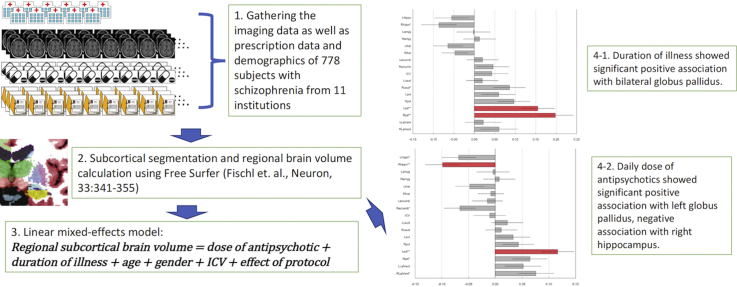
Abstract
Background
The effect of duration of illness and antipsychotic medication on the volumes of subcortical structures in schizophrenia is inconsistent among previous reports. We implemented a large sample analysis utilizing clinical data from 11 institutions in a previous meta-analysis.
Methods
Imaging and clinical data of 778 schizophrenia subjects were taken from a prospective meta-analysis conducted by the COCORO consortium in Japan. The effect of duration of illness and daily dose and type of antipsychotics were assessed using the linear mixed effect model where the volumes of subcortical structures computed by FreeSurfer were used as a dependent variable and age, sex, duration of illness, daily dose of antipsychotics and intracranial volume were used as independent variables, and the type of protocol was incorporated as a random effect for intercept. The statistical significance of fixed-effect of dependent variable was assessed.
Results
Daily dose of antipsychotics was positively associated with left globus pallidus volume and negatively associated with right hippocampus. It was also positively associated with laterality index of globus pallidus. Duration of illness was positively associated with bilateral globus pallidus volumes. Type of antipsychotics did not have any effect on the subcortical volumes.
Discussion
A large sample size, uniform data collection methodology and robust statistical analysis are strengths of the current study. This result suggests that we need special attention to discuss about relationship between subcortical regional brain volumes and pathophysiology of schizophrenia because regional brain volumes may be affected by antipsychotic medication.
Keywords: Schizophrenia, MRI, Globus pallidus, Hippocampus, Antipsychotic, Duration of illness
Graphical abstract
Highlights
-
•
The imaging data as well as prescription data and demographics from 778 patients with schizophrenia from 11 institutions were included.
-
•
The effect of protocol was cooperated as random-effect in the linear mixed-effect model.
-
•
Significant positive association were found between daily dose of antipsychotics and left globus pallidus volume.
-
•
Significant negative association was found between daily dose of antipsychotics and right hippocampus volume.
-
•
Significant positive associations were found between duration of illness and bilateral volumes of globus pallidus.
1. Introduction
Numerous systematic reviews and meta-analyses of magnetic resonance imaging (MRI) studies have shown the various regional volume changes in patients with schizophrenia (Fusar-Poli et al., 2013, Haijma et al., 2013, Ho et al., 2011, Honea et al., 2005, Huhtaniska et al., 2017b, Hulshoff Pol and Kahn, 2008, Moncrieff and Leo, 2010, Navari and Dazzan, 2009, Olabi et al., 2011, Steen et al., 2006, Torres et al., 2013, Vita et al., 2012, Woods et al., 2005, Wright et al., 2000). However, a meta-review of these review reports pointed out that the quality of evidence for morphological changes in subjects with schizophrenia is moderate due to unreliable data and inconsistent review methodology (Shepherd et al., 2012). In addition, not all collected data was fully explored in retrospective meta-analyses because of methodological inconsistency among the included studies, and the number of studies or subjects was often small in sub-group analyses such as those conducted on a particular anatomical region (Haijma et al., 2013, van Erp et al., 2016, Wright et al., 2000).
Prospective meta-analysis, which is the analysis of brain scans worldwide using standardized method, is a new attempt in this field, first adopted by ENIGMA (Enhancing Neuro Imaging Genetics by Meta-Analysis) schizophrenia Working Group (van Erp et al., 2016). In this type of study, brain scans are analyzed using standardized methods with automated imaging analysis software at each site, and summary statistics computed are collected. The large number of subjects in the analysis for a particular anatomical region, homogeneity of imaging and statistical analysis and quality assurance are particular strengths of this type of study. The first report by the ENIGMA consortium revealed that schizophrenia patients showed smaller hippocampus, amygdala, thalamus, accumbens, and intracranial volume (ICV), as well as larger globus pallidus and lateral ventricle. These findings were perfectly replicated by the second prospective meta-analysis conducted by Japanese COCORO (Cognitive Genetic Collaborative Research Organization) consortium, where the rank order and magnitude of the effect sizes for subcortical volumetric changes in schizophrenia was identical to the original (Okada et al., 2016). In addition, the study in Japan revealed schizophrenia specific leftward asymmetry for globus pallidus volume.
One major limitation of the study in Japan is that it did not take into account the effects of duration of illness and antipsychotic treatment (Okada et al., 2016). The original ENIGMA study showed that duration of illness was positively associated with putamen and globus pallidus group contrast effect sizes, and dose of antipsychotics and proportion of second generation antipsychotics were positively associated with lateral ventricle volume group contrast effect size (van Erp et al., 2016). However, their moderator analysis was implemented using meta-regression method where specific summary statistics of each sites were used to examine the effect of mediators such as other meta-analyses. The findings of previous meta-analyses were inconsistent on the effects of duration of illness and antipsychotic medication on the volume changes (Shepherd et al., 2012), and a new approach adhering to a higher degree of precision is warranted.
In this study, we gathered the clinical data of all subjects in previous prospective meta-analysis and analyzed it using uniform, robust statistical method. In this type of study, it is essential to obtain the consent of transfer of data from all subjects who are visiting in different institutions (van Erp et al., 2016), and close cooperation within the COCORO network made it possible. Our main objective is to examine whether the effect of duration of illness, total daily dose and type of antipsychotics on the brain volume changes would become evident with this new methodology.
2. Materials and methods
2.1. Sample subjects and imaging
Imaging data of 883 schizophrenia subjects was taken from the prospective meta-analysis conducted by COCORO consortium in Japan. Detailed information on subjects and imaging protocols was given in our previous report (Okada et al., 2016). Briefly, T1-weighted magnetic resonance images of 1117 subjects with schizophrenia from 11 sites (26 protocols) were gathered, visually checked and processed with FreeSurfer software version 5.3 (http://surfer.nmr.mgh.harvard.edu). After the quality control and exclusion of the subjects from the protocol with small sample size (lower than 50 total subjects or lower than 10 subjects in either diagnostic group), images of subcortical segmentation and regional volumes (for bilateral lateral ventricles, thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, accumbens and ICV) of 884 subjects from 15 protocols were used in the previous study. Among these 884 subjects, one subject withdrew their consent during preparation of current study. The remaining 883 subjects were included.
2.2. Sample demographics
Of 6 sample demographics (age, sex, ICV, duration of illness, total daily dose of antipsychotics, type of antipsychotics) that we used as moderators in the current analysis, age, sex and ICV information had been obtained in the previous study (Okada et al., 2016). Duration of illness (years) and antipsychotic medication (name and dose of all antipsychotics that were prescribed at the time of scanning) were checked for the current study at each site. The chlorpromazine equivalents of daily dose of all prescribed antipsychotics at the time of scanning were computed (daily dose of antipsychotics) (Inada and Inagaki, 2015) (Supplementary Table 1). In each analysis, among potential candidate protocols, protocols with lower than 5 participants in the groups to be compared were excluded to minimize the unfavorable effects of very small groups.
From 883 subjects with schizophrenia, 14 were excluded due to the lack of duration of illness information, 1 was excluded due to the lack of prescription information, 33 were excluded due to the lack of both, and 57 subjects were excluded because no antipsychotics were prescribed. The result was, 778 subjects with schizophrenia from 14 protocols (11 institutions) were included in the analysis of the effect of duration of illness and daily dose of antipsychotic on the volumes of subcortical structures (Table 1). In the analysis of the effect of type of antipsychotics, we compared subjects who were prescribed atypical antipsychotics only with the subjects who were prescribed typical antipsychotics only (Jorgensen et al., 2016). In this process, 195 subjects who were prescribed both typical and atypical antipsychotics were excluded, and 8 protocols with 209 subjects were excluded as subjects prescribed typical antipsychotics were lower than 5 in these protocols. As a result, 389 subjects from 6 protocols (5 institutions) were included in the second analysis (Supplementary Table 2).
Table 1.
Characteristics of the included protocols for analysis of the effects of duration of illness and daily dose of antipsychotics.
| Protocol name | Age |
Duration of illnessa |
Daily dose of antipsychoticsb |
Vendor | MFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Male | Female | Mean | s.d. | Mean | s.d. | Median | Mean | s.d. | Median | |||
| Osaka A | 123 | 71 | 52 | 35.9 | 12.2 | 11.8 | 9.7 | 10.0 | 595.7 | 505.4 | 425.0 | GE | 1.5 T |
| Tokyo A | 100 | 57 | 43 | 33.4 | 9.5 | 10.5 | 8 | 9.5 | 814.1 | 610.4 | 626.0 | GE | 1.5 T |
| Osaka B | 68 | 33 | 35 | 33.2 | 11.6 | 10.9 | 8.9 | 8.0 | 839.5 | 683 | 750.0 | GE | 3.0 T |
| Toyama A | 111 | 58 | 53 | 26.6 | 6.3 | 4.1 | 5.1 | 2.0 | 550 | 440.7 | 400.0 | Siemens | 1.5 T |
| Kyoto | 83 | 44 | 39 | 36.2 | 9.1 | 13 | 8.6 | 12.0 | 559.1 | 375.9 | 450.0 | Siemens | 3.0 T |
| Hokkaido | 92 | 32 | 60 | 35.2 | 12 | 8.6 | 9.7 | 4.9 | 749.3 | 582.6 | 600.0 | Siemens | 1.5 T |
| Tokyo B | 42 | 27 | 15 | 30.7 | 8.8 | 8.9 | 7.1 | 6.1 | 870.8 | 737.1 | 625.0 | GE | 3.0 T |
| Nagoya A | 43 | 26 | 17 | 43.6 | 10.6 | 19.8 | 11.4 | 18.0 | 610.3 | 439.3 | 600.0 | Siemens | 3.0 T |
| Kyushu A | 25 | 7 | 18 | 38.5 | 8.1 | 13.4 | 7.8 | 11.0 | 673.5 | 410.2 | 600.0 | Philips | 3.0 T |
| Kanazawa-med | 34 | 14 | 20 | 35.9 | 9.5 | 10.2 | 9.2 | 7.5 | 519.1 | 463.4 | 376.5 | Siemens | 3.0 T |
| UOEH | 11 | 4 | 7 | 29.4 | 14.4 | 0.7 | 1 | 0.0 | 239.7 | 164.8 | 200.0 | GE | 3.0 T |
| Yaesu A | 11 | 6 | 5 | 38.4 | 4.2 | 10.6 | 6.4 | 12.0 | 765 | 479.3 | 750.0 | Philips | 3.0 T |
| Tokyo C | 11 | 5 | 6 | 31.5 | 14.3 | 7.5 | 8.9 | 3.8 | 511.2 | 456.4 | 400.0 | GE | 3.0 T |
| Kyushu B | 24 | 13 | 11 | 35.1 | 11.3 | 8.3 | 7 | 5.0 | 576.9 | 491.5 | 387.5 | Philips | 3.0 T |
| Total | 778 | 397 | 381 | 34.5 | 10.1 | 9.9 | 7.8 | 8.0 | 633.9 | 488.6 | 504.5 | ||
Abbreviations: MFS, magnetic field strength.
Years.
Chlorpromazine equivalent daily dose of antipsychotics at the time of scanning.
2.3. Analysis of effect of moderators
Following the previous ENIGMA study, we adopted a general linear model to examine the effect of moderators. In addition, since the effect of brain volume caused by the scanner characteristics could be associated with the results (van Erp et al., 2014), we added the effect of protocol to moderators. As we were not interested in the size of the effect of protocols, and because 14 protocols was quite a large number for our total number of subjects, we incorporated the effect of protocol as random effect, not as fixed effect (Michael, 2015). We selected the linear mixed-effects model (LMM) where the volumes of subcortical structures were used as a dependent variable and age, sex, duration of illness, chlorpromazine equivalent daily dose of antipsychotics and ICV were used as independent variables, and type of protocol was incorporated as a random effect for intercept only (see supplementary information for details of model selection).
All dependent and independent variables were scaled before estimation. The statistical significance of the fixed-effect of duration of illness, daily dose of antipsychotics, and type of antipsychotics was assessed in t-test. The Satterthwaite approximated degree of freedom was obtained and a parameter-specific p value was calculated for each dependent variable (Satterthwaite, 1946). The significant level was set at 0.05, and Bonferroni correction was applied to the statistical results to reduce type-I errors generated by multiple comparisons (Okada et al., 2016). Further, we confirmed the result of t-test using parametric bootstrapping test to compensate for the asymptotic assumption of t-test for null distribution of parameter estimates in mixed effect model (Douglas Bates et al., 2015). In parametric bootstrapping test, the resampling number was set to 1000 and beta coefficients of moderators were estimated each time, and 95% confidence interval of beta coefficients was obtained from the null distribution (Douglas Bates et al., 2015). All LMM analyses were conducted using lmer function in the lme4 package (Douglas Bates et al., 2015) running on R statistics 3.1.2 (https://www.r-project.org). The coef function of lmerTest package was used to obtain the Satterthwaite approximated degree of freedom (Douglas Bates et al., 2015). The bootMer function of lme4 package was used to implement parametric bootstrapping in mixed linear model (Douglas Bates et al., 2015).
2.4. The effect of duration of illness and antipsychotic medication on laterality index (LI) of subcortical regional volumes
As we found the schizophrenia specific leftward asymmetry of globus pallidus volume in our previous study (Okada et al., 2016), we examined the effect of duration of illness, antipsychotic medication, type of antipsychotics, on the laterality of globus pallidus volumes. Laterality index (LI) defined as the ratio [(left − right) / (left + right)] can range from − 1 to 1 and a positive LI indicates a leftward asymmetry (Okada et al., 2016). The statistical significance of fixed-effects of duration of illness, daily dose of antipsychotics and type of antipsychotics was assessed using the same procedure as volume analysis. As LI is the ratio, we did not include ICV volume as an independent variable.
2.5. Comparison of medicated subjects with drug free subjects
To examine the regional brain volume of drug free subjects, we have compared their regional brain volumes with those of medicated subjects. We implemented this analysis using subjects at the site where there were 5 or more drug free subjects (Osaka A, Osaka B, Toyama A, Hokkaido) (Supplementary Tables 3, 4). To compare drug free subjects (n = 51) with medicated subjects (n = 394), we newly made binominal item (1 means medicated and 0 means drug free) and used it instead of chlorpromazine equivalent antipsychotic dose. Other than that, we followed the method that we had used to assess the effect of daily of antipsychotics.
3. Results
3.1. Analysis of the effect of duration of illness and daily dose of antipsychotics on the volumes
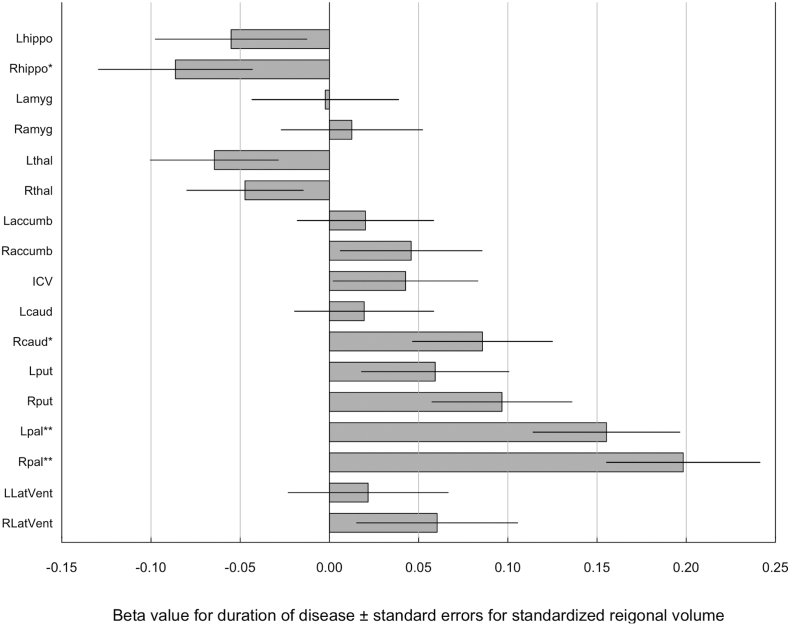
Duration of illness was significantly positively correlated with the volumes of bilateral globus pallidus after controlling the effects of age, sex, ICV and dose of antipsychotics (left: t = 3.8, p = 1.7 × 10− 4; right: t = 4.6, p = 4.7 × 10− 6) (Fig. 1, Supplementary Table 13). The effect was significant after Bonferroni correction. This significance was also confirmed by parametric bootstrapping. Duration of illness was also positively correlated with right putamen volume (t = 2.5, p = 0.01) and negatively correlated with right hippocampal volume (t = − 2.0, p = 0.05), though Bonferroni correction determined that correlations were not significant.
Fig. 1.
Beta value for duration of disease ± standard errors for standardized regional volume.
Beta value was calculated from mixed effect model where the volumes of subcortical structures, lateral ventricles and ICV were used as a dependent variable and age, sex, duration of illness, chlorpromazine equivalent daily dose of antipsychotics and ICV were used as independent variables, and type of protocol was incorporated as a random effect. accumb, accumbens; amyg, amygdala; caud, caudate; hippo, hippocampus; ICV, intracranial volume; L, left; LatVent, lateral ventricle; pal, pallidum; put, putamen; R, right; thal, thalamus.
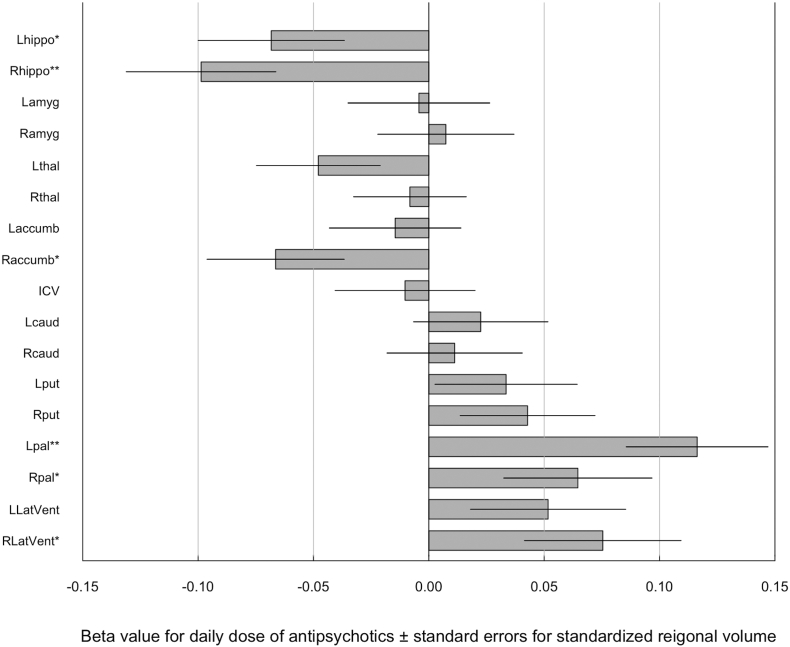
On the other hand, daily dose of antipsychotics was positively correlated with left globus pallidus volume (t = 3.8, p = 2.0 × 10− 4) and negatively correlated with right hippocampus volume (t = − 3.0, p = 2.4 × 10− 3) after controlling the effects of age, sex, ICV and duration of illness (Fig. 2, Supplementary Table 14). The significance was also confirmed by parametric bootstrapping. Daily dose of antipsychotics also showed a positive correlation with right globus pallidus (t = 2.0, p = 0.04) and right ventricular volume (t = 2.2, p = 0.03), and negative correlation with left hippocampus (t = − 2.2, p = 0.03) and right accumbens volumes (t = − 2.2, p = 0.03), though the correlations were not significant after Bonferroni correction. The results were not changed when we included drug free subjects. (Supplementary Table 15).
Fig. 2.
Beta value for daily dose of antipsychotics ± standard errors for standardized regional volume.
Beta value was calculated from mixed effect model where the volumes of subcortical structures, lateral ventricles and ICV were used as a dependent variable and age, sex, duration of illness, chlorpromazine equivalent daily dose of antipsychotics and ICV were used as independent variables, and type of protocol was incorporated as a random effect. accumb, accumbens; amyg, amygdala; caud, caudate; hippo, hippocampus; ICV, intracranial volume; L, left; LatVent, lateral ventricle; pal, pallidum; put, putamen; R, right; thal, thalamus.
3.2. Analysis of the effect of the type of antipsychotic
Type of antipsychotics did not show any significant association with the volumes of subcortical regions (Supplementary Table 16, the correlations were not significant after Bonferroni correction.).
3.3. The effect of duration of illness and antipsychotic medication on globus pallidus LI
Daily dose of antipsychotics had a significant effect on globus pallidus LI (t = 2.3, p = 0.02), confirmed by parametric bootstrapping (mean = 0.08, 95% confidence interval = (0.01–0.15). Duration of illness (t = − 0.2, p = 0.87) and antipsychotics (t = − 0.6, p = 0.58) did not show significant effect.
3.4. Comparison of medicated subjects with drug free subjects
The bilateral caudate were significantly larger in medicated subjects than drug free subjects (t = 3.6, p = 8.7 × 10− 3 for left and t = 2.7, p = 7.3 × 10− 3 for right). The difference was significant after Bonferroni correction. Right globus pallidus was also larger in medicated subjects (t = 2.0, p = 0.04), although the difference was not significant after Bonferroni correction. (Supplementary Table 17).
4. Discussion
In this study, we found significant positive association between bilateral globus pallidus volumes and duration of illness, and between left globus pallidus volume and daily dose of antipsychotics. Increment of globus pallidus volume in medicated subjects with chronic schizophrenia was consistent with previous studies (Gur et al., 1998, Lang et al., 2004, van Haren et al., 2016) and one meta-analysis (Haijma et al., 2013). Globus pallidus showed the largest effect size in comparison with chronic schizophrenia and control subjects in both the ENIGMA study (van Erp et al., 2016) and the COCORO study (Okada et al., 2016). However, the original ENIGMA study did not show significant effect of daily dose of antipsychotics on the globus pallidus volumes (van Erp et al., 2016). This suggested that our method may have a higher sensitivity than the meta-regression analysis adopted in the original ENIGMA study, though other factors such as the difference in moderator, may also have influenced the result.
Because studies with antipsychotic naïve schizophrenia patients failed to show the increase of globus pallidus volume (Gur et al., 1998, Lang et al., 2001), it seemed that globus pallidus volume increment was mainly induced by antipsychotics and duration of illness reflected the cumulative dose of antipsychotics that each patient had taken. The neurobiological underpinnings of basal ganglia volume increment associated with antipsychotic medication remain poorly understood. The striatum has particularly high density of D2 receptors (Black et al., 1997), and blockade of D2 receptor may affect the cell proliferation through modulation of the Akt signaling pathway (Beaulieu et al., 2007). In fact, blockade of D2 receptors induce cell proliferation in rodents has been reported, though the site was forebrain, and not striatum (Kippin et al., 2005, Wang et al., 2004). Increment of regional blood flow was another possible mechanism of enlargement. A previous PET study showed the increment of blood flow in striatum after single administration of haloperidol, and this effect was larger than that of olanzapine (Lahti et al., 2005). Although the meaning of regional brain volume changes associated with antipsychotic use is not fully elucidated (Huhtaniska et al., 2017b), previous study showed that larger brain volume change was associated with lower global function (Ho et al., 2011), more severe negative symptom (Nesvag et al., 2012), and lower IQ (Kubota et al., 2015). These findings may indicate that we have to care for the possible long-term undesirable effect of antipsychotic on the brain volume and function of the subjects with schizophrenia.
In contrast, we found a significant negative association of right and tendency of negative association of left hippocampus volume with daily dose of antipsychotics. As the hippocampus volume reduction was also shown in antipsychotic naïve schizophrenia subjects (Haijma et al., 2013), antipsychotics are not the only cause of hippocampus volume reduction. The relationship between antipsychotics and hippocampus volume was controversial. A 6-month longitudinal study of first episode schizophrenia treated with quetiapine showed significant volume loss which was more evident in the higher dose group (Ebdrup et al., 2011). In contrast, another longitudinal study showed that a higher dose of atypical antipsychotics was associated with less hippocampus volume reduction (Koolschijn et al., 2010). In the animal study, low dose of clozapine increased a marker of DNA synthesis in the dentate gyrus, but high dose of either clozapine or haloperidol did not produce the same result in adult rat hippocampus (Halim et al., 2004). These studies suggested that careful interpretation according to type and dose of antipsychotics is necessary to examine the association between antipsychotic and hippocampus volume.
The. LI of globus pallidus showed marginally significant association with daily dose of antipsychotics. Although few studies have examined the association of antipsychotic treatment and LI of globus pallidus, our result corresponded with the previous study by Gur and colleagues which showed that LI was higher in previously medicated schizophrenia subjects than in never medicated subjects or healthy comparison (Gur et al., 1998).
Current study did not find any effect of type of antipsychotics on the subcortical volumes. Previous studies showed that prescription of typical antipsychotics was associated with increment of caudate volume (Keshavan et al., 1994) and switching from typical antipsychotic to olanzapine (Lang et al., 2004) or clozapine (Frazier et al., 1996) reduces volume of caudate and globus pallidus. On the other hand, one meta-analysis with large sample size (Haijma et al., 2013) and original ENIGMA study did not find association between antipsychotics and subcortical structural volumes, as current results. Jorgensen and colleagues has examined the relationship between basal ganglia volume and type of antipsychotics and found that subjects with typical antipsychotics had larger putamen than those with atypical antipsychotic but this effect was specifically attributed to clozapine (Jorgensen et al., 2016). In this report subjects with clozapine has smaller putamen than control subjects and subjects with olanzapine. Taken together, drug type of antipsychotics may have effect on subcortical volumes but dividing all antipsychotics into typical and atypical antipsychotics may be too simple (Jorgensen et al., 2016). It is future consideration to examine the difference of effects on brain volumes among antipsychotics according to more detailed features like the strength of D2 blockade.
About the comparison of drug free subjects with medicated subjects, the bilateral caudate were significantly larger in medicated subjects. One previous meta-analysis showed that drug free subjects showed significantly smaller caudate than control. (Haijma et al., 2013) Because medication had positive effect on the volume of basal ganglia (caudate, putamen, globus pallidum), small volume of drug free subjects might be a reason why difference in caudate between drug free subjects and medicated subjects was more notable than in putamen or in globus pallidum. However, the number of drug free subjects was small and findings should be interpreted with caution.
There are several limitations in our study. Firstly, although we used the daily dose of antipsychotics at the time of scanning as a moderator, the lack of data on medication history is an important limitation. Andreasen and colleagues argued for the importance of use of cumulative antipsychotic exposure to assess the effect of antipsychotic on brain volume (Andreasen et al., 2010). Although prospective meta-analysis is very effective way for imaging study with large sample, it is inevitably cross-sectional, and as a result, cumulative dose is not available. In our opinion, using the daily dose of antipsychotics at the date of scan of each subject could be the second-best choice if we would like to examine the effect of antipsychotic on brain volume using large sample. The concordance of the current results with meta-analysis of longitudinal studies most of which examined the effect of cumulative dose (Huhtaniska et al., 2017b) might support our idea. In this meta-analysis, higher antipsychotic dose was associated with higher basal ganglia volume. Secondly, although we assessed the effect of 6 moderators on subcortical volumes, other cofounding factors can influence regional brain volume, including medication other than antipsychotics (van Erp et al., 2016). For example, cumulative dose of benzodiazepine was shown to have negative effect on the caudate volume (Huhtaniska et al., 2017a). Lack of assessment of illness severity is the third limitation of our study. However, the original ENIGMA study found the association of negative symptom severity with lateral ventricular volume, but not with subcortical regional volumes (van Erp et al., 2016).
5. Conclusions
In conclusion, we have demonstrated that daily dose of antipsychotics was positively associated with left globus pallidus volume, and negatively associated with right hippocampus volume, and that duration of illness was positively associated with bilateral globus pallidus volumes. A large sample size, uniform data collection methodology and robust statistical analysis are strengths of the current study. This result suggests that we need special attention to discuss about relationship between subcortical regional brain volumes and pathophysiology of schizophrenia because regional brain volumes may be affected by antipsychotic medication.
Acknowledgments
Acknowledgement
We thank everyone who participated in this study. A part of computations was performed using Research Center for Computational Science, Okazaki, Japan.
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributors
NH wrote the article and NH and YAI analyzed the data. NH, NO, HY, YY, MF, NK, AT, SS, HN, MY, AK, KO, SK, TT, TO gathered clinical data. KKT, FY, KN, MF, YW supervised the imaging analysis. RH supervised the study. All authors reviewed the article and approved submission.
Role of funding source
This work was supported by the Japan Agency for Medical Research and Development (17dm0207004h0004) (the Brain Mapping by Integrated Neurotechnologies for Disease Studies) and the Ministry of Education, Culture, Sports, Science and Technology (Advanced Bioimaging Support 16H06280, KAKENHI 16H05375). The funding sources had no role in the study design; data collection, analysis, and interpretation; writing of the report; or the decision to submit the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.11.004.
Appendix A. Supplementary data
Supplementary material
References
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.M., Tirotta E., Sotnikova T.D., Masri B., Salahpour A., Gainetdinov R.R., Borrelli E., Caron M.G. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J. Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K.J., Gado M.H., Perlmutter J.S. PET measurement of dopamine D2 receptor-mediated changes in striatopallidal function. J. Neurosci. 1997;17:3168–3177. doi: 10.1523/JNEUROSCI.17-09-03168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas Bates M.M., Bolker Benjamin M., Walker Steven C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67 [Google Scholar]
- Ebdrup B.H., Skimminge A., Rasmussen H., Aggernaes B., Oranje B., Lublin H., Baare W., Glenthoj B. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. Int. J. Neuropsychopharmacol. 2011;14:69–82. doi: 10.1017/S1461145710000817. [DOI] [PubMed] [Google Scholar]
- van Erp T.G., Greve D.N., Rasmussen J., Turner J., Calhoun V.D., Young S., Mueller B., Brown G.G., McCarthy G., Glover G.H., Lim K.O., Bustillo J.R., Belger A., McEwen S., Voyvodic J., Mathalon D.H., Keator D., Preda A., Nguyen D., Ford J.M., Potkin S.G., Fbirn A multi-scanner study of subcortical brain volume abnormalities in schizophrenia. Psychiatry Res. 2014;222:10–16. doi: 10.1016/j.pscychresns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp T.G., Hibar D.P., Rasmussen J.M., Glahn D.C., Pearlson G.D., Andreassen O.A., Agartz I., Westlye L.T., Haukvik U.K., Dale A.M., Melle I., Hartberg C.B., Gruber O., Kraemer B., Zilles D., Donohoe G., Kelly S., McDonald C., Morris D.W., Cannon D.M., Corvin A., Machielsen M.W., Koenders L., de Haan L., Veltman D.J., Satterthwaite T.D., Wolf D.H., Gur R.C., Gur R.E., Potkin S.G., Mathalon D.H., Mueller B.A., Preda A., Macciardi F., Ehrlich S., Walton E., Hass J., Calhoun V.D., Bockholt H.J., Sponheim S.R., Shoemaker J.M., van Haren N.E., Pol H.E., Ophoff R.A., Kahn R.S., Roiz-Santianez R., Crespo-Facorro B., Wang L., Alpert K.I., Jonsson E.G., Dimitrova R., Bois C., Whalley H.C., McIntosh A.M., Lawrie S.M., Hashimoto R., Thompson P.M., Turner J.A. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry. 2016;21:547–553. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Giedd J.N., Kaysen D., Albus K., Hamburger S., Alaghband-Rad J., Lenane M.C., McKenna K., Breier A., Rapoport J.L. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am. J. Psychiatry. 1996;153:564–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Smieskova R., Kempton M.J., Ho B.C., Andreasen N.C., Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013;37:1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.E., Maany V., Mozley P.D., Swanson C., Bilker W., Gur R.C. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am. J. Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haijma S.V., Van Haren N., Cahn W., Koolschijn P.C., Hulshoff Pol H.E., Kahn R.S. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr. Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim N.D., Weickert C.S., McClintock B.W., Weinberger D.R., Lipska B.K. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29:1063–1069. doi: 10.1038/sj.npp.1300422. [DOI] [PubMed] [Google Scholar]
- van Haren N.E., Schnack H.G., Koevoets M.G., Cahn W., Hulshoff Pol H.E., Kahn R.S. Trajectories of subcortical volume change in schizophrenia: a 5-year follow-up. Schizophr. Res. 2016;173:140–145. doi: 10.1016/j.schres.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Ho B.C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R., Crow T.J., Passingham D., Mackay C.E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Huhtaniska S., Jaaskelainen E., Heikka T., Moilanen J.S., Lehtiniemi H., Tohka J., Manjon J.V., Coupe P., Bjornholm L., Koponen H., Veijola J., Isohanni M., Kiviniemi V., Murray G.K., Miettunen J. Long-term antipsychotic and benzodiazepine use and brain volume changes in schizophrenia: the Northern Finland birth cohort 1966 study. Psychiatry Res. 2017;266:73–82. doi: 10.1016/j.pscychresns.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Huhtaniska S., Jaaskelainen E., Hirvonen N., Remes J., Murray G.K., Veijola J., Isohanni M., Miettunen J. Long-term antipsychotic use and brain changes in schizophrenia - a systematic review and meta-analysis. Hum. Psychopharmacol. 2017;32 doi: 10.1002/hup.2574. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol H.E., Kahn R.S. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr. Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin. Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- Jorgensen K.N., Nesvag R., Gunleiksrud S., Raballo A., Jonsson E.G., Agartz I. First- and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266:451–460. doi: 10.1007/s00406-015-0650-9. [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Bagwell W.W., Haas G.L., Sweeney J.A., Schooler N.R., Pettegrew J.W. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Kippin T.E., Kapur S., van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J. Neurosci. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C., van Haren N.E., Cahn W., Schnack H.G., Janssen J., Klumpers F., Hulshoff Pol H.E., Kahn R.S. Hippocampal volume change in schizophrenia. J. Clin. Psychiatry. 2010;71:737–744. doi: 10.4088/JCP.08m04574yel. [DOI] [PubMed] [Google Scholar]
- Kubota M., van Haren N.E., Haijma S.V., Schnack H.G., Cahn W., Hulshoff Pol H.E., Kahn R.S. Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiat. 2015;72:803–812. doi: 10.1001/jamapsychiatry.2015.0712. [DOI] [PubMed] [Google Scholar]
- Lahti A.C., Weiler M.A., Medoff D.R., Tamminga C.A., Holcomb H.H. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res. 2005;139:19–30. doi: 10.1016/j.pscychresns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lang D.J., Kopala L.C., Vandorpe R.A., Rui Q., Smith G.N., Goghari V.M., Honer W.G. An MRI study of basal ganglia volumes in first-episode schizophrenia patients treated with risperidone. Am. J. Psychiatry. 2001;158:625–631. doi: 10.1176/appi.ajp.158.4.625. [DOI] [PubMed] [Google Scholar]
- Lang D.J., Kopala L.C., Vandorpe R.A., Rui Q., Smith G.N., Goghari V.M., Lapointe J.S., Honer W.G. Reduced basal ganglia volumes after switching to olanzapine in chronically treated patients with schizophrenia. Am. J. Psychiatry. 2004;161:1829–1836. doi: 10.1176/ajp.161.10.1829. [DOI] [PubMed] [Google Scholar]
- Michael J.C. second edition. John Wiley & Sons Ltd; West Sussex: 2015. Statistics an Introduction Using R. [Google Scholar]
- Moncrieff J., Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol. Med. 2010;40:1409–1422. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
- Navari S., Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol. Med. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nesvag R., Bergmann O., Rimol L.M., Lange E.H., Haukvik U.K., Hartberg C.B., Fagerberg T., Soderman E., Jonsson E.G., Agartz I. A 5-year follow-up study of brain cortical and subcortical abnormalities in a schizophrenia cohort. Schizophr. Res. 2012;142:209–216. doi: 10.1016/j.schres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Okada N., Fukunaga M., Yamashita F., Koshiyama D., Yamamori H., Ohi K., Yasuda Y., Fujimoto M., Watanabe Y., Yahata N., Nemoto K., Hibar D.P., van Erp T.G., Fujino H., Isobe M., Isomura S., Natsubori T., Narita H., Hashimoto N., Miyata J., Koike S., Takahashi T., Yamasue H., Matsuo K., Onitsuka T., Iidaka T., Kawasaki Y., Yoshimura R., Watanabe Y., Suzuki M., Turner J.A., Takeda M., Thompson P.M., Ozaki N., Kasai K., Hashimoto R. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry. 2016:1460–1466. doi: 10.1038/mp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabi B., Ellison-Wright I., McIntosh A.M., Wood S.J., Bullmore E., Lawrie S.M. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Satterthwaite F.E. An approximate distribution of estimates of variance components. Biom. Bull. 1946;2:110–114. [PubMed] [Google Scholar]
- Shepherd A.M., Laurens K.R., Matheson S.L., Carr V.J., Green M.J. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci. Biobehav. Rev. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Steen R.G., Mull C., McClure R., Hamer R.M., Lieberman J.A. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br. J. Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Torres U.S., Portela-Oliveira E., Borgwardt S., Busatto G.F. Structural brain changes associated with antipsychotic treatment in schizophrenia as revealed by voxel-based morphometric MRI: an activation likelihood estimation meta-analysis. BMC Psychiatry. 2013;13:342. doi: 10.1186/1471-244X-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A., De Peri L., Deste G., Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry. 2012;2 doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.D., Dunnavant F.D., Jarman T., Deutch A.Y. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- Woods B.T., Ward K.E., Johnson E.H. Meta-analysis of the time-course of brain volume reduction in schizophrenia: implications for pathogenesis and early treatment. Schizophr. Res. 2005;73:221–228. doi: 10.1016/j.schres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Wright I.C., Rabe-Hesketh S., Woodruff P.W., David A.S., Murray R.M., Bullmore E.T. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material