Abstract
Until recently, the predominant pathology of chronic pelvic pain conditions was thought to reside in the peripheral tissues. However, mounting evidence from neuroimaging studies suggests an important role of the central nervous system in the pathogenesis of these conditions. In the present cross-sectional study, proton magnetic resonance spectroscopy (1H-MRS) of the brain was conducted in female patients with urologic chronic pelvic pain syndrome (UCPPS) to determine if they exhibit abnormal concentrations of brain metabolites (e.g. those indicative of heightened excitatory tone) in regions involved in the processing and modulation of pain, including the anterior cingulate cortex (ACC) and the anterior and posterior insular cortices. Compared to a group of age-matched healthy subjects, there were significantly higher levels of choline (p = 0.006, uncorrected) in the ACC of UCPPS patients. ACC choline levels were therefore compared with the region's resting functional connectivity to the rest of the brain. Higher choline was associated with greater ACC-to-limbic system connectivity in UCPPS patients, contrasted with lower connectivity in controls (i.e. an interaction). In patients, ACC choline levels were also positively correlated with negative mood. ACC γ-aminobutyric acid (GABA) levels were lower in UCPPS patients compared with controls (p = 0.02, uncorrected), but this did not meet statistical correction for the 4 separate regional comparisons of metabolites. These results are the first to uncover abnormal GABA and choline levels in the brain of UCPPS patients compared to controls. Low GABA levels have been identified in other pain syndromes and might contribute to CNS hyper-excitability in these conditions. The relationships between increased ACC choline levels, ACC-to-limbic connectivity, and negative mood in UCPPS patients suggest that this metabolite could be related to the affective symptomatology of this syndrome.
Keywords: Proton magnetic resonance spectroscopy, Interstitial cystitis, Choline, Gamma aminobutyric acid (GABA), Centralized pain, MAPP
Highlights
-
•
Chronic pelvic pain patients (UCPPS;n = 18) and healthy controls (HC;n = 20) participated
-
•
Anterior cingulate cortex (ACC) choline (Cho) levels significantly ↑ in UCPPS vs. HC.
-
•
ACC γ-aminobutyric acid (GABA) levels marginally ↓ in UCPPS vs. HC.
-
•
↑ ACC Cho related to ↑ negative mood and ↑ limbic system connectivity in UCPPS
-
•
ACC metabolic activity and functional connectivity may contribute to UCPPS pathology.
1. Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) are characterized by chronic pain in the pelvic region often accompanied by increased urinary frequency and urgency to void. These syndromes, referred to collectively here as urologic chronic pelvic pain syndrome (UCPPS) (Clemens et al., 2014, Landis et al., 2014), affect between 3 and 6% of women in the United States (Berry et al., 2011, Mathias et al., 1996) and contribute to a reduced quality of life in patients (Ayorinde et al., 2015). UCPPS was historically thought to be due solely to damage and inflammation in the pelvic region (Janicki, 2003); however, mounting evidence from neuroimaging suggests that for many UCPPS patients the pain may instead be related to alterations in the function and morphology of the central nervous system (CNS) (As-Sanie et al., 2016, Bagarinao et al., 2014, Farmer et al., 2015, Kilpatrick et al., 2014, Kleinhans et al., 2016, Kutch et al., 2017, Kutch et al., 2015, Martucci et al., 2015, Mayer et al., 2015, Woodworth et al., 2015). Similar abnormalities of the CNS occur in other chronic overlapping pain conditions that are frequently comorbid with UCPPS (Kuner and Flor, 2016, Maixner et al., 2016, Schmidt-Wilcke, 2015, Walitt et al., 2016), including fibromyalgia (FM), irritable bowel syndrome, chronic fatigue syndrome, and temporomandibular disorders.
In addition to changes in brain structure and function, neurochemical abnormalities have been identified in chronic pain conditions using proton magnetic resonance spectroscopy (1H-MRS). 1H-MRS is a non-invasive neuroimaging technique that can be used to measure concentrations of brain metabolites in vivo, such as glutamate, glutamine, choline, creatine, N-acetyl-aspartate (NAA), and γ-aminobutyric acid (GABA). For example, a recent study in a mixed sample of chronic pain patients found significantly increased Glx (i.e. glutamate + glutamine) in the anterior cingulate cortex (ACC) compared to pain-free controls (Ito et al., 2017). FM patients show increased Glx in the posterior insular cortex (pIC) (Harris et al., 2009) and decreased GABA (Foerster et al., 2012) in the anterior insular cortex (aIC) compared to healthy controls. Higher levels of Glx and lower levels of GABA were also found to be associated with increased clinical pain intensity and evoked pain sensitivity in FM patients (Foerster et al., 2012, Harris et al., 2009, Harte et al., 2013). As-Sanie et al. recently demonstrated increased insular Glx in patients with painful endometriosis, another form of chronic pelvic pain, compared to pain-free controls (As-Sanie et al., 2016). Furthermore, regional Glx levels were related to the resting functional connectivity between the insula and other brain regions. In chronic migraine patients, elevated GABA levels in the posterior cingulate cortex were associated with increased pain, but patients were studied at times when they were pain-free, so it is unclear whether ACC GABA levels differ in these patients while their pain is occurring (Aguila et al., 2016). In patients with ongoing knee osteoarthritis pain, there were negative correlations between ACC GABA levels and pain severity, but no overall differences between patients and controls (Reckziegel et al., 2016). Overall, these studies suggest that an imbalance of CNS inhibitory (i.e., GABA) and excitatory (i.e., glutamate, Glx) neurotransmission may play an important role in the pathophysiology of chronic pain (Foerster et al., 2012, Harris and Clauw, 2012, Harris et al., 2013, Harris et al., 2009, Harris et al., 2008, Harte et al., 2013, Ichesco et al., 2012, Petrou et al., 2012).
The aim of the present study was to characterize the brain metabolite status of the ACC and insula in patients with UCPPS compared to in healthy controls, and the relationship between these metabolite levels and UCPPS symptoms. In addition, we also examined whether neurochemical tone in these regions was related to resting state functional connectivity with other brain regions. We hypothesized that, similar to other chronic pain conditions, UCPPS patients would show increased Glx and decreased GABA levels in the ACC and/or insula, and that metabolite levels within and connectivity between these regions and others would be related to pain and symptoms.
2. Materials and methods
2.1. Participants
Recruitment was carried out at the University of Michigan (Ann Arbor, MI) Discovery Site of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network (Clemens et al., 2014, Landis et al., 2014). Eighteen females with UCPPS who met previously described (Landis et al., 2014) criteria to be enrolled in the parent multisite MAPP Network neuroimaging protocol (Alger et al., 2016) agreed to participate in a co-occurring, site-specific study examining the role of brain metabolites in the pathophysiology of UCPPS. Table 1 shows the frequency of comorbid conditions in our patient sample. An additional 20 females without chronic pelvic pain, who were also enrolled in the broader MAPP protocol, were recruited to undergo spectroscopy and serve as age-matched healthy controls (HC). Study procedures were approved by the University of Michigan Medical School's Institutional Review Board and all subjects gave written informed consent to participate.
Table 1.
Chronic overlapping pain conditions in UCPPS patient sample.
| n (%) | |
|---|---|
| Conditions | |
| Temporomandibular disorder | 6 (33) |
| Irritable bowel syndrome | 4 (22) |
| Fibromyalgia | 2 (11) |
| Chronic fatigue syndrome | 1 (6) |
| Number per patient | |
| 0 | 8 (44) |
| 1 | 6 (33) |
| 2 | 1 (6) |
| 3 | 3 (17) |
2.2. Clinical pain and psychological assessment
Clinical pain was assessed with the short form of the Brief Pain Inventory (BPI) which captures both pain severity and interference due to pain over the course of the previous week (Cleeland and Ryan, 1994). Participants also responded the short form of the McGill Pain Questionnaire (MPQ), which includes subscales of sensory and affective clinical pain levels (Melzack, 1987). The Hospital Anxiety and Depression Scale (HADS) was administered to obtain levels of depression and anxiety (Zigmond and Snaith, 1983). Mood over the previous week was assessed with the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988); to get a more recent indication of mood, subjects responded to a question before the scan asking them to rate their mood over the past 24 h on a scale from 0 to 10, where 0 meant “extremely good mood” and 10 meant “extremely bad mood” (Landis et al., 2014). Finally, genitourinary pain and symptoms were assessed with the Genitourinary Pain Index (GUPI) (Clemens et al., 2009).
2.3. Neuroimaging acquisition and preprocessing
2.3.1. Proton magnetic resonance spectroscopy (1H-MRS)
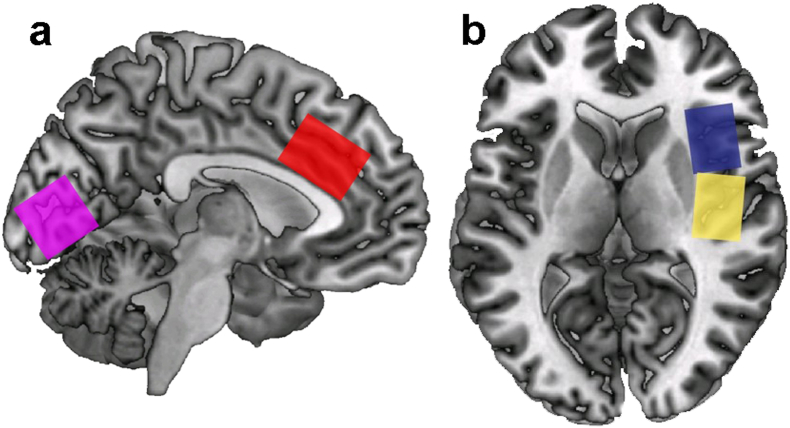
The neuroimaging scan for each participant was conducted within 48 h of the clinical and psychological visit. Brain imaging was conducted on a 3.0T scanner (Philips Achieva), using an 8-channel receiver head coil. We performed T1-weighted 3-dimensional MPRAGE imaging with an isotropic voxel resolution of 1.0 mm3. MR spectra were acquired from 3.0 × 2.0 × 3.0 cm voxels placed in the right anterior insula (aIC), right posterior insula (pIC), midline anterior cingulate cortex (ACC), and midline occipital cortex (OC). See Fig. 1. The ACC and insula were chosen for their well-described role in both acute and chronic pain (Bliss et al., 2016), and previous findings from our lab and others showing altered metabolites in these regions in chronic pain patients (As-Sanie et al., 2016, Cagnoli et al., 2013, Cao et al., 2016, Feraco et al., 2011, Foerster et al., 2012, Harris et al., 2013, Harris et al., 2009, Harris et al., 2008, Ito et al., 2017, Petrou et al., 2008, Petrou et al., 2012, Reckziegel et al., 2016, Valdes et al., 2010, Widerstrom-Noga et al., 2013, Zhao et al., 2017). The occipital cortex was selected as a control region. Single-voxel point-resolved spectroscopy (PRESS) spectra (time to recovery [TR]/time to echo [TE] 2000/33 ms) were acquired using VAPOR water suppression with 32 averages and a scan time of 1 min for each voxel. For the quantification of GABA, spectroscopy experiments using the Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) technique were performed, using the following parameters: TE 68 ms (TE1 15 ms, TE2 53 ms), TR 1.8 s, 256 transients of 2000 data points, spectral width 2 kHz, frequency-selective editing pulses (14 ms) applied at 1.9 ppm (the ON mode) and 7.46 ppm (the OFF mode). Refocusing was performed using the amplitude-modulated pulse GTST1203 (length 7 ms, bandwidth 1.2 kHz). The results from the conventional PRESS technique were analyzed using LCModel (Stephen Provencher, Oakville, Ontario, Canada). The MEGA-PRESS results were analyzed using in-house post-processing software in Matlab (Mathworks, Sherborn, MA, USA), with Gaussian curve fitting to the GABA and inverted N-acetylaspartate (NAA) peaks. We measured GABA relative to the NAA signal in the edited spectra (Stagg et al., 2009) to calculate a ratio based on the concentration of NAA, as generated by the MEGA-PRESS technique. After calculating this GABA:NAA ratio, we then multiplied this ratio by the NAA concentration, determined from LCModel analysis of a short-TE PRESS spectrum of the same voxel, which provides a concentration of NAA in arbitrary institutional units (AIU) relative to the values for water. Correction for cerebrospinal fluid (CSF) was performed for each voxel, using statistical parametric mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging) software. Metabolite concentrations were used for statistical analysis from the LCModel only if the Cramer-Rao bounds were < 20%, except for glutamine where a cutoff was not applied.
Fig. 1.
Voxel locations for measurements of brain metabolites. a) Sagittal view depicting the locations of the anterior cingulate cortex (red) and occipital (magenta) regions. b) Axial view depicting anterior (blue) and posterior (yellow) insula regions.
2.3.2. Resting state functional connectivity (fcMRI)
Resting state functional images were collected following the MAPP Network neuroimaging protocol (Alger et al., 2016). Sequence parameters during this scan included 30 whole brain volumes, slice thickness = 4 mm, slice gap = 0.5 mm, TR = 2000 ms, TE = 28 ms, flip angle = 77°, field of view (FOV) = 220 × 200, 2.75 × 2.75 mm in-slice voxel size, and 80 × 80 voxel matrices. Subjects were asked to keep their eyes closed during the 10-min scan. Data were preprocessed using the standard protocol in the SPM8 software through Matlab, including slice time correction, motion correction via realignment to the first volume in the series, normalization to the Montreal Neurological Institute (MNI) template, and smoothing with an 8 mm FWHM Gaussian kernel. Motion during the scan was assessed using three translational and rotational dimensions for each scan. Participants whose head motion during the functional scan exceeded ± 2 mm for translation and/or ± 1° for rotation were removed from further fcMRI analyses, which was the case for two UCPPS patients and one healthy control.
2.4. Data analysis
Pre-processed data were analyzed using the Functional Connectivity Toolbox v2.0 (CONN; available online at www.nitrc.org/projects/conn) in SPM8. The CONN toolbox utilizes a component-based noise correction method (CompCor), which increases sensitivity and selectivity and also allows for a high degree of interscan reliability (Whitfield-Gabrieli and Nieto-Castanon, 2012). A band pass filter (0.01–0.1 Hz) was used to remove high frequency noise and linear drift artifacts. Head motion, white matter, and CSF were included as regressors of no interest in first-level analyses. We assessed resting functional connectivity between the regions where metabolites were measured (i.e. 2 × 2 × 3 cm prisms placed on the already warped images) and the rest of the brain using seed-to-voxel whole brain correlations (first-level analysis).
Seed-to-voxel connectivity measures are based on correlation analyses, where Fisher z-transformed r values represent the degree of connectivity between the ROI and target region. First-level, subject-specific, connectivity maps for each seed (i.e. ACC, aIC, pIC) were obtained from the CONN toolbox and were then used in a second-level multiple regression, using metabolite levels in the seed region (e.g. ACC GABA) as covariates of interest (in separate models for each metabolite of interest) and age as a regressor of no interest. Significant clusters for fcMRI analyses were identified using a family-wise error (FWE) cluster-level significance of p < 0.05, corrected for multiple comparisons, derived from an uncorrected voxel-level threshold of p < 0.001.
Outside of the SPM-based analyses, all statistical tests were conducted using IBM SPSS Statistics 24 (Armonk, New York). Differences in clinical measures and metabolite levels between the groups were assessed using independent-samples t-tests. Pearson's correlations were utilized to test for relationships between metabolite levels and clinical variables. We were specifically interested in whether metabolites that differed significantly across groups are related to chronic pain levels, mood, and urologic symptoms. To get an indication of the degree to which patients experienced the affective dimension of pain relative to the sensory dimension of pain, affective-to-sensory ratios were calculated by dividing the MPQ affective pain score by the MPQ sensory pain score, as we have done previously (Schrepf et al., 2016). Since it measures the affective dimension of pain (controlling for variations in the intensive dimension), we consider this more a measure of affect than clinical pain in the present investigation, though it is certainly tied to both domains. Bonferroni corrections were employed to correct for multiple comparisons. The clinical measures were assessed in terms of 3 domains, pain, mood, and genitourinary symptoms, and thus corrected significance was p < 0.017. Spectroscopy data was collected in 4 separate brain regions, so the adjusted α level was 0.0125 for detecting group differences in metabolite levels.
3. Results
3.1. Pain and psychological assessment
Demographic and psychological outcomes are presented in Table 2. Groups did not differ on age. As expected, UCPPS patients scored significantly higher than controls on clinical pain and genitourinary measures. The difference between the groups in affective/mood measures was more mixed; UCPPS patients scored significantly higher on the HADS Depression and Recent Mood item. They also scored higher on the PANAS Negative subscale, but this was not significant after correction for multiple comparisons. The groups did not significantly differ in measures of anxiety or positive affect.
Table 2.
Participant demographics.
| Group means (SD) |
Inferential statistics |
||||
|---|---|---|---|---|---|
| Domains | Healthy (n = 20) | UCPPS (n = 18) | t | p | |
| Age | 34.7 (12.3) | 34.8 (11.0) | 0.03 | 0.973 | |
| Sympt Dur (yrs) | NA | 5.9 (6.5) | NA | NA | |
| Pain | GUPI – Pain | 0.2 (0.7) | 11.7 (4.7) | 10.93 | < 0.001⁎ |
| BPI Sev | 0.3 (0.6) | 4.6 (1.9) | 9.54 | < 0.001⁎ | |
| BPI Int | 0.1 (0.3) | 4.6 (2.7) | 7.31 | < 0.001⁎ | |
| MPQ Sens | 0.7 (1.9) | 10.7 (5.3) | 7.96 | < 0.001⁎ | |
| MPQ Affect | 0.1 (0.4) | 2.3 (2.8) | 3.51 | 0.001⁎ | |
| MPQ Total | 0.8 (1.9) | 13.0 (7.6) | 6.94 | < 0.001⁎ | |
| Affective | A/S Ratio | NA | 0.16 (0.19) | NA | NA |
| HADS Dep | 1.7 (2.3) | 4.7 (3.5) | 3.22 | 0.003⁎ | |
| HADS Anx | 4.9 (3.2) | 7.2 (5.3) | 1.62 | 0.114 | |
| PANAS Pos | 34.5 (8.4) | 31.9 (9.7) | 0.87 | 0.389 | |
| PANAS Neg | 13.9 (4.9) | 18.1 (7.1) | 2.18 | 0.036 | |
| Recent Mood | 2.2 (1.4) | 3.9 (2.5) | 2.65 | 0.012⁎ | |
| Genitourinary | GUPI – Urine | 0.9 (1.4) | 5.9 (3.2) | 6.48 | < 0.001⁎ |
Note: Sympt Dur = symptom duration; BPI = Brief Pain Inventory; Sev = severity; Int = interference; MPQ = McGill Pain Questionnaire; Sens = sensory subscore; Affect = affective subscore; A/S Ratio = affective-to-sensory ratio; HADS = Hospital Anxiety and Depression Scales; Dep = depression; Anx = anxiety; PANAS = Positive and Negative Affect Schedule; GUPI = Genitourinary Pain Index; bold = meets uncorrected statistical threshold of p < 0.05.
Meets correction for multiple comparisons (i.e. p < 0.017).
3.2. 1H–MRS
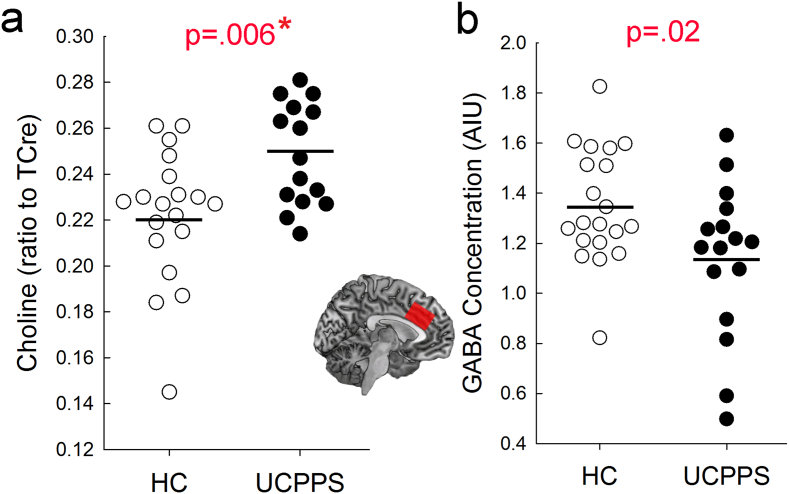
UCPPS patients, compared to HCs, exhibited significantly higher choline (UCPPS Cho:TCre = 0.25 ± 0.02) vs. HC Cho:TCre = 0.22 ± 0.03, p = 0.006) and lower GABA (UCPPS GABA = 1.14 ± 0.31 vs. HC GABA = 1.35 ± 0.23, p = 0.02) levels in the ACC (Fig. 2, Table 3), but the GABA finding does not meet statistical correction for multiple comparisons. ACC GABA was unusable from two UCPPS patients due to very poor spectra, meaning these comparisons included data from 20 controls and 16 patients. All of the GABA values of the remaining subjects had Cramer-Rao bounds < 20%. There were no significant differences between groups in gray matter, white matter, or CSF volumetric proportions within the voxel of interest. GABA levels were also not correlated with tissue proportions. Contrary to our hypothesis, there were no differences between the groups in any of the other metabolite levels, except Glu in the pIC (which was not statistically significant after correction for multiple comparisons), in any other brain region examined (Supplemental Table 1, Supplemental Table 2, Supplemental Table 3). Although LCModel provides estimates of metabolite levels for both Gln and Glu, the substantial overlap between these spectra prohibits their distinction with the pulse sequence we used. Thus, without a significant difference between the groups in pIC Glx levels along with the Glu result, we are unable to properly assess the meaning of this finding.
Fig. 2.
Anterior cingulate cortex (ACC) metabolite levels in healthy controls (HC) and urologic chronic pelvic pain syndrome (UCPPS) patients. a) Plot depicting individual choline levels relative to total creatine (TCre) in the two groups. b) Plot depicting individual GABA levels in the two groups in arbitrary institutional units (AIU). Open circles are healthy controls and closed circles represent patients. Horizontal lines represent mean metabolite levels within a group. P values from independent samples t-tests are shown above each figure. * indicates that the result survives correction for multiple comparisons (i.e. p < 0.0125).
Table 3.
Anterior cingulate cortex (ACC) metabolite levels.
| Group means (StdDev) |
Inferential statistics |
||||
|---|---|---|---|---|---|
| Metabolite | Control | UCPPS | df | t | p |
| GABA | 1.35 (0.23) | 1.14 (0.31) | 34 | 2.38 | 0.02 |
| Cho | 1.25 (0.17) | 1.38 (0.14) | 32 | − 2.27 | 0.03 |
| Cho:TCre | 0.22 (0.03) | 0.25 (0.02) | 32 | − 2.95 | 0.006⁎ |
| Glx | 7.50 (0.94) | 7.33 (1.34) | 36 | 0.47 | 0.64 |
| Glx:TCre | 1.33 (0.16) | 1.32 (0.22) | 36 | 0.25 | 0.81 |
| Gln | 1.33 (0.47) | 1.37 (0.51) | 36 | − 0.28 | 0.78 |
| Gln:TCre | 0.23 (0.08) | 0.25 (0.10) | 36 | − 0.52 | 0.61 |
| Glu | 6.18 (0.81) | 5.96 (1.35) | 36 | 0.62 | 0.53 |
| Glu:TCre | 1.10 (0.15) | 1.07 (0.23) | 36 | 0.49 | 0.63 |
| NAA | 6.97 (0.55) | 6.79 (0.75) | 36 | 0.85 | 0.40 |
| NAA:TCre | 1.24 (0.11) | 1.22 (0.11) | 36 | 0.58 | 0.65 |
| Cre | 2.70 (0.47) | 2.70 (0.52) | 34 | − 0.01 | 0.99 |
| Cre:TCre | 0.48 (0.07) | 0.48 (0.07) | 34 | − 0.18 | 0.86 |
| Ins | 4.67 (0.54) | 4.71 (0.58) | 36 | − 0.21 | 0.84 |
| Ins:TCre | 0.83 (0.09) | 0.85 (0.11) | 36 | − 0.65 | 0.52 |
Note: All metabolites from the regular PRESS sequence are cerebrospinal fluid (CSF)-corrected, or presented as a ratio to total creatine (TCre). Data were discarded on a test-by-test basis if the Cramer-Rao bounds were > 20, except in the case of glutamine (Gln), where a cutoff was not applied. GABA = gamma aminobutyric acid; Cho = choline; Glx = glutamate + glutamine; Glu = glutamate; NAA = N-acetylaspartate; Cre = Creatine; Ins = Inositol; bold = meets uncorrected statistical threshold of p < 0.05.
Meets correction for multiple comparisons (i.e. p < 0.0125).
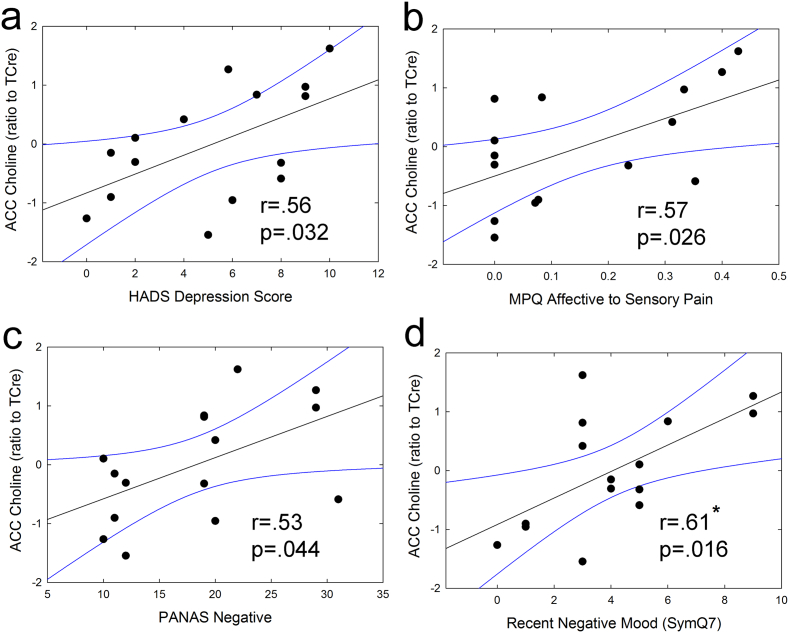
In the patient group, ACC choline levels were found to be positively correlated with several measures of negative mood, including HADS Depression (r = 0.56, p = 0.032), PANAS Negative Affect (r = 0.53, p = 0.044), and negative mood during the 24 h pre-scan (r = 0.61, p = 0.016). ACC choline was also positively correlated with greater affective-to-sensory pain (r = 0.57, p = 0.026). See Fig. 3. There were no correlations between ACC metabolite levels and psychosocial variables in healthy controls, and no correlations between ACC GABA levels and any clinical outcomes in either group.
Fig. 3.
Correlations between ACC choline levels and mood-related psychosocial measures, including a) HADS Depression, b) MPQ affective-to-sensory pain, c) PANAS Negative, and d) recent negative mood (past 24 h) in UCPPS patients. As negative mood and affective pain (relative to sensory pain) scores increased, ACC choline levels increased. Metabolite levels were corrected for age. * indicates that the result survives correction for multiple comparisons (i.e. p < 0.017).
3.3. fcMRI
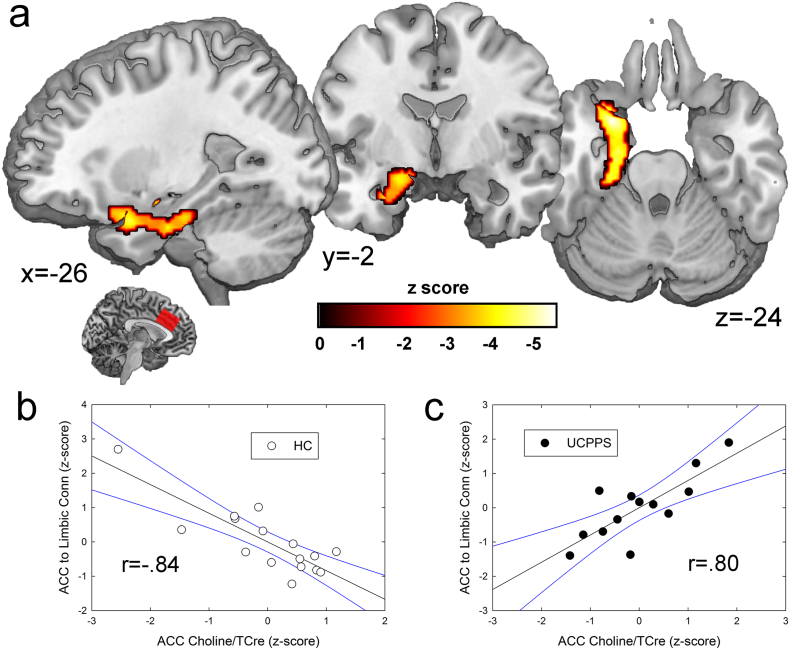
Given the significant differences in ACC choline levels between groups, connectivity between the ACC spectroscopy voxel and the rest of the brain was analyzed. There were no significant overall differences in the resting connectivity of the ACC voxel across the groups. However, choline levels were differentially (i.e. based on a seed-to-whole-brain interaction conducted in SPM) associated with ACC connectivity depending on group to a large cluster in the left hemisphere that was situated mostly along the parahippocampal gyrus and included the amygdala. The result showed that as ACC choline levels increased in HC participants, ACC connectivity to these limbic sites decreased. The opposite relationship was observed in UCPPS patients: as ACC choline levels increased, ACC connectivity to the limbic system increased. See Fig. 4 and Table 4.
Fig. 4.
Correlation between anterior cingulate cortex (ACC) choline levels and ACC spectroscopy voxel connectivity. (a) In urologic chronic pelvic pain (UCPPS) patients, as choline levels increased, connectivity between the ACC spectroscopy seed region and the depicted cluster, which encompasses the left amygdala and parahippocampal gyrus, increased. In controls, higher choline levels meant decreased connectivity between the ACC and this cluster. These results are significant at a familywise error-corrected (pFWE) value of p < 0.05, derived from an uncorrected voxel-wise p value < 0.001. (b) The scatterplot shows the amount of connectivity for each healthy control participant plotted against their ACC choline levels. (c) Scatterplot for UCPPS patients. ACC choline and connectivity values were corrected for age. Best-fit linear functions are depicted for each group, with 95% confidence intervals shown in blue. The r values depicted in the figure were obtained from SPSS-derived Pearson's correlations between the age-corrected variables. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Significant correlations between resting functional connectivity of a region and its metabolite level.
| Brain region | Coordinates (MNI) | Peak voxel (Z) | Finding | Cluster size (mm3) | Significance (pFWE) |
|---|---|---|---|---|---|
| ACC connectivity related to ACC Choline levels | |||||
| PHG, Amygdala | − 26,6,− 24 | 4.20 | UCPPS > HC | 3,848 | 0.001⁎ |
| − 24,− 12,− 30 | 4.06 | ||||
| − 18,− 6,18 | 3.75 | ||||
| pIC connectivity related to pIC Glx levels | |||||
| ACC | 10,36,34 | 4.35 | UCPPS > HC | 2,344 | 0.008⁎ |
| 0,38,30 | 3.61 | ||||
| − 6,44,20 | 3.19 | ||||
Note: MNI = Montreal Neurological Institute; ACC = Anterior cingulate cortex; PHG = parahippocampal gyrus; UCPPS = urologic chronic pelvic pain syndrome; HC = healthy controls; pIC = posterior insular cortex; pFWE = familywise error corrected cluster-level significance was set at p < 0.05, derived from voxel-level threshold set at p < 0.001 uncorrected.
Statistically significant following correction for multiple comparisons.
3.4. fcMRI exploratory analysis
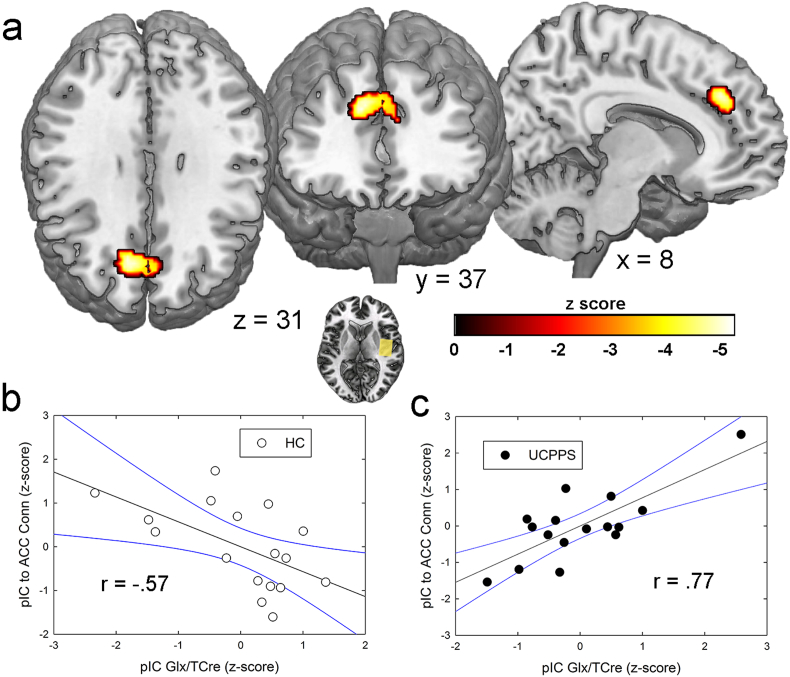
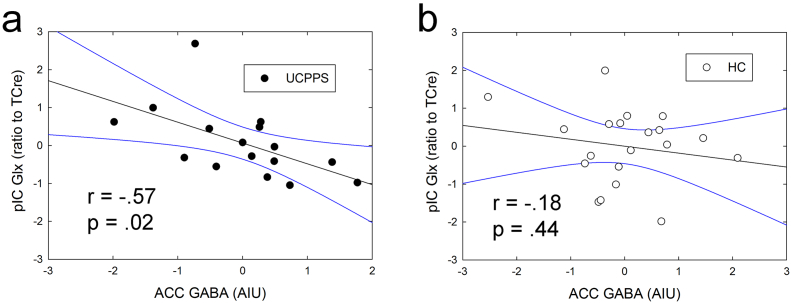
As an exploratory analysis, we examined whether Glx levels in either of the two insula voxels were related to different functional connectivity patterns in the two groups. Although Glx levels in the pIC were not significantly different between groups in the present study, our previous research has shown that heightened insular excitatory tone is associated with centralized chronic pain and its responsiveness to centrally targeted pharmacologic treatment (Harris et al., 2013). This analysis showed that pIC Glx levels were positively correlated with pIC connectivity to a cluster within the ACC spectroscopy voxel region. See Fig. 5 and Table 4. It should be noted that this ACC region was not specifically targeted in the analysis and was discovered in a seed-to-whole-brain analysis. In the patient group, but not in controls, pIC Glx concentrations were associated with ACC GABA levels, such that higher levels of Glx in the posterior insula were associated with lower GABA levels in the ACC. This relationship supports our hypothesis that an inhibitory:excitatory imbalance in brain chemistry is a possible mechanism of chronic pain pathology. See Fig. 6. There were no significant correlations between aIC Glx levels and that region's resting connectivity to others.
Fig. 5.
Correlations between right posterior insular cortex (pIC) connectivity and right pIC Glx levels in urologic chronic pelvic pain syndrome (UCPPS) patients compared with controls. a) Axial, coronal, and sagittal views of a cluster within the location of the anterior cingulate cortex (ACC) spectroscopy voxel whose connectivity to the right pIC was positively correlated with pIC Glx levels in patients, but negatively correlated in controls. These results are significant at a familywise error corrected (pFWE) value of p < 0.05, derived from an uncorrected voxelwise p value < 0.001. b) Scatterplot depicting correlation between pIC Glx levels and connectivity in healthy controls (HC). c) Relationship between Glx and connectivity in UCPPS patients. pIC Glx levels were corrected for age. Black lines represent linear association and blue lines depict 95% confidence intervals. The r values depicted in the figure were obtained from SPSS-derived Pearson's correlations between the age-corrected variables. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Correlation between ACC GABA levels and pIC Glx levels. ACC GABA levels were negatively correlated with pIC Glx levels in UCPPS patients (a), but not in controls (b). Metabolite levels were both corrected for age. These results were obtained in SPSS analyses. The r and p values were derived from Pearson's correlations between the variables. Due to the exploratory nature of this analysis, we have not used a statistical correction for multiple comparisons.
4. Discussion
In the present study, we were interested in determining whether brain metabolite levels in several pain processing regions differ in UCPPS patients and controls. Additionally, we assessed whether regional metabolite levels related to functional connectivity of these regions. The results showed lower GABA and higher choline levels in the ACC of UCPPS patients, compared to age- and gender-matched control participants. In patients, higher choline levels were related negative mood and higher ACC-to-limbic system connectivity. In addition, posterior insula Glx levels were related to higher pIC-to-ACC connectivity in patients compared to controls, in a region where significantly different metabolite levels were detected across groups. The present results add to the growing body of literature indicating central abnormalities in chronic pelvic pain and are the first to show possible deficits in inhibitory neurotransmission in UCPPS.
4.1. Evidence for pain centralization in pelvic pain patients
The evidence for centralized components of chronic pelvic pain has been provided by brain neuroimaging studies, many from the MAPP Research Network, showing significant structural and functional anomalies in patients including white and gray matter differences (Bagarinao et al., 2014, Farmer et al., 2015, Huang et al., 2016, Kairys et al., 2015, Woodworth et al., 2015), altered resting state functional connectivity (Kilpatrick et al., 2014, Kleinhans et al., 2016, Kutch et al., 2017, Kutch et al., 2015, Martucci et al., 2015), and heightened pain-evoked brain activity in and functional connectivity between pain-processing brain regions (Deutsch et al., 2016, Kleinhans et al., 2016). Furthermore, quantitative sensory testing has demonstrated widespread hyperalgesia, allodynia, and impaired endogenous analgesia in individuals with chronic pelvic pain, including UCPPS, (As-Sanie et al., 2016, Clauw et al., 1997, Grinberg et al., 2017, Iacovides et al., 2015, Ness et al., 2014, Ness et al., 2005, Powell-Boone et al., 2005, Slater et al., 2015, Stratton et al., 2015), which also strongly suggest involvement of the central nervous system. Altered levels of brain metabolites have been observed in chronic pain patients with endometriosis (As-Sanie et al., 2016) and in other non-pelvic pain conditions (Aguila et al., 2016, Cao et al., 2016, Chang et al., 2013, Fayed et al., 2010, Feraco et al., 2011, Foerster et al., 2012, Grachev et al., 2000, Harris and Clauw, 2012, Harris et al., 2013, Harris et al., 2009, Harris et al., 2008, Ito et al., 2017, Petrou et al., 2008, Petrou et al., 2012, Reckziegel et al., 2016, Valdes et al., 2010, Widerstrom-Noga et al., 2013, Zhao et al., 2017), but the present study is the first to test for these factors in UCPPS.
4.2. Metabolic changes in UCPPS
4.2.1. GABA
GABA is the brain's primary inhibitory neurotransmitter. Resting GABA levels have been shown, using spectroscopy in humans, to be negatively correlated with visual-evoked brain activity, suggesting that lower GABA levels may be associated with increased regional excitability (Muthukumaraswamy et al., 2009). GABA could likewise be important for attenuating central activations from pain, as the production of higher GABA concentrations the insula can cause analgesia to experimental pain in animals (Jasmin et al., 2003). Since decreases in GABA are associated with local excitability and targeted increases in GABA can cause reductions in pain, pain centralization (i.e. central nervous system changes that promote chronic pain) in some individuals could be due in part to reductions in inhibitory neurotransmission in pain-processing brain regions. Consistent with this idea, lower GABA levels have been identified in the anterior insula of patients with fibromyalgia (Foerster et al., 2012).
In the present study, we found lower concentrations of GABA in the ACC of UCPPS patients, which we broadly interpret as a deficit in inhibitory neurotransmission in this area. However, ACC GABA levels were not significantly correlated with resting functional connectivity of the ACC voxel region, nor were they associated with any clinical measures in the patient group. Therefore, it is unclear what effects (if any) that these reduced GABA levels have on the condition of those with UCPPS. It may be that lower GABA levels are reflective of a general increase in functional connections of this region to others, but none specific and strong enough to have withstood the stringent statistical correction for multiple comparisons we employed in this study.
4.2.2. Choline
Choline functions in the brain as part of cell's phospholipid bilayer and is more prevalent in white matter, due to its presence in myelin, compared to gray matter. High choline levels are thought to reflect injury and repair of myelin sheaths, and choline levels have been shown to increase following mild traumatic brain injury (Ross et al., 1998, Yeo et al., 2011). Elevated choline has been detected not only following mechanical head injuries, but also in a host of other conditions including bipolar disorder (Cao et al., 2016, Galinska-Skok et al., 2016), chronic fatigue syndrome (Chaudhuri et al., 2003, Puri et al., 2002), hepatitis C infection (Forton et al., 2002, Grover et al., 2012), and chronic pain (Widerstrom-Noga et al., 2013). Choline is also a glial metabolite, and thus higher levels may also reflect neuroinflammation and neuroimmune response (Chang et al., 2013). Using positron emission tomography (PET), higher levels of a glial metabolite that is thought to be a marker of neuroinflammation have been shown in chronic pain patients (Loggia et al., 2015). We previously found significantly higher variability of choline levels in the prefrontal cortex of FM patients compared to controls, and a positive correlation between this metabolite and clinical pain scores in FM patients (Petrou et al., 2008).
In the present study we found significantly elevated levels of choline in the ACC of UCPPS patients. The lack of significant differences between the groups in choline levels in the other spectroscopy regions suggests that this is a regionally specific difference that is not reflective of an overall higher brain metabolism or inflammatory response in patients. Instead, the regional specificity of this (and the GABA) finding, suggest that localized metabolic differences exist in the brains of UCPPS patients in the midACC, a region that is known to be important for processing pain and affect, among additional non-pain related functions (Fuchs et al., 2014, Shackman et al., 2011).
The correlations between choline levels and negative mood in the patient group suggest that this metabolite may be a marker for negative mood in patients. Furthermore, the correlation between choline levels in the ACC and the degree of functional connectivity between the ACC voxel and regions important for emotional processing (including the amygdala and parahippocampal gyrus (Frank et al., 2014)), suggests that the two may be causally related. A meta-analysis of literature on clinical depression found that the left amygdala and parahippocampal gyrus are two of the regions that most commonly show significant functional connectivity differences in depressed patients (Palmer et al., 2014). While speculative, it could be that higher choline levels are associated with changes in membrane structure in the ACC, including alterations in membrane channel properties or numbers, leading to increased ACC excitatory output to limbic regions and increased negative mood.
4.2.3. Glutamate and Glx
Previous studies in various chronic pain conditions have found altered levels of glutamatergic metabolites in the brain, but we did not observe any significant differences between the groups in Glx concentrations in the present study. One potential explanation of the discrepancy is that UCPPS patients differ in their degree of pain centralization, meaning for some the pain is more peripherally driven and is not the result of central abnormalities like increased insular Glx. If aberrant Glx is a marker of centralized pain as suggested by studies in a predominantly centralized condition (i.e. FM), then a group of UCPPS patients with more heterogeneous pain mechanisms might not significantly differ from controls. Alternatively, Glx levels might not be significantly different across groups in a brain region, while still exerting differential effects depending on the strength of functional connections to other brain regions. Our data are in line with the latter proposition, since high pIC Glx levels were associated with stronger connectivity to the ACC in UCPPS patients, while high Glx resulted in less pIC-to-ACC connectivity in controls. This and the differential effect of ACC choline levels on ACC connectivity depending on clinical status both suggest that brain metabolite levels are not only important in terms of helping detect regional differences in brain chemistry, but also in terms of their effects on the myriad networks with which neural signals from (or to) a brain region are shared. Future spectroscopic studies of chronic pain patients should therefore employ additional functional imaging techniques when possible, since understanding how brain chemistry affects connectivity (and vice versa) can aid in discovering new markers of centralized pain in patients.
4.3. Conclusions
UCPPS patients presented with significantly higher choline and lower GABA in the ACC, compared to healthy controls. ACC choline levels, given their difference between the groups, their relationship to negative mood and symptoms, and their association with functional connectivity, may represent an important marker of centralized changes in UCPPS. More research is needed to replicate and expand these findings. Future questions of interest include whether these metabolic changes and their relationships with functional connectivity are present across a variety of chronic pain conditions or if they are specific to UCPPS, and to what extent the differences are attributable to mood differences between groups, rather than clinical pain.
The following are the supplementary data related to this article.
Anterior insula metabolite levels.
Posterior insula metabolite levels.
Occipital cortex metabolite levels.
Acknowledgments
Acknowledgements
Authors Harper, Ichesco, Schrepf, Halvorson, and Puiu have no financial relationships to disclose. Dr. Clauw has consulted for Forest Laboratories, Pfizer, Inc., Cerephex Corporation, Eli Lilly and Company, Merck & Co., Nuvo Research Inc., Tonix Pharmaceuticals, Johnson & Johnson, Pierre Fabre, Cypress Biosciences, Wyeth Pharmaceuticals, UCB, AstraZeneca, Jazz Pharmaceuticals, Abbott Laboratories, and Iroko Pharmaceuticals. Dr. Harris has received research funding from and has consulted for Pfizer, Inc. Dr. Harte has received research funding from Aptinyx, Cerephex, Eli Lily, Forest Laboratories, and Merck; and served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics.
This MAPP Research Network study was funded by National Institutes of Health (NIH) (DK082345). Dr. Harper is and has been supported by grants from the National Institutes of Health (K99-DE026810, awarded to DEH; K12-DE023574, awarded to DJC).
References
- Aguila M.R., Rebbeck T., Leaver A.M., Lagopoulos J., Brennan P.C., Hubscher M., Refshauge K.M. The association between clinical characteristics of migraine and brain GABA levels: an exploratory study. J. Pain. 2016;17(10):1058–1067. doi: 10.1016/j.jpain.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Alger J.R., Ellingson B.M., Ashe-McNalley C., Woodworth D.C., Labus J.S., Farmer M., Huang L., Apkarian A.V., Johnson K.A., Mackey S.C., Ness T.J., Deutsch G., Harris R.E., Clauw D.J., Glover G.H., Parrish T.B., Hollander J., Kusek J.W., Mullins C., Mayer E.A. Multisite, multimodal neuroimaging of chronic urological pelvic pain: methodology of the MAPP research network. Neuroimage Clin. 2016;12:65–77. doi: 10.1016/j.nicl.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As-Sanie S., Kim J., Schmidt-Wilcke T., Sundgren P.C., Clauw D.J., Napadow V., Harris R.E. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J. Pain. 2016;17(1):1–13. doi: 10.1016/j.jpain.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayorinde A.A., Macfarlane G.J., Saraswat L., Bhattacharya S. Chronic pelvic pain in women: an epidemiological perspective. Women's Health (Lond. Engl.) 2015;11(6):851–864. doi: 10.2217/whe.15.30. [DOI] [PubMed] [Google Scholar]
- Bagarinao E., Johnson K.A., Martucci K.T., Ichesco E., Farmer M.A., Labus J., Ness T.J., Harris R., Deutsch G., Apkarian A.V., Mayer E.A., Clauw D.J., Mackey S. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP Network Study. PAIN®. 2014;155(12):2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S.H., Elliott M.N., Suttorp M., Bogart L.M., Stoto M.A., Eggers P., Nyberg L., Clemens J.Q. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J. Urol. 2011;186(2):540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L., Kaang B.K., Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016;17(8):485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- Cagnoli P., Harris R.E., Frechtling D., Berkis G., Gracley R.H., Graft C.C., Lowe S.E., Chenevert T.L., McCune W.J., Gebarski S., Sundgren P.C. Reduced insular glutamine and N-acetylaspartate in systemic lupus erythematosus: a single-voxel (1)H-MR spectroscopy study. Acad. Radiol. 2013;20(10):1286–1296. doi: 10.1016/j.acra.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Stanley J.A., Selvaraj S., Mwangi B., Passos I.C., Zunta-Soares G.B., Soares J.C. Evidence of altered membrane phospholipid metabolism in the anterior cingulate cortex and striatum of patients with bipolar disorder I: a multi-voxel (1)H MRS study. J. Psychiatr. Res. 2016;81:48–55. doi: 10.1016/j.jpsychires.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Chang L., Munsaka S.M., Kraft-Terry S., Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J. NeuroImmune Pharmacol. 2013;8(3):576–593. doi: 10.1007/s11481-013-9460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A., Condon B.R., Gow J.W., Brennan D., Hadley D.M. Proton magnetic resonance spectroscopy of basal ganglia in chronic fatigue syndrome. Neuroreport. 2003;14(2):225–228. doi: 10.1097/00001756-200302100-00013. [DOI] [PubMed] [Google Scholar]
- Clauw D.J., Schmidt M., Radulovic D., Singer A., Katz P., Bresette J. The relationship between fibromyalgia and interstitial cystitis. J. Psychiatr. Res. 1997;31(1):125–131. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- Cleeland C.S., Ryan K.M. Pain assessment: global use of the brief pain inventory. Ann. Acad. Med. Singap. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Clemens J.Q., Calhoun E.A., Litwin M.S., McNaughton-Collins M., Kusek J.W., Crowley E.M., Landis J.R. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74(5):983–987. doi: 10.1016/j.urology.2009.06.078. (quiz 987.e981-983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J.Q., Mullins C., Kusek J.W., Kirkali Z., Mayer E.A., Rodriguez L.V., Klumpp D.J., Schaeffer A.J., Kreder K.J., Buchwald D., Andriole G.L., Lucia M.S., Landis J.R., Clauw D.J., Group MRNS The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G., Deshpande H., Frolich M.A., Lai H.H., Ness T.J. Bladder distension increases blood flow in pain related brain structures in subjects with interstitial cystitis. J. Urol. 2016;196(3):902–910. doi: 10.1016/j.juro.2016.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer M.A., Huang L., Martucci K., Yang C.C., Maravilla K.R., Harris R.E., Clauw D.J., Mackey S., Ellingson B.M., Mayer E.A., Schaeffer A.J., Apkarian A.V., Network M.R. Brain white matter abnormalities in female interstitial cystitis/bladder pain syndrome: a MAPP network neuroimaging study. J. Urol. 2015;194(1):118–126. doi: 10.1016/j.juro.2015.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N., Garcia-Campayo J., Magallon R., Andres-Bergareche H., Luciano J.V., Andres E., Beltran J. Localized 1H-NMR spectroscopy in patients with fibromyalgia: a controlled study of changes in cerebral glutamate/glutamine, inositol, choline, and N-acetylaspartate. Arthritis Res. Ther. 2010;12(4):R134. doi: 10.1186/ar3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraco P., Bacci A., Pedrabissi F., Passamonti L., Zampogna G., Pedrabissi F., Malavolta N., Leonardi M. Metabolic abnormalities in pain-processing regions of patients with fibromyalgia: a 3T MR spectroscopy study. AJNR Am. J. Neuroradiol. 2011;32(9):1585–1590. doi: 10.3174/ajnr.A2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster B.R., Petrou M., Edden R.A., Sundgren P.C., Schmidt-Wilcke T., Lowe S.E., Harte S.E., Clauw D.J., Harris R.E. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64(2):579–583. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton D.M., Thomas H.C., Murphy C.A., Allsop J.M., Foster G.R., Main J., Wesnes K.A., Taylor-Robinson S.D. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology (Baltimore, Md) 2002;35(2):433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., Schaeffer D.J., Ball B.H., Schwarz N.F., Hussein A.A., Smart L.M., Sabatinelli D. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Fuchs P.N., Peng Y.B., Boyette-Davis J.A., Uhelski M.L. The anterior cingulate cortex and pain processing. Front. Integr. Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinska-Skok B., Konarzewska B., Kubas B., Tarasow E., Szulc A. Neurochemical alterations in anterior cingulate cortex in bipolar disorder: a proton magnetic resonance spectroscopy study (1H-MRS) Psychiatr. Pol. 2016;50(4):839–848. doi: 10.12740/PP/58749. [DOI] [PubMed] [Google Scholar]
- Grachev I.D., Fredrickson B.E., Apkarian A.V. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Grinberg K., Granot M., Lowenstein L., Abramov L., Weissman-Fogel I.A. Common pronociceptive pain modulation profile typifying subgroups of chronic pelvic pain syndromes is interrelated with enhanced clinical pain. Pain. 2017;158(6):1021–1029. doi: 10.1097/j.pain.0000000000000869. [DOI] [PubMed] [Google Scholar]
- Grover V.P., Pavese N., Koh S.B., Wylezinska M., Saxby B.K., Gerhard A., Forton D.M., Brooks D.J., Thomas H.C., Taylor-Robinson S.D. Cerebral microglial activation in patients with hepatitis C: in vivo evidence of neuroinflammation. J. Viral Hepat. 2012;19(2):e89–96. doi: 10.1111/j.1365-2893.2011.01510.x. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Clauw D.J. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci. Lett. 2012;520(2):192–196. doi: 10.1016/j.neulet.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Sundgren P.C., Pang Y., Hsu M., Petrou M., Kim S.H., McLean S.A., Gracely R.H., Clauw D.J. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58(3):903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Sundgren P.C., Craig A.D., Kirshenbaum E., Sen A., Napadow V., Clauw D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Napadow V., Huggins J.P., Pauer L., Kim J., Hampson J., Sundgren P.C., Foerster B., Petrou M., Schmidt-Wilcke T., Clauw D.J. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- Harte S.E., Clauw D.J., Napadow V., Harris R.E. Pressure pain sensitivity and insular combined glutamate and glutamine (Glx) are associated with subsequent clinical response to sham but not traditional acupuncture in patients who have chronic pain. Med. Acupunct. 2013;25(2):154–160. doi: 10.1089/acu.2013.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Kutch J.J., Ellingson B.M., Martucci K.T., Harris R.E., Clauw D.J., Mackey S., Mayer E.A., Schaeffer A.J., Apkarian A.V., Farmer M.A. Brain white matter changes associated with urological chronic pelvic pain syndrome: multisite neuroimaging from a MAPP case-control study. Pain. 2016;157(12):2782–2791. doi: 10.1097/j.pain.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovides S., Avidon I., Baker F.C. Women with dysmenorrhoea are hypersensitive to experimentally induced forearm ischaemia during painful menstruation and during the pain-free follicular phase. Eur. J. Pain (Lond. Engl.) 2015;19(6):797–804. doi: 10.1002/ejp.604. [DOI] [PubMed] [Google Scholar]
- Ichesco E., Clauw D.J., Harte S.E., Kairys A.E., Hampson J.P., Schmidt-Wilcke T., Harris R.E. Posterior insula combined glutamate and glutamineis associated with pain in fibromyalgia: a replication study. Arthritis Rheum. 2012;64(10):S350–S351. [Google Scholar]
- Ito T., Tanaka-Mizuno S., Iwashita N., Tooyama I., Shiino A., Miura K., Fukui S. Proton magnetic resonance spectroscopy assessment of metabolite status of the anterior cingulate cortex in chronic pain patients and healthy controls. J. Pain Res. 2017;10:287–293. doi: 10.2147/JPR.S123403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki T.I. Chronic pelvic pain as a form of complex regional pain syndrome. Clin. Obstet. Gynecol. 2003;46(4):797–803. doi: 10.1097/00003081-200312000-00009. [DOI] [PubMed] [Google Scholar]
- Jasmin L., Rabkin S.D., Granato A., Boudah A., Ohara P.T. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424(6946):316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Kairys A.E., Schmidt-Wilcke T., Puiu T., Ichesco E., Labus J.S., Martucci K., Farmer M.A., Ness T.J., Deutsch G., Mayer E.A., Mackey S., Apkarian A.V., Maravilla K., Clauw D.J., Harris R.E. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in patients with interstitial cystitis/painful bladder syndrome. J. Urol. 2015;193(1):131–137. doi: 10.1016/j.juro.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L.A., Kutch J.J., Tillisch K., Naliboff B.D., Labus J.S., Jiang Z., Farmer M.A., Apkarian A.V., Mackey S., Martucci K.T., Clauw D.J., Harris R.E., Deutsch G., Ness T.J., Yang C.C., Maravilla K., Mullins C., Mayer E.A. Alterations in resting state oscillations and connectivity in sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J. Urol. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Yang C.C., Strachan E.D., Buchwald D.S., Maravilla K.R. Alterations in connectivity on functional magnetic resonance imaging with provocation of lower urinary tract symptoms: a MAPP research network feasibility study of urological chronic pelvic pain syndromes. J. Urol. 2016;195(3):639–645. doi: 10.1016/j.juro.2015.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R., Flor H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016;18(1):20–30. doi: 10.1038/nrn.2016.162. [DOI] [PubMed] [Google Scholar]
- Kutch J.J., Yani M.S., Asavasopon S., Kirages D.J., Rana M., Cosand L., Labus J.S., Kilpatrick L.A., Ashe-McNalley C., Farmer M.A., Johnson K.A., Ness T.J., Deutsch G., Harris R.E., Apkarian A.V., Clauw D.J., Mackey S.C., Mullins C., Mayer E.A. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: a MAPP: research network neuroimaging study. Neuroimage Clin. 2015;8:493–502. doi: 10.1016/j.nicl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch J.J., Labus J.S., Harris R.E., Martucci K.T., Farmer M.A., Fenske S., Fling C., Ichesco E., Peltier S., Petre B., Guo W., Hou X., Stephens A.J., Mullins C., Clauw D.J., Mackey S.C., Apkarian A.V., Landis J.R., Mayer E.A., Network M.R. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158(6):1069–1082. doi: 10.1097/j.pain.0000000000000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J.R., Williams D.A., Lucia M.S., Clauw D.J., Naliboff B.D., Robinson N.A., van Bokhoven A., Sutcliffe S., Schaeffer A.J., Rodriguez L.V., Mayer E.A., Lai H.H., Krieger J.N., Kreder K.J., Afari N., Andriole G.L., Bradley C.S., Griffith J.W., Klumpp D.J., Hong B.A., Lutgendorf S.K., Buchwald D., Yang C.C., Mackey S., Pontari M.A., Hanno P., Kusek J.W., Mullins C., Clemens J.Q., Group MRNS The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia M.L., Chonde D.B., Akeju O., Arabasz G., Catana C., Edwards R.R., Hill E., Hsu S., Izquierdo-Garcia D., Ji R.R., Riley M., Wasan A.D., Zurcher N.R., Albrecht D.S., Vangel M.G., Rosen B.R., Napadow V., Hooker J.M. Evidence for brain glial activation in chronic pain patients. Brain J. Neurol. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W., Fillingim R.B., Williams D.A., Smith S.B., Slade G.D. Overlapping chronic pain conditions: implications for diagnosis and classification. J. Pain. 2016;17(9 Suppl):T93–t107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci K.T., Shirer W.R., Bagarinao E., Johnson K.A., Farmer M.A., Labus J.S., Apkarian A.V., Deutsch G., Harris R.E., Mayer E.A., Clauw D.J., Greicius M.D., Mackey S.C. The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network-a resting-state study from the MAPP research network. Pain. 2015;156(9):1755–1764. doi: 10.1097/j.pain.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S.D., Kuppermann M., Liberman R.F., Lipschutz R.C., Steege J.F. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet. Gynecol. 1996;87(3):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Gupta A., Kilpatrick L.A., Hong J.Y. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl. 1):S50–63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Edden R.A., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness T.J., Powell-Boone T., Cannon R., Lloyd L.K., Fillingim R.B. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J. Urol. 2005;173(6):1983–1987. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- Ness T.J., Lloyd L.K., Fillingim R.B. An endogenous pain control system is altered in subjects with interstitial cystitis. J. Urol. 2014;191(2):364. doi: 10.1016/j.juro.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S.M., Crewther S.G., Carey L.M., Team S.P. A meta-analysis of changes in brain activity in clinical depression. Front. Hum. Neurosci. 2014;8:1045. doi: 10.3389/fnhum.2014.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M., Harris R.E., Foerster B.R., McLean S.A., Sen A., Clauw D.J., Sundgren P.C. Proton MR spectroscopy in the evaluation of cerebral metabolism in patients with fibromyalgia: comparison with healthy controls and correlation with symptom severity. AJNR Am. J. Neuroradiol. 2008;29(5):913–918. doi: 10.3174/ajnr.A0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M., Pop-Busui R., Foerster B.R., Edden R.A., Callaghan B.C., Harte S.E., Harris R.E., Clauw D.J., Feldman E.L. Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Acad. Radiol. 2012;19(5):607–612. doi: 10.1016/j.acra.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Boone T., Ness T.J., Cannon R., Lloyd L.K., Weigent D.A., Fillingim R.B. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J. Urol. 2005;174(5):1832. doi: 10.1097/01.ju.0000176747.40242.3d. [DOI] [PubMed] [Google Scholar]
- Puri B.K., Counsell S.J., Zaman R., Main J., Collins A.G., Hajnal J.V., Davey N.J. Relative increase in choline in the occipital cortex in chronic fatigue syndrome. Acta Psychiatr. Scand. 2002;106(3):224–226. doi: 10.1034/j.1600-0447.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- Reckziegel D., Raschke F., Cottam W.J., Auer D.P. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol. Pain. 2016;12 doi: 10.1177/1744806916650690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B.D., Ernst T., Kreis R., Haseler L.J., Bayer S., Danielsen E., Bluml S., Shonk T., Mandigo J.C., Caton W., Clark C., Jensen S.W., Lehman N.L., Arcinue E., Pudenz R., Shelden C.H. 1H MRS in acute traumatic brain injury. J. Magnet. Resonan. Imaging: JMRI. 1998;8(4):829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T. Neuroimaging of chronic pain. Best Pract. Res. Clin. Rheumatol. 2015;29(1):29–41. doi: 10.1016/j.berh.2015.04.030. [DOI] [PubMed] [Google Scholar]
- Schrepf A., Harper D.E., Harte S.E., Wang H., Ichesco E., Hampson J.P., Zubieta J.K., Clauw D.J., Harris R.E. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. 2016;157(10):2217–2225. doi: 10.1097/j.pain.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater H., Paananen M., Smith A.J., O'Sullivan P., Briggs A.M., Hickey M., Mountain J., Karppinen J., Beales D. Heightened cold pain and pressure pain sensitivity in young female adults with moderate-to-severe menstrual pain. Pain. 2015;156(12):2468–2478. doi: 10.1097/j.pain.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Best J.G., Stephenson M.C., O'Shea J., Wylezinska M., Kincses Z.T., Morris P.G., Matthews P.M., Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009;29(16):5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton P., Khachikyan I., Sinaii N., Ortiz R., Shah J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet. Gynecol. 2015;125(3):719–728. doi: 10.1097/AOG.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes M., Collado A., Bargallo N., Vazquez M., Rami L., Gomez E., Salamero M. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 2010;62(6):1829–1836. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- Walitt B., Ceko M., Gracely J.L., Gracely R.H. Neuroimaging of central sensitivity syndromes: key insights from the scientific literature. Curr. Rheumatol. Rev. 2016;12(1):55–87. doi: 10.2174/1573397112666151231111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga E., Pattany P.M., Cruz-Almeida Y., Felix E.R., Perez S., Cardenas D.D., Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154(2):204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth D., Mayer E., Leu K., Ashe-McNalley C., Naliboff B.D., Labus J.S., Tillisch K., Kutch J.J., Farmer M.A., Apkarian A.V., Johnson K.A., Mackey S.C., Ness T.J., Landis J.R., Deutsch G., Harris R.E., Clauw D.J., Mullins C., Ellingson B.M. Network MR. unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: a MAPP network neuroimaging study. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo R.A., Gasparovic C., Merideth F., Ruhl D., Doezema D., Mayer A.R. A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J. Neurotrauma. 2011;28(1):1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Xu M., Jorgenson K., Kong J. Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: a systematic review. Neuroimage Clin. 2017;13:33–38. doi: 10.1016/j.nicl.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anterior insula metabolite levels.
Posterior insula metabolite levels.
Occipital cortex metabolite levels.