Abstract
Purpose:
Community oncology practices frequently manage chemotherapy-associated toxicities, which may disrupt treatment, impair quality of life, and induce unplanned service use. We sought to understand the patterns and correlates of unplanned health care service use among patients receiving first-cycle chemotherapy at five community-based ambulatory oncology practices.
Patients and Methods:
A survey study examined the dichotomous outcome of unplanned service use, defined as oncologist visits, emergency department visits, and hospitalizations, resulting from toxicity-related factors. Newly diagnosed patients with breast, lung, head and neck, or colorectal cancer or non-Hodgkin lymphoma were recruited during the first chemotherapy cycle. Before beginning the second cycle of chemotherapy, patients completed a questionnaire that measured unplanned service use and overall distress, plus severity of nausea, vomiting, diarrhea, constipation, mouth sores, intravenous catheter problems, pain, fever and chills, extremity edema, and dyspnea on a 5-point scale (1, did not experience; 5, disabling). Medical record reviews captured chemotherapy doses, comorbid conditions, and supportive care interventions. Mixed-effects logistic regression was used to identify factors associated with unplanned service use, with random effects specified for each clinic.
Results:
Among 106 patients (white, 98%; female, 74.5%; mean age ± standard deviation, 60 ± 11 years), frequently reported toxicities were pain, nausea, diarrhea, and constipation. Thirty-six patients (34%) reported unplanned service use: 29% reported oncologist visits, 14% reported emergency department visits, and 8% reported hospitalizations. Factors significantly associated with unplanned service use were high patient-reported distress and receipt of colony-stimulating factor.
Conclusion:
Service use resulting from toxicity-related factors occurs frequently in community oncology settings. Monitoring toxicity patterns and outcomes can inform proactive symptom management approaches to reduce toxicity burden between scheduled visits.
INTRODUCTION
Little is known about the experiences of patients undergoing cancer treatment in community settings. Most therapeutic clinical trials that inform oncologists about expected toxicity incidence are conducted in academic settings with relatively healthy and homogeneous patient samples. However, most oncology care is delivered in community-based practices.1,2 Chemotherapy toxicities are common and may cause disruptions in treatment, impaired health-related quality of life, and unplanned health care service use.2 Few studies have examined the patient experience as chemotherapy begins, despite data that suggest toxicities associated with chemotherapy treatment occur most often in the initial treatment cycle.1 Systematic measurement of chemotherapy toxicity from the patient perspective could improve patient–clinician decision making surrounding treatment and prioritize implementation of evidence-based practice initiatives for toxicity prevention and management.
Typically, toxicity measurement is restricted to clinician reports in clinical trials. Published toxicity incidence ranges from 9% in pooled trial data3 to 35% in a recently published observational study.4 Because of concerns of clinician bias in toxicity grading and increased attention to patient-centered care, patient-reported outcomes are used increasingly in both clinical trials and health services research. Traditional chemotherapy reporting is subject to bias, because most clinical trials use the Common Terminology Criteria for Adverse Events (CTCAE), a clinician-reported measure that is not routinely used in practice.5 Compared with patients, clinicians report chemotherapy toxicities at lower severity and later in the treatment course.6 To addresses these biases, the National Cancer Institute has developed a patient-reported version of the CTCAE (PRO-CTCAE).7 To date, 80 toxicities associated with cancer therapy have been translated into patient-reported items, in both paper and electronic forms, with excellent validity and reliability in a large national sample of patients at community and academic medical centers.8 However, patient-reported toxicity measures are not frequently used in community oncology settings and rarely inform treatment decisions or prompt clinical quality improvement efforts.2
In systematic review of 18 studies examining emergency department (ED) visits among oncology patients, more than half of ED visits resulted in hospital admissions.9 However, reports of toxicities experienced by oncology patients were inadequate to synthesize data across studies, highlighting the need for consistent use of patient-reported toxicity measures.9 Furthermore, regular assessment of toxicities among oncology patients can predict ED visits. In a cohort study of more than 45,000 oncology patients in Canada, the patient-reported Edmonton Symptom Assessment Scale was used to predict subsequent ED visits within 7 days of assessment.10 Predictors of ED visits included nausea, drowsiness, dyspnea, pain, fatigue, poor appetite, and low patient-reported well-being.10 Consistent use of patient-reported assessments can help clinicians identify and address worsening toxicities.10
In this context, a descriptive study was conducted to examine patient-reported toxicities among patients receiving first-cycle chemotherapy treatment in community-based oncology practices. The proposed research plan was motivated by two factors. First, patient perspectives are crucial to reduce symptom burden and improve the quality of chemotherapy delivered in community oncology practices. Second, our community partner, the Michigan Cancer Research Consortium (MCRC), which includes nine community-based oncology settings, has placed strategic emphasis on developing capacity to conduct cancer care delivery research in the community oncology settings served. Such capacity has the potential to support future studies that improve outcomes for patients treated in community oncology settings. This analysis was driven by two research questions: First, what are the frequency and severity of patient-reported, chemotherapy-related toxicities in a sample of adults receiving their first cycle of chemotherapy for cancer, compared with the frequency of toxicities documented by clinicians? Second, what factors are associated with unplanned health care service use resulting from toxicities reported by patients after first-cycle chemotherapy?
PATIENTS AND METHODS
Sample and Recruitment
For this prospective pilot study, a convenience sample of newly diagnosed patients was recruited from the St Joseph Mercy Health System Cancer Program and the Genesys Hurley Cancer Institute in Michigan. Treating physicians received an e-mail introducing the study and had the option to decline or to allow MCRC research staff to approach their patients. Inclusion criteria included: age 21 years or older; able to read, write, and speak English (or had a caregiver who could meet these criteria); and diagnosis of non-Hodgkin lymphoma or female breast, colorectal, non–small-cell lung, or head and neck cancer. Patients with these cancer diagnoses were identified in previous (unpublished) pilot studies to be most at risk for toxicities from high doses of chemotherapy. Exclusion criteria included prior history of cancer, psychiatric diagnoses, and concurrent clinical trial participation, because clinical trial participants may have received additional assessment beyond routine care. All patients provided informed consent and completed surveys at the second-cycle return visit. This study was approved by both the University of Michigan and St Joseph Mercy Health System Institutional Review Boards.
To inform our sample size calculations, we anticipated a 41.2% rate of chemotherapy-associated toxicity and a 19% rate of unplanned health care service use resulting from toxicity-related factors based on our previous pilot studies. With a sample size of 106, we estimated 95% CIs of 32% to 51% for toxicity occurrence and 11% to 27% for unplanned service use.11 Thus, our sample size of 106 participants enabled us to examine frequencies of toxicities and service use with reasonable precision for the study.
To minimize clinic disruptions, all recruitment activities were performed by MCRC research staff at the respective oncology clinics. Study staff reviewed the daily schedule for the outpatient infusion centers with the charge nurses each day. Patients were identified during their first cycle of chemotherapy. Staff then approached eligible study participants in or near the outpatient infusion center to seek their interest in participation on arrival for their second cycle of chemotherapy. On average, six to eight patients per day were preparing for chemotherapy treatment. If any treating physician did not want patients to participate, the patients were not approached by research staff. Recruitment continued for 9 months until the desired sample of 100 patients was reached.
Study Procedures
After obtaining informed consent from participants, research staff created a tracking database with patient name, diagnosis, and planned chemotherapy treatments and schedule. On completion of the first chemotherapy cycle, at the first regularly scheduled visit for cycle two, research staff members provided patients with a study packet consisting of: a letter written at sixth grade reading level indicating that he or she had agreed to participate in the study and describing the purpose of the study, instructions about completing the questionnaire, and a telephone number to call with questions or concerns, along with a questionnaire. Patients completed the questionnaire while waiting for their treatment to finish. All survey data were entered by research staff at the University of Michigan, and double-entry procedures assured accurate entry.
Measures
Patient surveys measured 10 common chemotherapy-associated toxicities informed by descriptors for toxicities in the PRO-CTCAE.7 The survey instrument included a patient-reported set of toxicity measures derived from the PRO-CTCAE but did not assess all domains specified for the PRO-CTCAE measure. On the basis of clinical consultation and review of the literature, 10 prevalent and clinically significant toxicities were selected for assessment: nausea, vomiting, diarrhea, constipation, mouth or throat sores, pain, intravenous catheter site problem, fever and chills, shortness of breath, and extremity edema.1,3,4,6,12-14 Patients rated severity of toxicities on a 5-point Likert scale: 1, did not occur; 2, mild compared with baseline; 3, moderate difficulty performing normal daily activities; 4, severe interference with normal daily activities; and 5, disabling. Patients also completed a visual analog scale of current distress (“How are you doing overall?”), rated from 0 to 10 (0, high distress; 10, low distress). To measure unplanned health care service use, patients reported whether they had unplanned oncologist visits, ED visits, or unplanned hospitalizations related to each type of toxicity (Data Supplement provides survey instrument).
Detailed treatment and demographic data were collected from medical record reviews performed by research staff at each site using a standardized abstraction form (Data Supplement). Clinician-documented toxicities were abstracted from progress notes and infusion notes in the medical record; toxicities were documented at providers’ discretion. Chemotherapy regimens, dosages, and supportive care medications were included. Comorbidity data were obtained from the review of systems and history and physical examination documented at the first patient visit. We selected the following comorbid conditions based on prior evidence that these conditions were most significantly associated with adverse events related to chemotherapy: diabetes, cardiac disease, pulmonary disease, hepatic disease, renal disease, and active wounds.15,16
Statistical Analysis
Descriptive statistics were calculated to describe patient demographic and clinical characteristics. To answer the first research question, descriptive statistics were computed to describe frequency and severity of patient-reported chemotherapy toxicities, as well as frequency of clinician-documented toxicities. To answer the second research question, descriptive statistics, bivariable analyses, and mixed-effects logistic regression were used to describe the frequency and correlates of unplanned service use related to toxicities. All analyses were completed using R statistical computing software (https://www.r-project.org/).
The dichotomous outcome of unplanned service use related to toxicities (yes v no) during first-cycle chemotherapy was the primary outcome. First, to identify correlates of unplanned service use for treatment-related toxicities, patients were sorted into two groups: unplanned service use (one or more unplanned visit) or no unplanned service use (zero unplanned visits). Bivariable analyses to examine correlation of the independent variables with unplanned service use were performed using logistic regression models. Variables with a marginal P value less than .2 in bivariable analyses were included in the multivariable regression model. Independent variables examined in bivariable analyses included age, sex, comorbid conditions, Karnofsky performance score, and patient-reported level of distress (patient factors); type and stage of cancer (disease factors); and treatment with anthracyclines, taxanes, trastuzumab, or colony-stimulating factor (treatment factors). The R package lme4 (https://cran.r-project.org/web/packages/lme4/index.html) was used to estimate a mixed-effects logistic regression model for the dichotomous outcome of unplanned service use, with random effects specified for each clinic to account for correlations of patients treated in the same clinics.
RESULTS
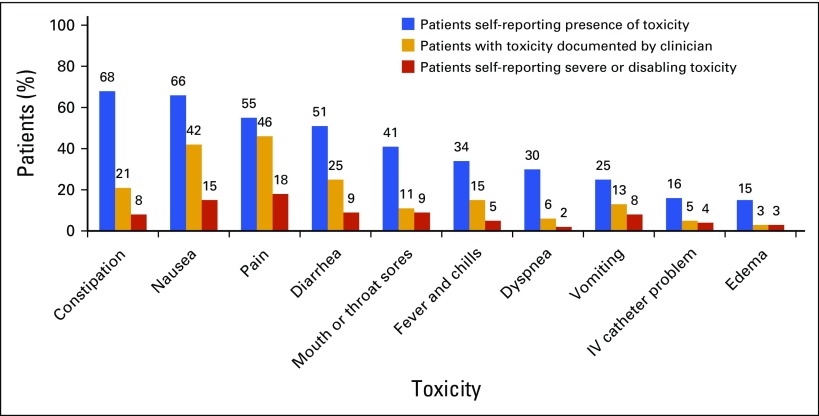
Of 117 eligible patients approached, 106 (91%) participated and completed questionnaires. No patients withdrew their consent. The sample was mostly white (98%) and female (74.5%), with a mean (± standard deviation) age of 60 (± 11) years. The most common diagnoses were breast (43%), lung (21%), and colorectal cancers (13%), followed by non-Hodgkin lymphoma (13%). Patients had varying stages of cancer, including stage I (13.2%), II (30.2%), III (25.5%), and IV or metastatic (31.1%). Chemotherapy regimens are listed in Appendix Table A1 (online only). All patients reported some degree of toxicity, rated at least mild. Among all study patients, 45% reported at least one toxicity as severe or disabling. The most frequent toxicities reported during cycle one of chemotherapy were constipation (68%), nausea (66%), pain (54%), and diarrhea (51%; Fig 1). The toxicities most commonly rated severe or disabling were pain (18%) and nausea (15%). For all 10 toxicities assessed, patient-reported toxicities were reported more frequently than clinician-documented toxicities (Fig 1). Disparities between patient-reported and clinician-documented toxicity frequency ranged from 9% (pain) to 47% (constipation).
FIG 1.
Frequency of patient-reported chemotherapy toxicities. IV, intravenous.
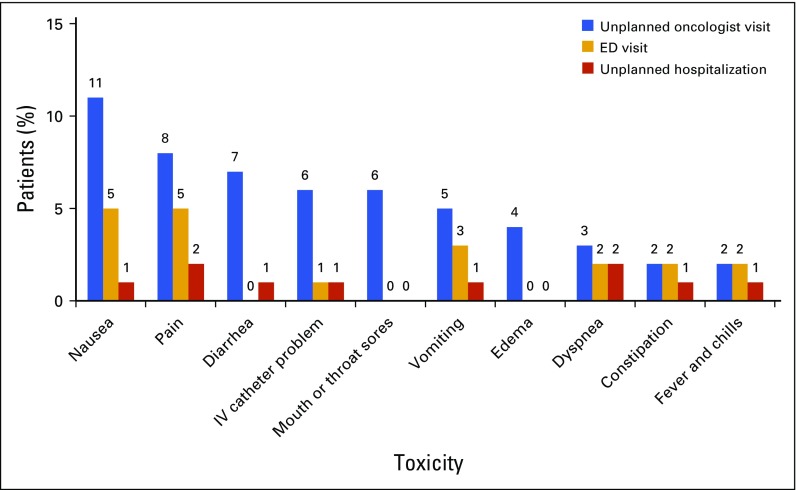
Thirty-six patients (34%) in the sample reported unplanned service use, defined as one or more additional events related to toxicities, including 31 patients (29%) with unplanned oncologist visits, 15 (14%) with ED visits, and eight (7.5%) with unplanned hospitalizations. Among the 31 patients who reported unplanned oncologist visits, the most frequent reasons for the visit were nausea (39%), pain (29%), and diarrhea (23%). Among the 15 patients who reported ED visits, the most frequent reasons for the visit were nausea (33%) and pain (33%). Among the eight patients who reported unplanned hospitalizations, pain (25%) and dyspnea (25%) were most the frequent reasons for inpatient admission. Of the 36 patients with unplanned service use, 89% reported toxicities rated severe or disabling. Frequency of unplanned service use is shown in Figure 2.
FIG 2.
Frequency of patient-reported unplanned service use for chemotherapy toxicities. ED, emergency department; IV, intravenous.
In bivariable analyses, eight of the independent variables listed previously were significantly associated with unplanned service use (P < .20): patient age, female sex, cancer stage, patient-reported level of distress (measured on a numeric 0 to 10 scale), receipt of CSF, comorbid cardiovascular disease, breast cancer, and lung cancer. These variables were entered into the multivariable logistic regression model. The final model was selected using backward selection. Age, female sex, and breast cancer were not significant and were deleted from the final model. Cancer stage was not a significant predictor but was retained in the final model as a potential confounder. Predictors in the final model included cancer stage, receipt of CSF, patient-reported distress level, cardiovascular disease, and lung cancer. Lung cancer was marginally significant (P = .06) and was retained in the final model. Three variables were significantly associated with unplanned service use (P < .05): receipt of CSF (coefficient, 1.88; SE, 0.66; P < .01), higher distress level (coefficient, 0.38; SE, 0.10; P < .001), and cardiovascular disease (coefficient, −1.74; SE, 0.80; P = .03; Table 1). Unplanned service use did not vary significantly among the five clinics (P = 1). The 58 patients (54.7%) who received CSF had the following diagnoses: breast cancer (69%), non-Hodgkin lymphoma (14%), lung cancer (7%), colon cancer (3%), head and neck cancer (2%), and other cancer (5%).
Table 1.
Factors Associated With Unplanned Service Use (N = 106)
| Factor | Bivariable Models* | Multivariable Regression Models† | ||
|---|---|---|---|---|
| Coefficient (SE) | P | Coefficient (SE) | P | |
| Sex | 0.76 (0.52) | .14 | ||
| Age | −0.03 (0.02) | .14 | ||
| Karnofsky score | 0.01 (0.38) | .97 | ||
| Pulmonary disease (COPD, asthma, sleep apnea) | 0.32 (0.51) | .53 | ||
| Hepatic disease (history of hepatitis, cirrhosis) | −0.03 (1.24) | .98 | ||
| Diabetes | 0.66 (0.54) | .22 | ||
| Received taxanes | 0.34 (0.42) | .42 | ||
| Received trastuzumab | 0.54 (0.53) | .31 | ||
| Received anthracyclines | −0.04 (0.52) | .95 | ||
| Breast cancer | 0.58 (0.41) | .16 | ||
| Colon cancer | −0.72 (0.69) | .30 | ||
| Head and neck cancer | 0.69 (1.02) | .50 | ||
| Non-Hodgkin lymphoma | −0.29 (0.63) | .65 | ||
| Lung cancer | −1.02 (0.60) | .09 | −1.60 (0.84) | .06 |
| Cardiac disease (history of myocardial infarction, angina, coronary artery disease, coronary artery bypass graft, arrhythmia, valve disorder) | −1.10 (0.67) | .10 | −1.74 (0.80) | .03 |
| Cancer stage | −0.27 (0.20) | .18 | 0.27 (0.28) | .35 |
| Received CSF | 1.33 (0.45) | < .01 | 1.88 (0.66) | < .01 |
| Distress score | −0.20 (0.07) | < .01 | 0.38 (0.10) | < .001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CSF, colony-stimulating factor.
No patients had comorbid renal disease or presence of active wound, so these variables were dropped from the model.
Random intercept for clinic not significant.
DISCUSSION
Chemotherapy-associated toxicities are considered important patient-centered indicators of quality cancer care.17 Our study addressed several issues critical to optimal delivery of patient care in community oncology settings, including the feasibility of toxicity reporting in a community oncology population and the relationship between toxicities and unplanned health care service use. With a 91% participation rate among eligible patients and a 100% survey completion rate among patients who consented to participate, this study demonstrated that brief patient surveys are a feasible method for systematic assessment of patient-reported toxicities and associated unplanned service use.
Lack of a standard template for routine assessment of toxicities underscores the need for a brief patient-reported measure to grade toxicities. Systematic, proactive symptom assessments may help clinicians manage patients’ toxicities that may otherwise not be addressed during clinic visits. Determining the frequency and severity of patient-reported, chemotherapy-related toxicities is crucial to development of clinical practice guidelines to improve care of community-based oncology patients.2,8,18 Nausea, pain, diarrhea, and constipation were reported by the majority of patients and should be anticipated and addressed early in treatment. Patients should be counseled about the type of toxicities they may experience and when to seek medical attention, even for more rare symptoms. Mouth or throat sores, vomiting, and fever and chills were reported by fewer than half of patients but were severe or disabling for 9%, 8%, and 5% of patients, respectively. Although severe toxicities warrant medical attention, routine use of symptom assessment tools may help clinicians manage toxicities between visits and address toxicities before they become severe and ED visits or hospitalizations occur.
Understanding the frequency and correlates of unplanned, toxicity-related service use is important not only for improving care but also for reducing costs in community oncology settings. In a national study of more than 3,200 patients treated for metastatic breast cancer, adverse events resulting from chemotherapy toxicities were associated with higher treatment expenditures, including both inpatient and outpatient costs.19 Unmanaged toxicities were important drivers of health care service use in our study, resulting in unplanned service use for more than one-third of patients. The finding that patients who received CSF were more likely to have unplanned service use was expected, given that patients who require CSF are immunocompromised from high doses of chemotherapy and more susceptible to infection and drug toxicities.20 Receipt of CSF may be considered a proxy measure for intensity of the chemotherapy regimen. Patients receiving CSF for neutropenia may need more frequent monitoring of chemotherapy toxicities, despite appropriate prophylactic therapy. Patient-reported level of distress was also positively correlated with unplanned service use. Toxicities are anxiety inducing for many patients, and anxiety may increase health care–seeking behaviors. Lung cancer was a marginally significant predictor of unplanned service use, which may be related to advanced cancer stage (III or IV) in nearly all of the patients with lung cancer. Unexpectedly, cardiovascular disease was negatively associated with unplanned service use. This finding may reflect clinician attention to underlying cardiovascular issues in the patients studied. Further exploration in a larger, more diverse sample is needed to determine how patient distress, comorbidities, and disease factors affect health care service use related to toxicities.
This study had some limitations, including recruitment of a convenience sample and exclusion of patients with previous cancer diagnoses. However, targeting first-time chemotherapy patients was desirable for this analysis. A broader sample of tumor types and chemotherapy regimens would generate new knowledge on toxicities, particularly for patients receiving multiple drugs. Some eligible patients refused participation. Lack of diversity was a limitation; the sample consisted predominantly of white, non-Hispanic participants. With a sample of 75% women, sex was not balanced among participants. A limitation of the survey instrument was inability to ascertain pain source, such as tumor-related pain or arthralgia related to CSF. This limitation may apply to other toxicities assessed in the survey, because patients may experience various symptoms unrelated to chemotherapy. Comorbidities were not assessed using a standardized measure such as the Charlson comorbidity index.21 In addition, toxicity reporting was not uniform across sites and providers. In their routine clinical practice, participating clinicians did not routinely grade toxicity severity. Recall bias may have affected patient survey responses, as well as clinician documentation of toxicities. Despite these limitations, this study is one of the few multisite studies based in community cancer centers that have been conducted to solicit patient-reported, chemotherapy-associated toxicities soon after the first cycle of treatment.
Chemotherapy toxicities and associated unplanned service use were prevalent in this community oncology population. With 45% of the total sample and 89% of patients with unplanned service use who reported at least one toxicity rated severe or disabling, there is a clear need for practical symptom assessment tools to evaluate patient-reported toxicities early in the course of treatment. Although improvements are needed in the management of patient toxicities, the results of this study are encouraging for future cancer care delivery research in community settings. Strategies to reduce unplanned service use may include implementation of patient-reported toxicity measures to screen for toxicities at routine clinic visits, as well as regular screening of patient-reported distress. With further exploration of the most frequent and significant toxicities in a larger sample of patients, strategies can be developed for implementation of clinical practice guidelines in community oncology practices.
Acknowledgment
Supported by pilot funds from the University of Michigan Institute for Clinical and Health Research Outreach, Partnerships, and Implementation Science Program (National Institutes of Health [NIH] Grant No. UL1TR000433) and through resources supported by the Michigan Cancer Research Consortium. The views expressed in this article are those of the authors and do not reflect the view of the NIH.
Appendix
Table A1.
Chemotherapy Regimens
| Type of Chemotherapy Received | No. of Patients (%) |
|---|---|
| Taxane (paclitaxel or docetaxel) plus platinum (carboplatin or cisplatin) | 37 (34.9) |
| Docetaxel plus cyclophosphamide | 16 (15.1) |
| FOLFOX | 13 (12.3) |
| R-CHOP | 11 (10.4) |
| Doxorubicin plus cyclophosphamide | 10 (9.4) |
| Docetaxel, cisplatin, and fluorouracil | 3 (2.8) |
| Permetrexed plus platinum (carboplatin or cisplatin) | 3 (2.8) |
| Taxane only (paclitaxel or docetaxel) | 3 (2.8) |
| Bendamustine plus rituximab | 3 (2.8) |
| Other | 7 (6.6) |
Abbreviations: FOLFOX, fluorouracil, leucovorin, and oxaliplatin; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone.
AUTHOR CONTRIBUTIONS
Conception and design: Philip J. Stella, Beth LaVasseur, Paul T. Adams, Lauren Swafford, JoAnn Lewis, Christopher R. Friese
Financial support: Kari Mendelsohn-Victor, Christopher R. Friese
Administrative support: Christopher R. Friese
Provision of study materials or patients: Philip J. Stella, Paul T. Adams, Christopher R. Friese
Collection and assembly of data: All authors
Data analysis and interpretation: Jordan M. Harrison, Christopher R. Friese
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Toxicity-Related Factors Associated With Use of Services Among Community Oncology Patients
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Jordan M. Harrison
No relationship to disclose
Philip J. Stella
Employment: Physician Resource Management
Leadership: Physician Resource Management
Stock or Other Ownership: Physician Resource Management
Beth LaVasseur
No relationship to disclose
Paul T. Adams
No relationship to disclose
Lauren Swafford
No relationship to disclose
JoAnn Lewis
No relationship to disclose
Kari Mendelsohn-Victor
No relationship to disclose
Christopher R. Friese
No relationship to disclose
References
- 1.Hassett MJ Rao SR Brozovic S, etal: Chemotherapy-related hospitalization among community cancer center patients Oncologist 16:378–387,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzyzanowska MK Treacy J Maloney B, etal: Development of a patient registry to evaluate hospital admissions related to chemotherapy toxicity in a community cancer center J Oncol Pract 1:15–19,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont EB Herndon JE II Weeks JC, etal: Measuring clinically significant chemotherapy-related toxicities using Medicare claims from Cancer and Leukemia Group B (CALGB) trial participants Med Care 46:303–308,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrischilles EA Pendergast JF Kahn KL, etal: Adverse events among the elderly receiving chemotherapy for advanced non–small-cell lung cancer J Clin Oncol 28:620–627,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain A Chen A Ivy P, etal: The importance of clinical grading of heart failure and other cardiac toxicities during chemotherapy: Updating the common terminology criteria for clinical trial reporting Heart Fail Clin 7:373–384,2011 [DOI] [PubMed] [Google Scholar]
- 6.Basch E Iasonos A McDonough T, etal: Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study Lancet Oncol 7:903–909,2006 [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute : Patient-reported outcomes version of the CTCAE (PRO-CTCAE) https://wiki.nci.nih.gov/display/PROCTCAE/Patient-Reported+Outcomes+version+of+the+CTCAE+PRO-CTCAE
- 8.Dueck AC Mendoza TR Mitchell SA, etal: Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol 1:1051–1059,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandyk AD Harrison MB Macartney G, etal: Emergency department visits for symptoms experienced by oncology patients: A systematic review Support Care Cancer 20:1589–1599,2012 [DOI] [PubMed] [Google Scholar]
- 10.Barbera L Atzema C Sutradhar R, etal: Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis Ann Emerg Med 61:427.e5–437.e5,2013 [DOI] [PubMed] [Google Scholar]
- 11.Wilson E: Probable inference, the law of succession, and statistical inference J Am Stat Assoc 22:209–212,1927 [Google Scholar]
- 12.Basch E Artz D Iasonos A, etal: Evaluation of an online platform for cancer patient self-reporting of chemotherapy toxicities J Am Med Inform Assoc 14:264–268,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basch E Jia X Heller G, etal: Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes J Natl Cancer Inst 101:1624–1632,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sikorskii A Given CW Given B, etal: Differential symptom reporting by mode of administration of the assessment: Automated voice response system versus a live telephone interview Med Care 47:866–874,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman GH Morrison VA Dale DC, etal: Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy Leuk Lymphoma 44:2069–2076,2003 [DOI] [PubMed] [Google Scholar]
- 16.Friese CR, Silber JH, Aiken LH: National Cancer Institute Cancer Center designation and 30-day mortality for hospitalized, immunocompromised cancer patients Cancer Invest 28:751–757,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider EC Malin JL Kahn KL, etal: Developing a system to assess the quality of cancer care: ASCO’s national initiative on cancer care quality J Clin Oncol 22:2985–2991,2004 [DOI] [PubMed] [Google Scholar]
- 18.Basch E: The missing voice of patients in drug-safety reporting N Engl J Med 362:865–869,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helwick C: Chemotherapy-related toxicity adds to economic burden in metastatic breast cancer http://www.ahdbonline.com/issues/2012/august-2012-vol-5-no-5-special-issue-asco-2012-payers-perspective/1079-article-1079
- 20.Metcalf D: The colony-stimulating factors and cancer Cancer Immunol Res 1:351–356,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME Charlson RE Peterson JC, etal: The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients J Clin Epidemiol 61:1234–1240,2008 [DOI] [PubMed] [Google Scholar]