Abstract
Introduction:
Patients, providers, and payers are striving to identify where value in cancer care can be increased. As part of the Choosing Wisely (CW) campaign, ASCO and the American Society for Therapeutic Radiology and Oncology have recommended against specific, yet commonly performed, treatments and procedures.
Methods:
We conducted a retrospective analysis of Medicare claims data to examine concordance with CW recommendations across 12 cancer centers in the southeastern United States. Variability for each measure was evaluated on the basis of patient characteristics and site of care. Hierarchical linear modeling was used to examine differences in average costs per patient by concordance status. Potential cost savings were estimated on the basis of a potential 95% adherence rate and average cost difference.
Results:
The analysis included 37,686 patients with cancer with Fee-for-Service Medicare insurance. Concordance varied by CW recommendation from 39% to 94%. Patient characteristics were similar for patients receiving concordant and nonconcordant care. Significant variability was noted across centers for all recommendations, with as much as an 89% difference. Nonconcordance was associated with higher costs for every measure. If concordance were to increase to 95% for all measures, we would estimate a $19 million difference in total cost of care per quarter.
Conclusion:
These results demonstrate ample room for reduction of low-value care and corresponding costs associated with the CW recommendations. Because variability in concordance was driven primarily by site of care, rather than by patient factors, continued education about these low-value services is needed to improve the value of cancer care.
INTRODUCTION
Because health care costs are rising at an unsustainable rate,1 patients, providers, and payers are collectively striving to identify where value in cancer care can be increased and how the triple aim of better health, better health care, and lower cost can be achieved.2 The American Board of Internal Medicine’s Choosing Wisely (CW) campaign aims to improve value by targeting low-value services in medicine and thus increase quality of care while lowering cost.3 These services include tests, procedures, and medications that are routinely used despite lacking evidence of benefit. Both ASCO and the American Society for Therapeutic Radiology and Oncology (ASTRO) have recommended against specific, yet commonly performed, practices.4-6
The extent of adoption is different for each recommendation, but early evidence is emerging that concordance with these recommendations is overall suboptimal and that opportunities exist for improvement.7 Certain patient and provider factors may contribute to the observed variability in concordance with specific CW recommendations. Previous research has demonstrated that certain groups of patients are less likely to receive guideline-concordant care for cancer, ie, they are more likely to underuse medical services.8-11 Data on factors influencing overused, low-value medical services are limited. Inappropriate imaging of early breast and prostate cancers was driven primarily by regional differences rather than by patient factors in one study, suggesting provider or system influence on concordance with imaging recommendations.12
Nonconcordance with CW recommendations may be costly. Ramsey et al7 reported that concordance with the 2012 ASCO top five CW recommendations was associated with substantial differences in cost of patient care. No data are available on cost associated with concordance with the ASTRO-supported recommendations and 2013 ASCO recommendations.
The primary purpose of this study was to evaluate the concordance with six ASCO and three ASTRO CW recommendations in one cancer care network composed of 12 cancer centers of varying size and practice structure across five states in the southeastern United States. The second objective was to identify the variability of guideline concordance across patient factors (age, race, and comorbid conditions) and across the network’s institutions. Finally, we aimed to determine whether the concordance with recommendations was associated with lower total Medicare costs of care and to calculate the potential savings associated with optimal concordance levels (95%). Overall, our purpose was to identify opportunities for improving value within our network.
METHODS
Study Design and Data Source
We conducted a retrospective cohort study using data collected from patients with cancer within the University of Alabama at Birmingham (UAB) Health System Cancer Community Network (CCN) as part of a 2012 Center for Medicare and Medicaid Innovation award. The CCN includes the UAB Comprehensive Cancer Center and 11 cancer centers located in five southeastern states (Alabama, Georgia, Florida, Mississippi, and Tennessee).13,14 The CCN comprises academic medical centers and community cancer centers located in geographically distinct regions, both rural and urban. The practice structures vary among sites and include hospital-based practices and affiliated traditional private practices.13,14 As part of this Center for Medicare and Medicaid Innovation project, a database was created to link clinical information from local tumor registries with Medicare claims for inpatient, outpatient, and hospice care. Claims data were provided by the Centers for Medicare & Medicaid Services Chronic Condition Data Warehouse. This study was approved by the institutional review boards of UAB and affiliate sites.
Sample population
Our linked database contained patients with cancer, with Medicare Fee-for-Service Parts A and B coverage, who received care at an affiliated hospital between 2012 and 2015. Patients were identified using each institution’s financial records merged with their cancer registry data. Claims data were merged with patient records, and beneficiaries without continuous enrollment and Health Maintenance Organization coverage were excluded. Patients were categorized as having no, one, or at least two comorbidities on the basis of Charlson comorbidity weights using National Cancer Institute guidelines from the claims data. Each beneficiary’s score was determined from all claims available between 2012 and 2015.
Defining guideline concordance
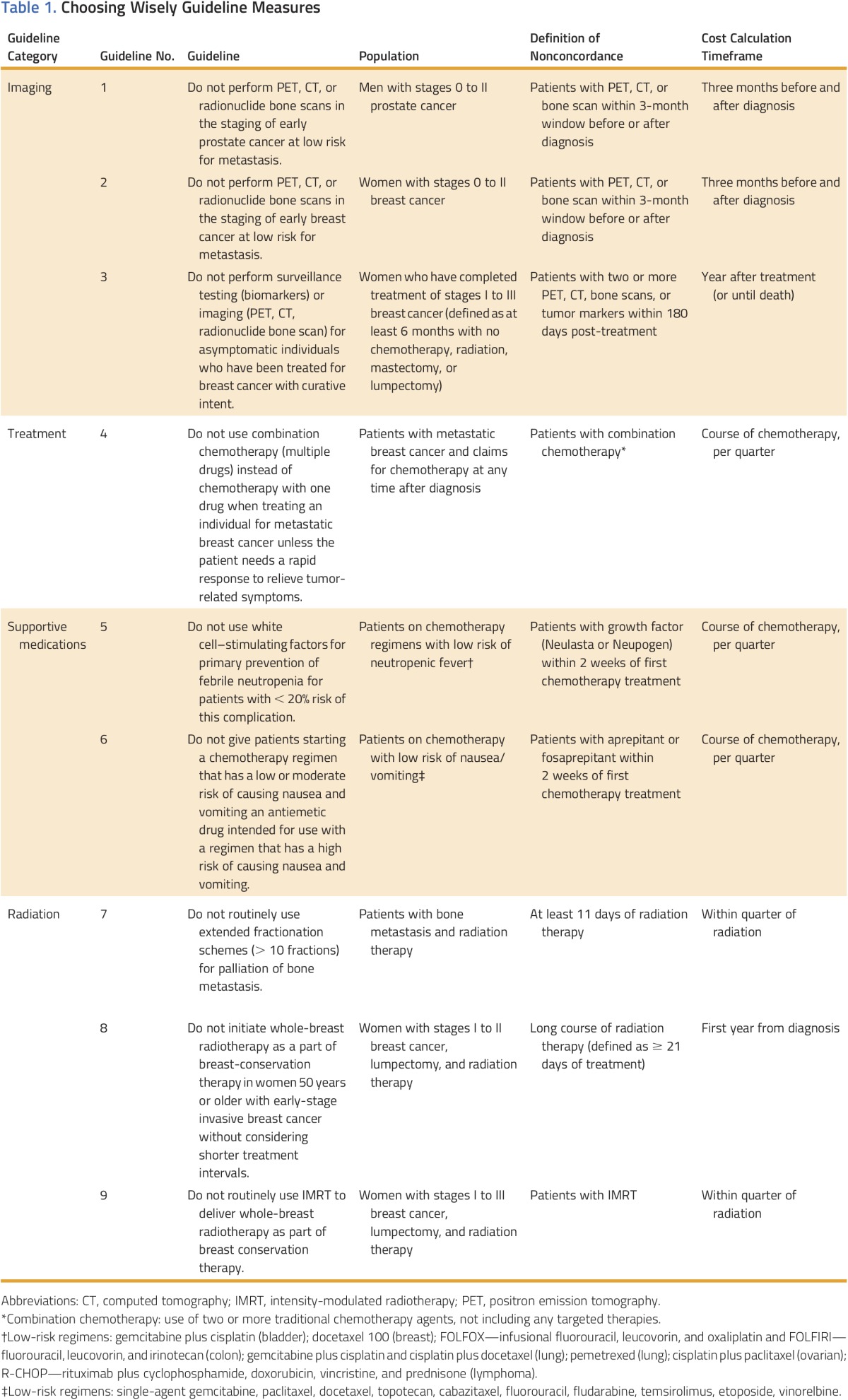
Criteria for guideline concordance were derived from ASCO and ASTRO CW recommendations from 2012 and 2013.4-6 For each guideline, the CCN database was used to identify patients who were eligible for each CW guideline measure between January 1, 2012 and December 31, 2015. Table 1 lists the specific criteria used to define the population and concordance with each measure.
Table 1.
Choosing Wisely Guideline Measures
Assessing Variability in Guideline Concordance
A dichotomous variable (yes v no) for receiving guideline-concordant care was created for each of the nine CW guideline measures. Concordance was assessed by calculating the percentage of patients with care concordant with guidelines for each measure. The percentage of patients with concordant care within each measure was stratified by race (white v other), age (63 to 74 years v ≥ 75 years), Charlson comorbidity category (0 v 1 v ≥ 2), site size (small v large), and site (12 sites within the UAB CCN).13 Patient age was defined as age at the time of receipt of services associated with the specific measure. The four sites with the greatest number of patients with cancer (> 4,000) in the data set were considered large sites, and the others were considered small sites. In addition, because some of the CW guidelines were not issued until 2013, we examined concordance over the periods 2012 to 2013 and 2014 to 2015.
Estimating Costs
Costs of care were calculated using total amounts reimbursed by Medicare to providers. All medical care received was included in the calculations, excluding Part D claims. Total Medicare costs were calculated over different periods depending on the CW recommendation (Table 1). Costs were then stratified by concordance status (concordant v nonconcordant) for each measure. We estimated the potential cost savings if subjects received care concordant with each measure guideline. We would not expect 100% concordance for any measure, because there are always exceptions to guidelines. We therefore estimated the best feasible adherence to be 95%. To calculate total projected savings, we initially estimated the cost per quarter as if 95% of patients within the study received concordant care and 5% received nonconcordant care. We then subtracted this from the actual observed costs to estimate the projected savings per quarter.
Statistical Analysis
Overall sample statistics for the UAB CCN population were calculated using frequencies and percentages. To address the primary aim, we estimated the overall concordance for each CW guideline measure, as well as stratum-specific estimates for site, race, age, and comorbidity category. All 12 UAB CCN sites were assessed in the analyses; the highest and lowest site-concordance percentages are shown to indicate range of concordance. Between-strata differences in concordance were assessed using χ2 and Fisher’s exact tests. To examine differences between concordance status and average cost per patient, we used hierarchical linear modeling to account for the within-site correlation of observations. Different correlation structures were examined and the optimum model fit was assessed using the Akaike Information Criterion (lower scores indicate better fit15). All analyses were performed using SAS version 9.4 (Cary, NC).
RESULTS
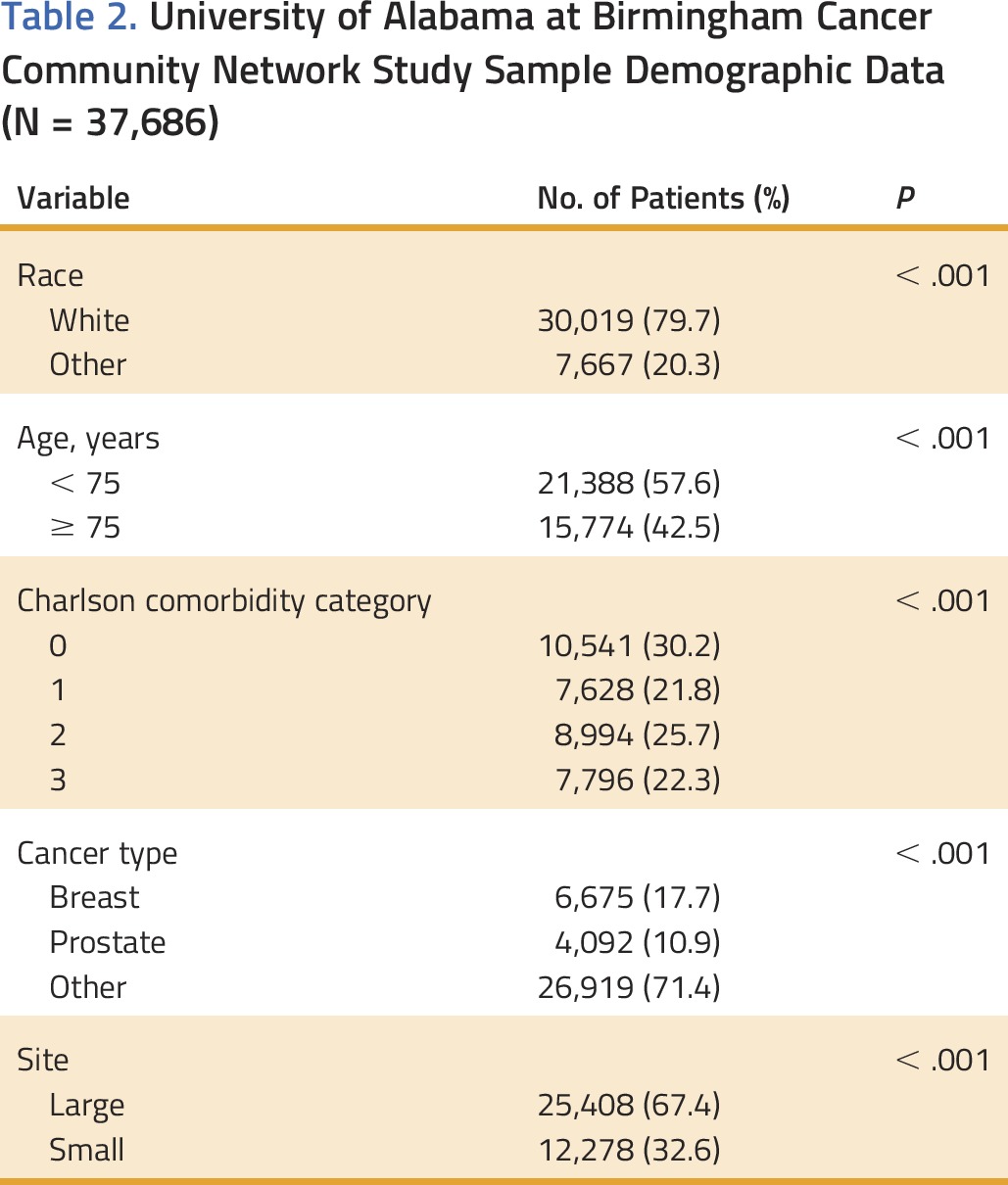
The UAB CCN database included 37,686 unique Medicare patients, with a known cancer diagnosis date after 2008, who received care between 2012 and 2015. Twenty percent of patients were nonwhite, 43% were ≥ 75 years old, and 48% had two or more major comorbidities (Table 2). Thirty-three percent of patients were treated within small sites.
Table 2.
University of Alabama at Birmingham Cancer Community Network Study Sample Demographic Data (N = 37,686)
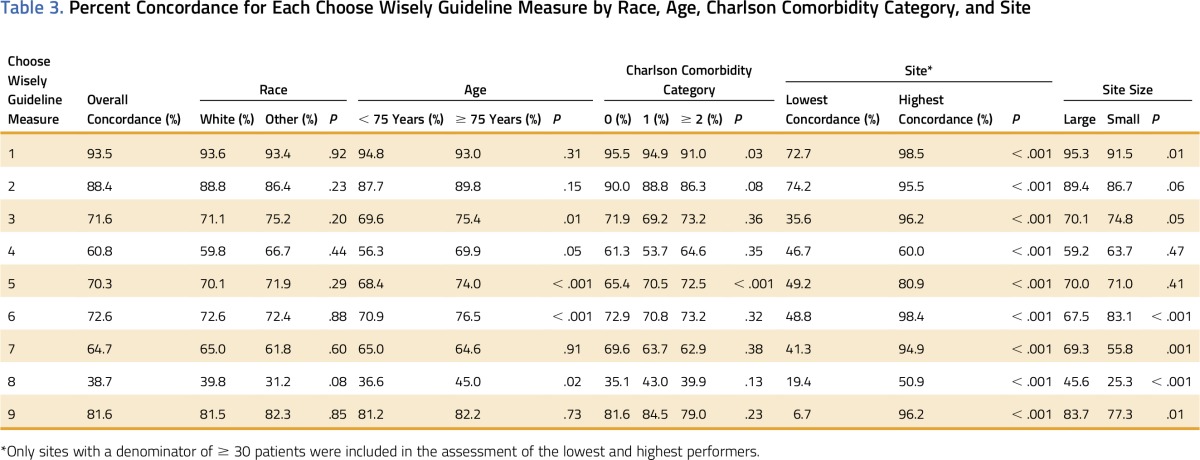
Concordance varied by CW guideline measure (Table 3). Overall, concordance was lowest for CW guideline measure 8, with only 39% of women with stages I to II breast cancer having received a short course of radiotherapy after lumpectomy. Concordance with CW guideline measure 1 was the highest, with 94% of men with stages 0 to II prostate cancer not receiving a positron emission tomography, computed tomography, or bone scan within 3 months before or after diagnosis. Concordance was similar for 2012 to 2013 and 2014 to 2015 (P ≥ .1), with the exception of guidelines regarding radiation for breast-conserving therapy (CW measures 8 and 9), which increased from 33.6% to 45.6% (P < .001) and from 79.1% to 85.0% (P = .02), respectively.
Table 3.
Percent Concordance for Each Choose Wisely Guideline Measure by Race, Age, Charlson Comorbidity Category, and Site
Site Variability
Significant variability was noted in concordance rates across sites for all CW guideline measures (Table 3). The highest variability was for use of intensity-modulated radiotherapy to deliver whole-breast radiotherapy as part of breast-conservation therapy (CW measure 9), with sites ranging from 6.7% to 96.2% concordance (P < .001). CW guideline measure 4, the use of combination chemotherapy for metastatic breast cancer, showed the lowest variability across sites (46.7% to 60.0%; P < .001), although overall concordance was low. All sites had at least one measure in which they were among the top performers and other measures in which their concordance was low.
Patient Variability
Patient-level factors (race, age, and comorbidities) had modest influence on CW concordance variability, particularly for medication-related measures. Compared with patients 75 years or older, those 63 to 74 years old were less likely to have treatment concordant with CW measures 3 to 6 and 8, ie, surveillance testing/imaging within 180 days of breast cancer treatment, combination chemotherapy for patients with metastatic breast cancer, patients with growth factor prophylaxis (receipt of antiemetics within the first 2 weeks of chemotherapy treatment) or long course of radiation therapy in women with early-stage breast cancer (Table 3; all P < .05).
We also noted a significant difference in concordance with CW measure 5, with younger adults and patients with fewer comorbidities being more likely to receive nonconcordant care, that is, to receive growth factor prophylaxis within the first 2 weeks of chemotherapy treatment (P < .001). For CW measure 1, which assesses the use of advanced imaging in early-stage prostate cancer, patients with multiple comorbidities were more likely to receive nonconcordant care (P = .03). Race had no statistically significant influence on any measure.
Cost
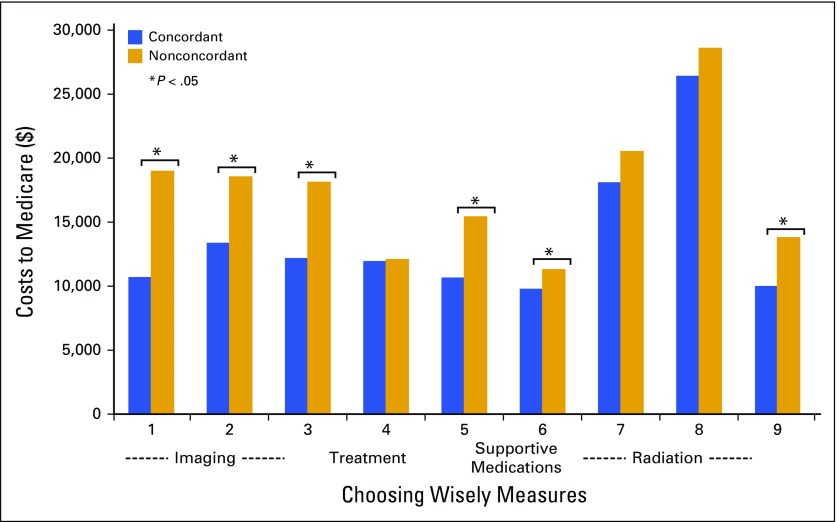
Differences in the average total Medicare cost per patient by concordance status varied within each CW guideline measure (Fig 1). Overall, the average cost for patients with nonconcordant treatment was higher than for patients with concordant treatment for every measure. Significant cost differences by concordance status were most often seen for CW measures that include imaging (CW measures 1, 2, and 3) and supportive medications (CW measures 5 and 6). Cost differences by concordance status for radiation therapy measures were significant only for CW measure 9, where costs were higher for women with stages I to III breast cancer receiving intensity-modulated radiotherapy versus other radiation therapy after lumpectomy (P < .001). If concordance was 95% for all measures, we estimated that it would result in a $19 million reduction in total cost of care per quarter.
FIG 1.
Concordant versus nonconcordant average cost per patient.
DISCUSSION
In this cohort of older adults receiving cancer care from several different cancer centers in the southeastern United States, concordance with CW recommendations was suboptimal and nonconcordance was associated with higher total costs of care for every recommendation. Our findings support the notion that these low-value practices, which do not improve patient outcomes, are common among both medical and radiation oncologists, with concordance ranging from 39% to 94% of patients receiving concordant care depending on the measure.7,12,16 Moreover, although a few patient factors were associated with concordance, the considerable variation by cancer center indicates that provider factors may be more important. This study also indicates that there is potential to enhance value by targeting concordance with CW recommendations, because we found that most of the measures we examined were associated with substantially higher costs of care.
The CW measures were initially created to address the rising cost of health care, while maintaining patient outcomes. Our study identified growth factor use among approximately 30% of patients, similar to previous reports.7 However, our study had lower rates of inappropriate imaging for prostate (6.5%; CW 1) and breast cancer (11.2%; CW 2) than previously reported rates of 22% to 44%.7,12 We also reported lower rates of surveillance testing, with 28% of patients with early-stage breast cancer having tumor markers or advanced imaging compared with 53% reported by Ramsey et al.7
Differences may be secondary to regional practice patterns, patient population, or insurance preauthorization. Our study evaluated older patients living in the Deep South with primary Medicare insurance, whereas Ramsey et al assessed younger patients with Blue Cross insurance in the state of Washington. Physicians may be more likely to order computed tomography scans to evaluate for metastatic disease in younger patients with breast cancer as a result of the perception that younger patients are at higher risk.17 In addition, differences in preauthorization for advanced imaging may have influenced frequency of receipt of this service. Further exploration of driving forces behind regional variation is needed.
To our knowledge, this is the first study on concordance with ASTRO CW recommendations. Lower concordance (36% to 80%) may be the result, in part, of the more recent nature of these recommendations, which were published in 2014. This is supported by the improvement in concordance we observed from 2012 to 2013 to 2014 to 2015 for two of the three measures. In addition, concordance was higher for radiation oncology measures at the larger centers, which included both academic cancer centers. This contrasts with the medical oncology measures, for which the smaller practices had performance similar to that of the larger sites. Thus, concordance with these radiation oncology measures may improve as knowledge of these guidelines is disseminated to smaller, community practices.
Our findings are also consistent with results by Ramsey et al7 that showed marked differences in total costs for patients who received care consistent with CW guidelines. The costs of care we calculated included those for all of the care received by patients. If there were savings associated with preventing adverse effects of chemotherapy, for example, these would be captured. However, our results clearly show that if there were such savings, there were not enough to compensate for the cost of the testing, medication, or procedure. The potential cost reduction associated with a wide-scale adoption of CW recommendations in our network could exceed $19 million; thus, concordance with CW recommendations will be a likely target for improving the value of our cancer care.
A common criticism of guidelines is that there are exceptions on the basis of patient factors. Our analysis suggests that patient factors are not the primary drivers of nonconcordance. The greatest source of variability in concordance with CW recommendations was site of treatment. For individual recommendations, the difference in concordance rate between the lowest and highest performer was as high as 89% (96% v 7%; CW measure 9). Even for recommendations in which performance was overall excellent, such as imaging at diagnosis, we observed a 26% difference between the highest and lowest performers. It was noteworthy, however, that no site had high or low concordance with all CW measures, suggesting that each site has both potential for improvement and an opportunity to share best practices.
These results are important because they provide an example of how claims data can be used to inform the transition to value-based cancer care. The CCN participating sites are not part of a large group practice; they share data, but do not share ownership or institutional policies. Thus, these variable practice structures are representative of different oncology practices that could be found across the United States. Networks, such as our CCN, allow institutions to partner in data-driven quality improvement, and to have the opportunity to share best practices and collectively enhance value within the region. We anticipate that these measures will be integrated into future pay-for-performance programs; thus assessment of policies and practices that enhance concordance are critical.
Low-value medical services, such as those evaluated within this analysis, have implications for quality and cost of care, but also the potential to lead to an inferior patient experience and health outcomes. For example, consider the case of a patient with breast cancer who comes to the clinic for radiation once per day for 6 weeks rather than receiving the shorter course. The shorter course is associated with fewer toxicities and better ability to meet family needs.18 In addition, these low-value procedures can cause harm. Low-value imaging is associated with increased radiation, patient inconvenience, and the need for further imaging and biopsies for findings that might never have become clinically significant.16 Therefore, providing care concordant with CW measures will likely lead to improving quality of life and satisfaction with care.
This study has some limitations. Claims-based analysis is often criticized for the inability to discriminate between when lack of concordance is the result of appropriate clinical indications or of patient and family preferences. However, given that site of care is likely the major contributor to guideline concordance rather than patient factors, systemic practice patterns are influencing nonconcordance. We did not conduct multivariable analysis to adjust for potential confounders, such as psychosocial needs or personal financial resources. However, given the lack of association between patient factors and concordance, adjusting for the available confounders would not have explained the variation by site.
In addition, data on participation in clinical trials were not available, which may have influenced concordance if protocols included treatment or surveillance inconsistent with the CW guidelines. This analysis also focuses exclusively on Medicare patients in the southeastern United States; therefore, findings may not be applicable for a younger population or patients in other geographic regions. Additionally, this analysis considers care received between 2012 and 2015. Given the recent press associated with the CW campaign, concordance may have improved over time as clinicians’ awareness increased.
In conclusion, depending on the CW measure, as many as 60% of older adults with cancer may be receiving low-value care. These results demonstrate ample room for improvement in clinical practice and reduction in costs of care. Because variability in concordance was driven primarily by site of care, rather than by patient factors, continued education about these recommendations is needed to improve the value of cancer care and to transition to value-based cancer care.
ACKNOWLEDGMENT
We acknowledge the Patient Care Connect Group for its contribution to this project and manuscript. This publication was made possible by Grant Number 1C1CMS331023 from the Department of Health & Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Department of Health & Human Services or any of its agencies. The research presented here was conducted by the awardee. Findings might or might not be consistent with or confirmed by the findings of the independent evaluation contractor. G.B.R. is supported by a Walter B. Frommeyer Jr Fellowship in Investigative Medicine. This funding source had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript for publication. From the Patient Care Connect Group. The members and affiliations of the writing committee are listed in the online-only Appendix.
Appendix
PATIENT CARE CONNECT GROUP
Memorial Hospital, Chattanooga, TN: Lee Jackson, MD, Zoe Scott
Northside Hospital Cancer Institute, Atlanta, GA: Guilherme Cantuaria, MD, Debbie Bickes, Tina Berry
Gulf Coast Regional Medical Center, Panama City, FL: George Reiss, MD, Hang Mai
Ft Walton Beach Medical Center, Ft Walton Beach, FL: Ming Chang, MD, Louiz Gomez, Rhonda Meeker
Singing River Health System, Pascagoula, MS: James Clarkson, MD, Maggie Clarkson
SE Alabama Medical Center, Dothan, AL: Steven Stokes, MD, Tina Newman
Russell Medical Center, Alexander City, AL: Mary Sheffield, MD
NE Alabama Regional Medical Center, Anniston, AL: Ellen Spremulli, MD, Wendy Watson
Marshall Medical Center, Albertville, AL: Tom Payne, MD, Hanna Bright, Stacey Holman
Mitchell Cancer Institute, Mobile, AL: Thomas Butler, MD, Cathy Tinnea
Medical Center Navicent Health, Macon, GA: Fred Schnell, MD, Cyndi Pyle
University of Alabama at Birmingham Comprehensive Cancer Center, Birmingham, AL: Gabrielle B. Rocque, MD, Richard Taylor, DNP, Aras Acemgil, Xuelin Li, PhD, Kelly M. Kenzik, PhD, Bradford E. Jackson, PhD, Karina I. Halilova, MD, Maria Pisu, PhD, Wendy Demark-Wahnefried, PhD, Karen Meneses, PhD, Yufeng Li, PhD, Michelle Y. Martin, PhD, Carol Chambless, Nedra Lisovicz, PhD, Valeria Pacheco-Rubi, Terri L. Salter, Warren Smedley, Mona Fouad, MD, Elizabeth A. Kvale, MD, Edward E. Partridge, MD
AUTHOR CONTRIBUTIONS
Conception and design: Gabrielle B. Rocque, Audrey S. Wallace, Karina I. Halilova, Edward E. Partridge, Maria Pisu
Collection and assembly of data: Gabrielle B. Rocque, Courtney P. Williams, Bradford E. Jackson, Audrey S. Wallace, Kelly M. Kenzik, Maria Pisu
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Choosing Wisely: Opportunities for Improving Value in Cancer Care Delivery?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Gabrielle B. Rocque
Honoraria: Medscape
Research Funding: PackHealth, Medscape, Carevive Systems, Genentech
Travel, Accommodations, Expenses: Medscape
Courtney P. Williams
No relationship to disclose
Bradford E. Jackson
No relationship to disclose
Audrey S. Wallace
No relationship to disclose
Karina I. Halilova
No relationship to disclose
Kelly M. Kenzik
No relationship to disclose
Edward E. Partridge
No relationship to disclose
Maria Pisu
No relationship to disclose
REFERENCES
- 1.American Society of Clinical Oncology The state of cancer care in America, 2015: A report by the American Society of Clinical Oncology. J Oncol Pract. 2015;11:79–113. doi: 10.1200/JOP.2015.003772. [DOI] [PubMed] [Google Scholar]
- 2.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 3.Cassel CK, Guest JA. Choosing Wisely: Helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- 4.Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31:4362–4370. doi: 10.1200/JCO.2013.53.3943. [DOI] [PubMed] [Google Scholar]
- 5.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 6.Hahn C, Kavanagh B, Bhatnagar A, et al. Choosing Wisely: The American Society for Radiation Oncology’s top 5 list. Pract Radiat Oncol. 2014;4:349–355. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey SD, Fedorenko C, Chauhan R, et al. Baseline estimates of adherence to American Society of Clinical Oncology/American Board of Internal Medicine Choosing Wisely initiative among patients with cancer enrolled with a large regional commercial health insurer. J Oncol Pract. 2015;11:338–343. doi: 10.1200/JOP.2014.002717. [DOI] [PubMed] [Google Scholar]
- 8.Kimmick G, Fleming ST, Sabatino SA, et al. Comorbidity burden and guideline-concordant care for breast cancer. J Am Geriatr Soc. 2014;62:482–488. doi: 10.1111/jgs.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwentner L, Wöckel A, König J, et al. Adherence to treatment guidelines and survival in triple-negative breast cancer: A retrospective multi-center cohort study with 9,156 patients. BMC Cancer. 2013;13:487. doi: 10.1186/1471-2407-13-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wöckel A, Kurzeder C, Geyer V, et al. Effects of guideline adherence in primary breast cancer—A 5-year multi-center cohort study of 3976 patients. Breast. 2010;19:120–127. doi: 10.1016/j.breast.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Chang J, Ziogas A, et al. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125:833–842. doi: 10.1097/AOG.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: Potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1:185–194. doi: 10.1001/jamaoncol.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocque GB, Taylor RA, Acemgil A, et al. Guiding lay navigation in geriatric patients with cancer using a distress assessment tool. J Natl Compr Canc Netw. 2016;14:407–414. doi: 10.6004/jnccn.2016.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rocque GB, Partridge EE, Pisu M, et al: The Patient Care Connect Program: Transforming health care through lay navigation. J Oncol Pract 12:e633-642, 2016. [DOI] [PMC free article] [PubMed]
- 15.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 16.Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157:574–576. doi: 10.7326/0003-4819-157-8-201210160-00535. [DOI] [PubMed] [Google Scholar]
- 17.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 18.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]