in 0.1% hydrogen peroxide for 20 minutes. Ultra V block (Cat.No: 85-9043; Invitrogen, Carlsbad, CA, USA) was applied for 10 minutes prior to the application of primary antibodies VEGF antibody (dilution rate, 1/100), cluster of differentiation 68 (CD68) antibody (dilution rate, 1/100) and Phosphorylation p38 MAPK Antibody (dilution rate, 1/100) overnight. Secondary antibody (Cat.No: 85-9043; Invitrogen) was applied for 20 minutes. Slides were then exposed to streptavidin-peroxidase for 20 minutes. Chromogen diaminobenzidine (DAB; Invitrogen) was used. Control slides were prepared as mentioned above, but omitting the primary antibodies. After counterstaining with hematoxylin, and washing in tap water for 8 minutes and holding in distilled water for 10 minutes, the slides were mounted with entellan.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 4.0 software (Graphpad Software Inc., San Diego, CA, USA). All data were presented as mean±standard deviation (SD). Groups of data were compared with an analysis of variance followed by Tukey's multiple comparison tests. Values of p<0.05 were considered as significant.
Results
Biochemical analysis
Tissue MDA levels
Comparing groups, MDA values in trauma group significantly higher than control group (p<0.001). This value significantly decreased in trauma + G. lucidum group than trauma group (p<0.01). No differences were statistically observed between G. lucidum and control group.
Tissue GSH levels
A significant decrease was observed in trauma group after TBI when compared with control and G. lucidum group (p<0.001). GSH levels significantly increased in group treated with G. lucidum after trauma (p<0.01). There was no statistical difference between G. lucidum and control group.
Tissue MPO activity
In analysis of tissue MPO activity, no significant differences were observed between G. lucidum and control groups. However, there was statistically significant differences; tissue MPO activity increased after TBI in trauma (p<0.01). Tissue MPO activity decreased in G. lucidum group after treatment, compared with trauma and trauma+ G. lucidum groups (p<0.05).
EB assay for BBB
Tissue EB content in trauma group was significantly higher than both in control and in G. lucidum groups (p<0.001). trauma + G. lucidum group had significantly lower value than trauma group (p<0.05). No differences were statistically observed between G. lucidum and control groups.
Brain water content
Brain water content, also an indicator of edema in brain, increased in trauma significantly than G. lucidum and control group (p<0.01). In trauma + G. lucidum group, brain water content was significantly lower than trauma group (p<0.05). There was no statistical difference between G. lucidum and control group in terms of brain water content. Results of biochemical analysis were shown in Table 1. There was no significant difference between the G. lucidum and the control groups (p=0.381, inflammation and edema; p=0.397, neuronal injury) (Table 1).
TABLE 1. Biochemical results relevant to the study groups.
The data is presented as mean±standard deviation (min, max). *p-values were calculated by one-way analysis of variance, †p-values were calculated by posthoc comparison using Tukey's method. MDA: malondialdehyde, GSH: glutathione, MPO: myeloperoxidase, BBB: blood-brain barrier, G: G. lucidum, T: Trauma
Histopathological evaluations
Light microscopic examinations of the brain tissue samples from the control and the G. lucidum groups revealed nothing remarkable. Histological alterations, such as inflammation and edema, neuronal injury, were found in the trauma group. When the pathological scores of the experimental groups were analyzed, the trauma group exhibited significantly higher scores compared with the controlgroup (p<0.001 for both). Rats in the trauma + G. lucidum showed significantly lower scores than rats in the trauma group (p<0.05 for both).
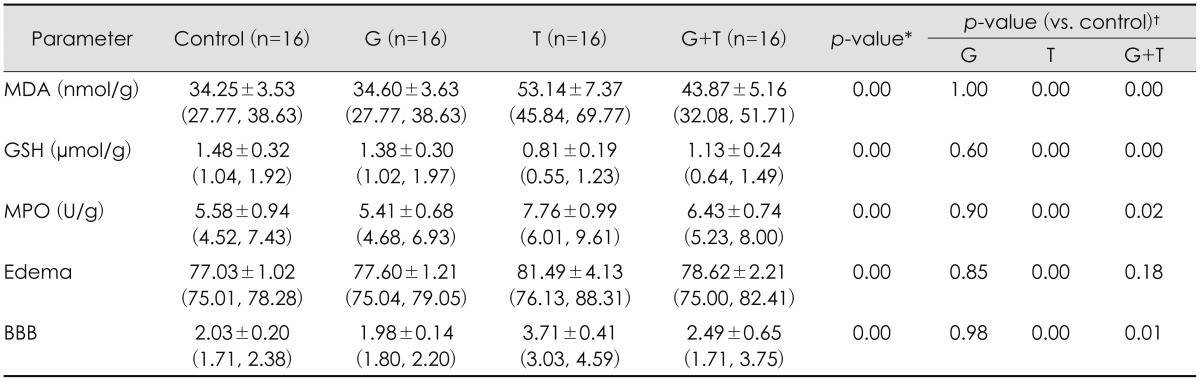
No structural changes were observed in neurons and glial cells of cerebral cortex in control group (Figure 1A). Hemorrhage and dilatation in blood vessels and edema near blood vessels were observed in trauma group. Slight degeneration in neuronal cells and glial cells and picnosis in their nuclei were seen. Hemorrhage and dilatation in blood vessels and edema in pericellular areas were observed (Figure 1B). Slight neuronal degeneration, blood dilatation, and decrease in hemorrhage, edema and inflammation were detected in trauma + G. lucidum group (Figure 1C). Although G. lucidum treatment doesn't fully heal injury, it is accepted that in group treated with G. lucidum decrease in neuronal injury and inflammation could be said to show protective effects of G. lucidum.
FIGURE 1. (A) Normal appearance of regular cells and vascular structures in brain cortex, hematoxylin and eosin (H & E) staining bar 50 µm (control group). (B) Dilation in blood vessels and hemorrhage (arrowhead), degeneration in some neuron (arrow) H & E staining bar 50 µm (trauma group). (C) Reduction in vascular dilatation, regular structure of the nucleus and cytoplasm in neurons and glial cells, H & E staining bar 50 µm (trauma + Ganoderma group). (D) Expression of phosphorylated p38 mitogen activated protein kinase (MAPK) in neuron nucleus (arrow), phosphorylated p38 immunohistochemistry (IHC) staining bar 50 µm (control group). (E) Positive expression of phosphorylated p38 MAPK in nucleus of neuron and glia cells (arrows), phosphorylated p38 IHC staining bar 50 µm (trauma group). (F) Increased p38 expression in neuronal membranes (yellow arrow) and nucleus of glial cells phosphorylated p38 (red arrow) IHC staining bar 50 µm (trauma+Ganoderma group).
IHC findings
In IHC sections of control group, phosphorylated p38 MAPK expression was mostly observed in neuronal nuclei (Figure 1D). In neuronal cells, micro glial cells and endothelial cells of trauma group, p38 expression was detected (Figure 1E). The p38 expression was observed in both neuronal and glial cell nuclei of G. lucidum + trauma group (Figure 1F).
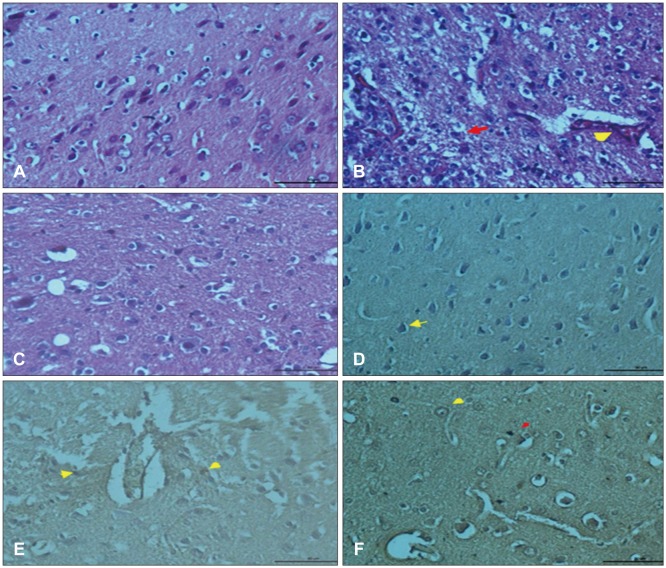
In control group, vascular endothelial cells in cortex showed VEGF expression (Figure 2A). in trauma group, vascular endothelial cells and inflammatory cells reflected positive VEGF expression (Figure 2B). In post-traumatic treatment group, VEGF expression increased in vascular endothelial cells (Figure 2C). CD68 expression was observed in glial cells around blood vessels (Figure 2D). however, in trauma group CD68 expression was weakly observed in glial cells (Figure 2E) while CD68 expression increased in glial cells of G. lucidum groups (Figure 2F).
FIGURE 2. (A) Vascular endothelial growth factor (VEGF) expression of vascular endothelial cells in the cortex (arrow) VEGF immunohistochemistry staining bar 50 µm (control group). (B) Positive VEGF expression in endothelial cells and inflammatory cells (arrows) VEGF immunohistochemistry staining bar 50 µm (trauma group). (C) Increased VEGF expression in endothelial cells (arrow), VEGF immunohistochemistry staining bar 50 µm (trauma+Ganoderma group). (D) Positive cluster of differentiation 68 (CD68) expression in glial cells surrounding of the blood vessels, CD68 immunohistochemistry staining bar 50 µm (control group). (E) Weak CD68 expression in glial cells (arrow) CD68 immunohistochemistry staining bar 50 µm (trauma group). (F) Positive CD68 expression in glia cells around blood vessels (CD68 immunohistochemistry staining bar 50 µm) (trauma+Ganoderma group).
Western blot results
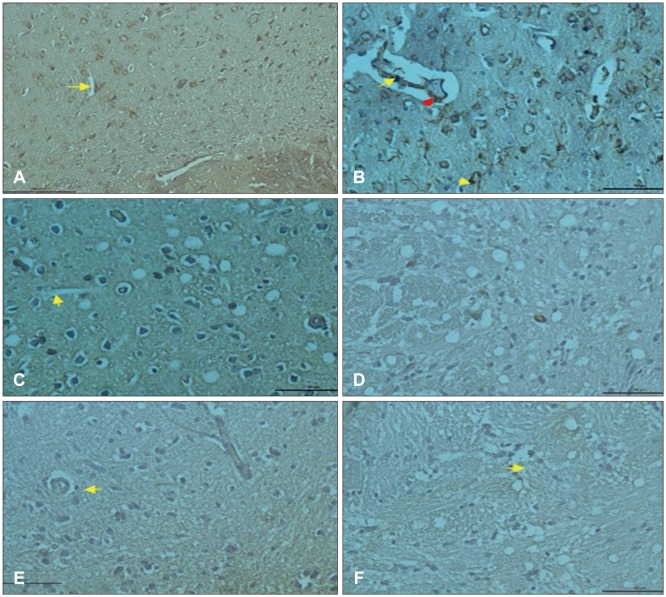
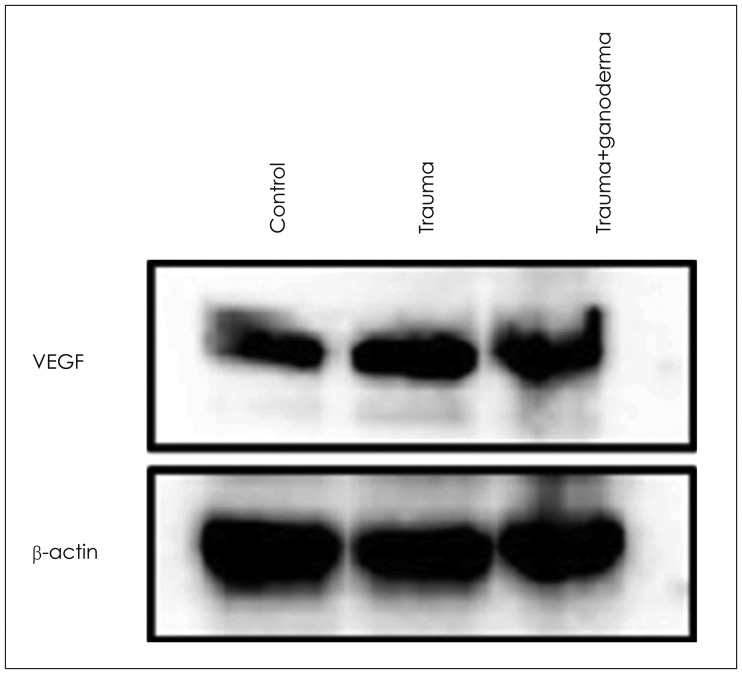
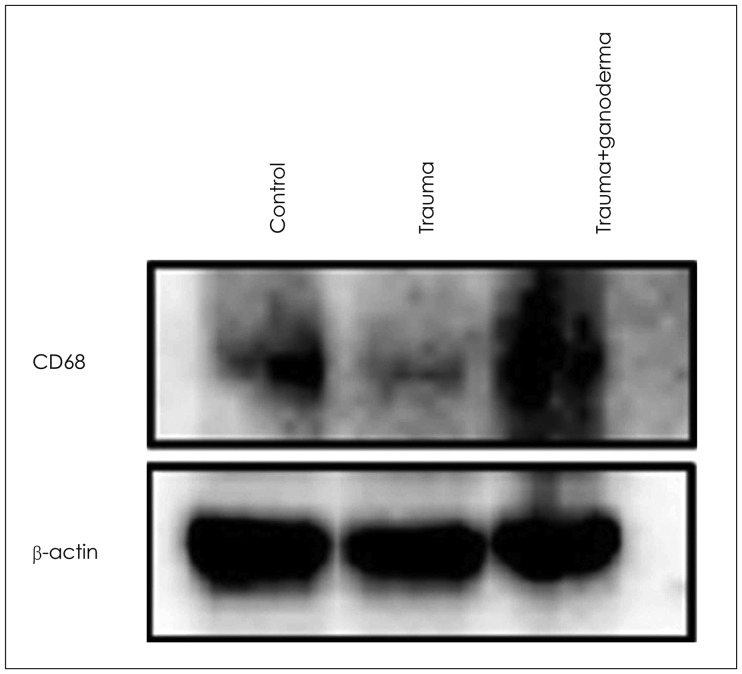
Comparing control, trauma and trauma + G. lucidium groups; it was observed p38 expression was increased in trauma group and trauma + G. lucidum group (Figure 3). VEGF expression. In the trauma group and trauma + G. lucidum group, VEGF expression gradually increased (Figure 4). CD68 expression in microglial cells increased in treatment group, while it decreases in trauma group (Figure 5).
FIGURE 3. Ganoderma treatment did not affect trauma-induced phosphorylation of p38 in brain tissue. Equal amounts of total proteins were run on the gel and analyzed by Western blot analysis for VEGE and β-actin. The β-actin was used as loading control.
FIGURE 4. Western blot analysis for vascular endothelial growth factor (VEGF) and β-actin. Ganoderma treatment did not affect trauma-induced expression of VEGF in brain tissue. Equal amounts of total proteins were run on the gel and analyzed by Western blotting using anti-VEGF and anti-β-actin antibodies. The β-actin was used as loading control.
FIGURE 5. Trauma-induced reduced cluster of differentiation 68 (CD68) expression in brain tissue was dramatically increased by ganoderma treatment. Equal amounts of total proteins were run on the gel and analyzed by Western blot analysis for CD68 and β-actin. The β-actin was used as loading control.
Discussion
Brain is particularly vulnerable organ to oxidative stress due to high oxygen consumption, abundant polyunsaturated fatty acids and low endogenous antioxidant levels. Free radicals may attack proteins of plasma membrane and other proteins in cellular membranes, thus disrupting organelle functionality. Therefore, it may be useful treatment for ischemic incidents to induce antioxidative effect. Superoxide dismutase is primary protective molecule against tissue damaged caused by reactive oxygen species. It catalyzes superoxide anion into hydrogen peroxide and prevents formation of hydroxyl radicals. It has been shown superoxide dismutase activity decreased in serum of stroke patients. Increasing anti oxidative activity may be beneficial in treatment of acute cerebral ischemia. In present study, we observed superoxide dismutase activity decreased after trauma-caused cerebral injury while its activity got close to usual level after post-traumatic G. lucidum treatment. Brain MDA is one of the most sensitive indicators of lipid peroxidation.
In trauma group of present study, MDA level significantly increased, GSH levels decreased, and existence of lipid peroxidation was seen after cerebral injury. After G. lucidum treatment, GSH levels were to be restored. Brain could accelerate its endogenous defensive capacity and induce its protective mechanism to battle with oxidative stress caused by TBI. G. lucidum has shown anti oxidative effect on lipid peroxidation. G. lucidum has been used in Eastern Asia for thousands years as a protective antioxidant drug. Polysaccharides derived from fruiting parts of G. lucidum reportedly have had antioxidant, immunomodulatory, and antitumor effects. Those polysaccharides have been reported to have protective effect against cerebral ischemic injury. G. lucidum extract reduced expression of cytotoxic and pro-inflammatory factors from activated microglia cells, and protected dopaminergic neurons against oxidative stress and inflammation. According to those results, GLPs' extract has neuronal protection and antioxidant properties. SB203580 DBI study states that p38 MAPK activity significantly decreased in cortical neuronal morphology and injury, causing neuronal loss and neuronal activation. In present study, p38 MAPK expression increased after post-traumatic treatment. Zhang et al.39) says that they observed CD163 macrophages, M2 marker, were detected in lesions after day 2 and day 4 of trauma in their head-traumatized rats by weight-drop model.
Inflammation formed in acute phase as a cause of TBI. After disruption of BBB balance, microglial activation in damaged tissue increased.17) In postmortem studies, reactive microglia cells in white matter of corpus callosum and frontal lobe of TBI patients up regulated for a long time after trauma.7,13,31). In our study, CD68 expression in microglial cells decreased due to trauma. After post-traumatic G. lucidum treatment, CD68 expression in phagocytic microglial cells around vessel was positively seen. It was thought that G. lucidum induces phagocytosis, influences cellular activity, and inhibits inflammation and edema. It was stated that increased VEGF ligands in brain tissue induces angiogenesis and neurogenesis by stimulating vascular endothelial cells and neuronal progenitors to restores injured neuronal tissues.
Krum and Khaibullina16) showed that inhibition of VEGF signals including VEGF-R1 receptors reduced number of reactive astrocytes and prevented glial scar formation in TBI models. Those observations indicate role of VEGFs astrocytic proliferation in brain pathologies. Due to traumatic damage, in the cerebral cortex, dilatation and hemorrhage in blood vessels, edema and inflammation around vessels were seen. These histopathological observations may cause BBB structural changes. Our study showed that inflammation, edema, dilatation and hemorrhage in blood vessels led to disruption of BBB due to traumatic injury in brain cortex. When we compare group of control, trauma and trauma + G. lucidum groups; we saw that p38 was increasingly expressed in trauma group and trauma + G. lucidum group (Figure 3). In the trauma group and trauma + G. lucidum group, VEGF expression gradually increased (Figure 4). CD68 expression in microglial cells increased in treatment group, while it decreases in trauma group (Figure 5). Brain edema causes formation of free radicals, proteases, inflammatory mediators, and bradykinin like arachidonic metabolites.1,35) VEGF was shown to be upregulated in parallel with hippocampal neurogenesis in acute period after TBI.19) In present study, in trauma group endothelial cells degenerated and vascular dilatation increased.
TBI led to cerebral edema by accelerating intracranial pressure and reducing blood flow. As a result, cerebral ischemia formed. It is known that activity of leukocytes is critical in hours following head trauma and this activity initiates inflammatory response. MPO activity is seen in neutrophils, monocytes and macrophages in lower levels. In our study, MPO activity measured in traumatized rats' brains increased while MPO activity was decreased lower in treatment groups. After increase in neutrophils infiltration and inflammatory pathways activation, high levels of free radicals in cell membrane caused lipid peroxidation.3) Continuing on lipid peroxidation, GSH levels decreased in trauma group. Following trauma, activation of inflammatory pathways and accelerated free radicals level broke down Na+, K+, -ATPase pumps, leading to edema.4) Intercellular edema and increased pressure caused opening of tight junctions and acceleration in blood brain permeability. In present study, both brain water content and BBB permeability significantly increased in trauma group. In contrast, GLPs treatment significantly protected BBB integrity and partially prevented cerebral edema. In histological analysis, degenerative neurons were observed less in number in GLPs group than in trauma group.
Comparing trauma and treatment group, it could be thought GLPs was effective in repairing brain injury due to increase of necrotic cells in number, and larger perineuronal edema and myelin damage were seen in trauma group.
In summary, edema and inflammation increased because of disrupted permeability of endothelial cells and degenerative changes in neuronal cells and glial cells of cortex were observed in brain cortex after trauma. These all were considered important histopathological findings in traumatic model. After GLPs treatment, dilation and hemorrhage decreased in blood vessels affected by damage and endothelial cells were observed structurally usual. Additionally, inflammation and edema outside vessels and degenerative changes in neuronal cells also decreased. In the analysis of IHC and western blot, expressions of p38, VEGF and CD68 changed because of angiogenic and neurogenic effects in a way parallel with and supporting histopathologic and biochemical results after GLPs treatment.
We propose that GLPs treatment after brain injury could be an alternative treatment to decraseing inflammation and edema, preventing neuronal and glial cells degeneration if given in appropriate dosage and in particular time intervals.
Conclusion
In conclusion, we point out that after brain injury, GLPs treatment could be an alternative treatment to reduce inflammation and edema, and preventing neuronal and glial cells degeneration if it is administrated in appropriate dosage and particular time intervals.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci. 2002;9:113–124. doi: 10.1054/jocn.2001.1031. [DOI] [PubMed] [Google Scholar]
- 2.Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–296. doi: 10.1016/s0014-5793(00)02317-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- 4.de Lores Arnaiz GR, Ordieres MG. Brain Na(+), K(+)-ATPase activity In aging and disease. Int J Biomed Sci. 2014;10:85–102. [PMC free article] [PubMed] [Google Scholar]
- 5.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Zhou S, Wen J, Huang M, Xu A. Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sci. 2002;72:731–745. doi: 10.1016/s0024-3205(02)02301-9. [DOI] [PubMed] [Google Scholar]
- 7.Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Hakan T, Toklu HZ, Biber N, Ozevren H, Solakoglu S, Demirturk P, et al. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol Res. 2010;32:629–635. doi: 10.1179/016164109X12464612122731. [DOI] [PubMed] [Google Scholar]
- 9.Hall R, Murdoch J. Brain protection: physiological and pharmacological considerations. Part II: The pharmacology of brain protection. Can J Anaesth. 1990;37:762–777. doi: 10.1007/BF03006535. [DOI] [PubMed] [Google Scholar]
- 10.Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 11.Hu ZL, Wen SG, Yu RJ, Zhu Y. Effects of Ganoderma lucidum fungus mixtureon immune enhancement in mice. Shandong Zhongyiyao Daxue Xuebao. 2003;27:683–687. [Google Scholar]
- 12.Ji Z, Tang Q, Zhang J, Yang Y, Jia W, Pan Y. Immunomodulation of RAW264.7 macrophages by GLIS, a proteopolysaccharide from Ganoderma lucidum. J Ethnopharmacol. 2007;112:445–450. doi: 10.1016/j.jep.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Janardhanan KK. Antioxidant and antitumor activity of ganoderma lucidum (Curt.: Fr.) P. Karst.-Reishi (Aphyllophoromycetideae) from South India. Int J Med Mushrooms. 2000;2:195–200. [Google Scholar]
- 15.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krum JM, Khaibullina A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: roles for VEGF in brain repair. Exp Neurol. 2003;181:241–257. doi: 10.1016/s0014-4886(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34:1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- 20.Lakshmi B, Ajith TA, Sheena N, Gunapalan N, Janardhanan KK. Antiperoxidative, anti-inflammatory, and antimutagenic activities of ethanol extract of the mycelium of Ganoderma lucidum occurring in South India. Teratog Carcinog Mutagen. 2003;Suppl 1:85–97. doi: 10.1002/tcm.10065. [DOI] [PubMed] [Google Scholar]
- 21.Li JM, Zhao YN, Chen CX, Li SX. Ischemic preconditioning on the sand mouse ischemia-reperfusion injury p38MAPK activation function. Zhong Hua Shen Jing Wai Ke Za Zhi. 2011;27:741–745. [Google Scholar]
- 22.Lin ZB, Zhang HN. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin. 2004;25:1387–1395. [PubMed] [Google Scholar]
- 23.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 24.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 25.Mizushina Y, Takahashi N, Hanashima L, Koshino H, Esumi Y, Uzawa J, et al. Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum. Bioorg Med Chem. 1999;7:2047–2052. doi: 10.1016/s0968-0896(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 26.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev. 2007;56:322–330. doi: 10.1016/j.brainresrev.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Shi YE, Wen RS, Cao XL, Wang RB, Wang XG. Effect of Ganoderma lucidum rich with selenium on the brain ischemia in rat. J Hebei Chinese Drug. 1998;4:27–28. [Google Scholar]
- 29.Shibuya M. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. Febs j. 2009;276:4636–4643. doi: 10.1111/j.1742-4658.2009.07175.x. [DOI] [PubMed] [Google Scholar]
- 30.Sköld MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma. 2005;22:353–367. doi: 10.1089/neu.2005.22.353. [DOI] [PubMed] [Google Scholar]
- 31.Smith C, Gentleman SM, Leclercq PD, Murray LS, Griffin WS, Graham DI, et al. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol. 2013;39:654–666. doi: 10.1111/nan.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Z, Liao Z, Shi Q, Xie Y, He Z, Zhan Y. Blocking p38 signal pathway lowers MMP-9 expression and reduces brain edema in rats with traumatic brain injury. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:928–931. [PubMed] [Google Scholar]
- 34.Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S. Modified experimental mild traumatic brain injury model. J Trauma. 2006;60:558–565. doi: 10.1097/01.ta.0000209172.75637.db. [DOI] [PubMed] [Google Scholar]
- 35.Wang ML, Huang XJ, Fang SH, Yuan YM, Zhang WP, Lu YB, et al. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem Biophys Res Commun. 2006;350:399–404. doi: 10.1016/j.bbrc.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 36.Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, et al. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699–705. doi: 10.1002/(sici)1097-0215(19970317)70:6<699::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- 38.Wittko-Schneider IM, Schneider FT, Plate KH. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol Life Sci. 2013;70:1705–1725. doi: 10.1007/s00018-013-1279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Lesional accumulation of CD163+ macrophages/microglia in rat traumatic brain injury. Brain Res. 2012;1461:102–110. doi: 10.1016/j.brainres.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 40.Zhao HB, Lin SQ, Liu JH, Lin ZB. Polysaccharide extract isolated from ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J Pharmacol Sci. 2004;95:294–298. doi: 10.1254/jphs.sc0040011. [DOI] [PubMed] [Google Scholar]
- 41.Zhao W, Jiang X, Deng W, Lai Y, Wu M, Zhang Z. Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Chem Toxicol. 2012;50:303–309. doi: 10.1016/j.fct.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 42.Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, et al. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol. 2010;131:154–164. doi: 10.1016/j.jep.2010.06.023. [DOI] [PubMed] [Google Scholar]