Abstract
[Purpose] To determine age-related differences in the subjective vertical in the frontal plane in healthy adults. [Subjects and Methods] The subjects were 26 healthy adults. For the subjective visual vertical (SVV), subjects were presented with a visual indicator in front of them that was rotated. For the subjective postural vertical-eyes open (SPV-EO) and subjective postural vertical (SPV), subjects sat in a seating device that was tilted right or left. The subjects gave a signal when they perceived true verticality. Each task was performed eight times. The items examined were the mean (tilt direction) and standard deviation (variability) of the eight trials, then the mean of four trials that started from the right or left side position. These items were compared between the young (age: 22–30 years [range]) and elderly (age: 60–74 years) groups. [Results] As for variability, the elderly group demonstrated significantly higher values of SPV-EO and SPV. As for the starting point effect, the elderly group demonstrated greater bias toward the starting direction than did the young group in SPV-EO and SPV in frontal plane. [Conclusion] The postural vertical was shown to change with age. Consideration of age-related changes and the starting point effect was indicated to be important.
Keywords: Healthy adults, Subjective vertical, Aging
INTRODUCTION
Humans orient their bodies vertically by integrating visual, somatosensory, and vestibular system information in their gravity environment1). The important cognitive aspects related to this vertical orientation are the subjective visual vertical (SVV), the subjective postural vertical-eyes open (SPV-EO), and the subjective postural vertical (SPV)2,3,4). The mean bias demonstrating the direction of tilt (hereafter “tilt direction”) and the standard deviation demonstrating instability in the perception of verticality (hereafter “variability”) are considered important indicators of the characteristics of the above-described perceptions of verticality. These parameters have been indicated to change as a result of cerebrovascular accidents and other neurological disorders2,3,4,5). Declines in postural balance and activities of daily living are also indicated to be associated with these parameters6,7,8). Recently, SPV in the sagittal plane has been shown to deviate backward with age9). Furthermore, in a study that investigated SPV in the sagittal plane on the influence of starting direction, the characteristics of this tilt were indicated to differ in young adults and elderly adults9). Although this result indicates that already-acquired verticality perception declines with age, age-related changes in the subjective vertical and starting direction characteristics in the frontal plane have not been determined. The purpose of the present study was to determine age-related differences in the subjective vertical perception in the frontal plane in healthy adults.
SUBJECTS AND METHODS
The subjects comprised 13 young adults (age: 25.1 ± 2.3 years [mean ± SD], 22–30 years [range]; 6 men, 7 women; all right-handed) and 13 elderly adults (age: 67.0 ± 5.1 years, 60–74 years; 7 men, 6 women; all right-handed). The inclusion criteria consisted of no past history of bone and joint disease, neurological disorders, psychiatric disorders, or dementia. All subjects in the elderly group walked without a cane and had no history of falls. The present study was conducted with the approval of the Saitama Medical University International Medical Center Institutional Review Board (approval number: 14-117). Recruitment for study participation was conducted openly. All subjects received an explanation of the study and provided informed consent in writing.
SVV was measured using computer software (Fig. 1). Measurements were conducted with the subjects seated and their feet flat. Two computers were used for measurements. These two computers were linked with an USB cable so that the image displayed on the computer controlling the SVV was also displayed on the computer screen that the subjects watched. In accordance with the method described by Pavan et al.10), a cylindrical tube was placed in such a manner that the vertical portion of the screen frame could not provide clues to verticality. The subjects’ trunks were fixed, and their feet were flat. The visual indicator was at the subject’s eye level, 50 cm in front of them. Subjects watched the visual indicator through the cylindrical tube placed in front of the computer screen. The SVV was controlled as follows. The experimenter rotated the visual indicator from a horizontal position leftward or rightward toward a vertical position at a rate of 5°/s. The rotation was stopped when the subject verbally reported that he had perceived true verticality, and the deviation from true verticality was recorded. Eight trials were performed in an ABBABAAB sequence.
Fig. 1.
Assessment of subjective visual vertical. These two computers were linked with an USB cable so that the image displayed on the computer controlling the SVV was also displayed on the computer screen that the subjects watched. a cylindrical tube was placed in such a manner that the vertical portion of the screen frame could not provide clues to verticality.
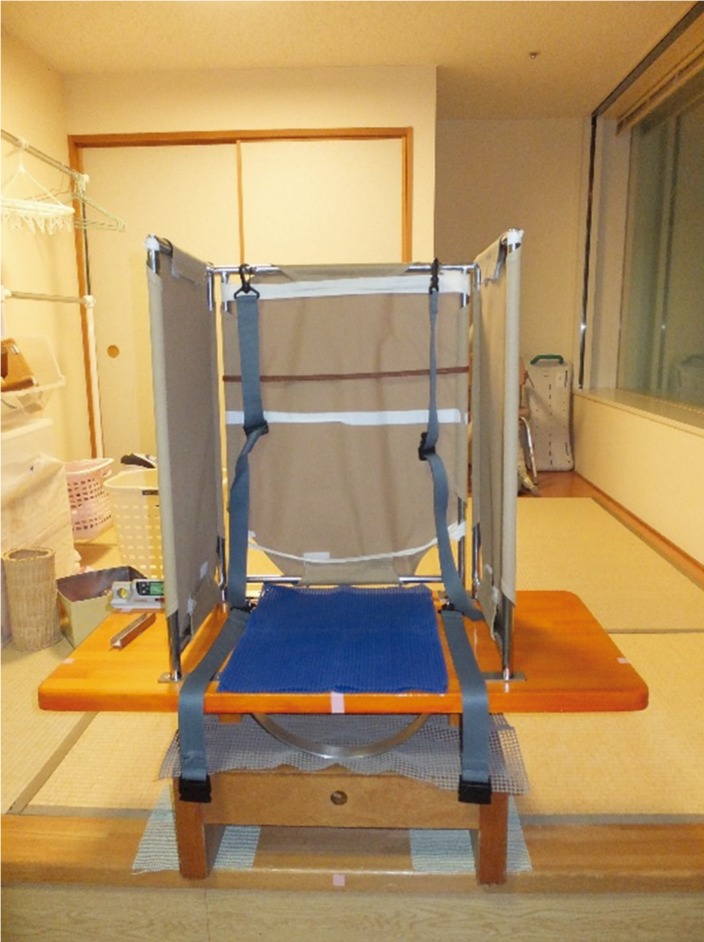
SPV-EO and SPV were measured using a vertical board (VB) that had a semicircular rail attached to the bottom (Fig. 2). The sides and backs of the subjects’ trunks were covered with non-stretchable cloth, and the subjects sat on the VB with their feet not in contact with the ground. The subjects’ trunks were fixed, their arms were crossed in front of their chest, and the positions of their heads and legs were not fixed. The VB was controlled by two experimenters. The experimenters rotated the seat of the VB from a position of 15° or 20° tilt toward a vertical position at a rate of approximately 1.5°/s. The tilt of the seat when the subjects verbally reported that he had reached a true verticality was recorded with a digital inclinometer. Eight trials were performed in an ABBABAAB sequence so that the starting position and angle would be pseudo-random. Trials for SPV-EO and SPV were conducted with the subjects’ eyes open and closed, respectively.
Fig. 2.
Measurement of subjective postural vertical and subjective postural vertical-eyes open. SPV-EO and SPV were measured using a vertical board (VB) that had a semicircular rail attached to the bottom.
A true vertical position was considered 0°, while rightward tilt and leftward tilt were treated as positive and negative, respectively. The mean (Tilt direction) and the SD (Variability) of the eight trials were calculated. In order to determine the starting point effect, means were calculated for the four trials that started from the right (Right side position) and the four trials that started from the left (Left side position), respectively.
Verticality perception parameters were compared between the young group and the elderly group using the unpaired t-test. Statistics were processed using PASW Statistics ver. 18.0 (SPSS Inc., Tokyo, Japan), with the level of significance set at 5%.
RESULTS
Results for tilt direction and variability are shown in Table 1. Significant differences in tilt direction were not observed in any parameter. In terms of variability, no difference was observed between young and elderly subjects in SVV. However, the elderly group demonstrated significantly higher variability in SPV-EO and SPV (p<0.05). Table 2 indicated results for starting point effects. In SVV, there was no difference between the groups in right and left side position, while the elderly group demonstrated significant bias against starting position in SPV-EO and SPV (p<0.05). In addition, the tilt of SPV-EO and SPV tended to occur in the starting direction.
Table 1. Results for tilt direction and variability.
| Variable | Young (n=13) | Elderly (n=13) | p value | |
|---|---|---|---|---|
| Tilt direction | ||||
| SVV | –0.3 ± 1.3 | –0.5 ± 1.8 | ||
| SPV-EO | 0.5 ± 0.5 | 0.5 ± 0.9 | ||
| SPV | 0.1 ± 0.6 | –0.1 ± 1.1 | ||
| Variability | ||||
| SVV | 0.9 ± 0.4 | 1.1 ± 0.5 | ||
| SPV-EO | 1.7 ± 0.8 | 2.9 ± 0.8 | * | |
| SPV | 1.9 ± 0.8 | 3.2 ± 1.1 | * | |
SVV: subjective visual vertical; SPV-EO: subjective postural vertical-eyes open; SPV: subjective postural vertical, *p<0.05
Table 2. Results for starting point effect.
| Variable | Young (n=13) | Elderly (n=13) | p value | |
|---|---|---|---|---|
| Right side position | ||||
| SVV | –0.7 ± 1.3 | –0.7 ± 1.6 | ||
| SPV-EO | 1.7 ± 1.2 | 2.8 ± 1.3 | * | |
| SPV | 1.6 ± 1.1 | 2.7 ± 1.5 | * | |
| Left side position | ||||
| SVV | 0.1 ± 1.2 | 0.1 ± 1.8 | ||
| SPV-EO | –0.8 ± 1.3 | –2.0 ± 1.3 | * | |
| SPV | –1.3 ± 1.2 | –2.9 ± 1.5 | * | |
SVV: subjective visual vertical; SPV-EO: subjective postural vertica-eyes open; SPV: subjective postural vertical, *p<0.05
DISCUSSION
In the present study, examinations of differences in the subjective vertical in the frontal plane between elderly adults and young adults revealed that elderly adults demonstrated significantly greater variability in SPV-EO and SPV, as well as greater bias toward the starting direction.
Mean tilt direction was not significantly different between the young and elderly groups. Pérennou et al.4) have reported that the normal range of SVV and SPV in healthy adults is −2.5° to 2.5°. While nothing was demonstrated with regard to the normal range of SPV-EO, all of the verticality perception data obtained in the present study was near perfect. Although visual, somatosensory, and vestibular system functions have long been known to decline with age, it was indicated that these declines do not bring about any specific directional abnormalities in verticality perception.
As for variability, although there was no difference between young and elderly subjects in SVV, the elderly group demonstrated significantly greater variability in SPV-EO and SPV. Saeys et al.11) investigated the association between SPV and somatosensory system function in stroke patients. They reported that deviation in SPV is related to somatosensory loss and is associated not with deep sensation, but with superficial sensation. In addition, Bisdorff et al.5) demonstrated that while SPV tilt direction is perfectly vertical in patients with vestibular disorders, SPV variability in patients with vestibular disorder is significantly greater among elderly patients. Bergmann et al.12) examined SPV in the sagittal and frontal planes during standing. They found that elderly subjects demonstrated significantly greater variability than young subjects in the sagittal plane; however, they found no difference between groups in SPV variability in the frontal plane. In the present study, the elderly group demonstrated significantly higher SPV variability while seated; this result differs from previous studies, which found no difference in variability while standing. These divergent results indicate that information processing for verticality perception differs between standing and sitting.
In examinations of verticality perception using starting direction, the young and elderly groups demonstrated no difference in SVV; however, for SPV-EO and SPV, both young and elderly subjects tilted in the starting direction. These findings on SPV-EO and SPV are consistent with the results reported by Mazibrada et al.13) reported that SPV was slightly deviated to stating position in normal subjects. This phenomenon of verticality perception tilting in the direction of postural tilt may demonstrate the Aubert effect. Barbieri et al.9) examined verticality perception in the sagittal plane by measurement of the starting direction. In young subjects, SPV tilted backward when measurement started in a backward position relative to the vertical, while SPV tilted forward when measurement started in a forward position. The phenomenon of these subjects’ perceptions of the postural vertical conforming to the starting direction was surmised as a possible manifestation of the Aubert effect. In elderly adults, however, SPV-EO and SPV were tilted backward regardless of the starting direction. In the present study, SPV-EO and SPV were tilted in the measurement starting direction regardless of age; therefore, the frontal plane was surmised to possess tilt characteristics that differ from those in the sagittal plane. Additionally, the greater bias toward the starting direction in the elderly group than in the young group may have been due to age-related degenerative changes in vestibular and somatosensory function. The vestibular and somatosensory systems have long been indicated to have great influence on the Aubert effect14,15,16). Degenerative changes in vestibular and somatosensory function have been reported in elderly individual17, 18); thus, elderly subjects may have been more affected by the Aubert effect than young individuals. In contrast, SVV is measured in an upright position, which may have nullified the Aubert effect.
Based on the above results, tilt direction in SPV-EO and SPV were offset by left and right bias, thus indicating that it is necessary to consider different analysis methods for different starting directions. Moreover, variability in verticality perception is believed to reflect the characteristics of the starting direction. It is conceivable that this is why the elderly group demonstrated greater bias toward the starting direction than did the young group, therefore demonstrating greater variability.
The present study showed that age increases variability in the postural vertical and results in greater bias toward starting direction. This finding is considered important, as it suggests that starting direction characteristics and age-related changes should be taken into account when assessing the characteristics of verticality perception in patients with cerebrovascular accidents and other neurological disorders.
One limitation of the present study is that the sample size was small. Additionally, the subjects comprised adults aged 20–39 years and 60–79 years; therefore, the characteristics of verticality perception in adults aged 40–59 years remains unknown. Going forward, it may be necessary to include larger numbers of subjects and investigate verticality perception characteristics with respect to age.
In conclusion, the present study examined age-related differences in the subjective vertical in the frontal plane in healthy individuals. The results demonstrated that elderly adults show greater bias in the postural vertical than do young adults, suggesting that the postural vertical declines with age. We hope to use the present study as a foundation for determining the characteristics of the subjective vertical in patients with cerebrovascular accidents.
Conflict of interest
The authors declare no conflicts of interest.
Funding
No financial support was received for this study.
REFERENCES
- 1.Barra J, Marquer A, Joassin R, et al. : Humans use internal models to construct and update a sense of verticality. Brain, 2010, 133: 3552–3563. [DOI] [PubMed] [Google Scholar]
- 2.Karnath HO, Ferber S, Dichgans J: The origin of contraversive pushing: evidence for a second graviceptive system in humans. Neurology, 2000, 55: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 3.Lafosse C, Kerckhofs E, Vereeck L, et al. : Postural abnormalities and contraversive pushing following right hemisphere brain damage. Neuropsychol Rehabil, 2007, 17: 374–396. [DOI] [PubMed] [Google Scholar]
- 4.Pérennou DA, Mazibrada G, Chauvineau V, et al. : Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain, 2008, 131: 2401–2413. [DOI] [PubMed] [Google Scholar]
- 5.Bisdorff AR, Wolsley CJ, Anastasopoulos D, et al. : The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain, 1996, 119: 1523–1534. [DOI] [PubMed] [Google Scholar]
- 6.Bonan IV, Guettard E, Leman MC, et al. : Subjective visual vertical perception relates to balance in acute stroke. Arch Phys Med Rehabil, 2006, 87: 642–646. [DOI] [PubMed] [Google Scholar]
- 7.Bonan IV, Hubeaux K, Gellez-Leman MC, et al. : Influence of subjective visual vertical misperception on balance recovery after stroke. J Neurol Neurosurg Psychiatry, 2007, 78: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggio JA, Mazin SS, Alessio-Alves FF, et al. : Verticality perceptions associate with postural control and functionality in stroke patients. PLoS One, 2016, 11: e0150754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbieri G, Gissot AS, Pérennou D: Ageing of the postural vertical. Age (Dordr), 2010, 32: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavan TZ, Funabashi M, Carneiro JA, et al. : Software for subjective visual vertical assessment: an observational cross-sectional study. Rev Bras Otorrinolaringol (Engl Ed), 2012, 78: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeys W, Vereeck L, Truijen S, et al. : Influence of sensory loss on the perception of verticality in stroke patients. Disabil Rehabil, 2012, 34: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann J, Kreuzpointner MA, Krewer C, et al. : The subjective postural vertical in standing: reliability and normative data for healthy subjects. Atten Percept Psychophys, 2015, 77: 953–960. [DOI] [PubMed] [Google Scholar]
- 13.Mazibrada G, Tariq S, Pérennou D, et al. : The peripheral nervous system and the perception of verticality. Gait Posture, 2008, 27: 202–208. [DOI] [PubMed] [Google Scholar]
- 14.Bronstein AM, Yardley L, Moore AP, et al. : Visually and posturally mediated tilt illusion in Parkinson’s disease and in labyrinthine defective subjects. Neurology, 1996, 47: 651–656. [DOI] [PubMed] [Google Scholar]
- 15.Dichgans J, Brandt T, Held R: The role of vision in gravitational orientation. Fortschr Zool, 1975, 23: 255–263. [PubMed] [Google Scholar]
- 16.Lechner-Steinleitner S: Interaction of labyrinthine and somatoreceptor inputs as determinants of the subjective vertical. Psychol Res, 1978, 40: 65–76. [DOI] [PubMed] [Google Scholar]
- 17.Bruce MF: The relation of tactile thresholds to histology in the fingers of elderly people. J Neurol Neurosurg Psychiatry, 1980, 43: 730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenhall U, Rubin W: Degenerative changes in the human vestibular sensory epithelia. Acta Otolaryngol, 1975, 79: 67–80. [DOI] [PubMed] [Google Scholar]