Abstract
Introduction
We are developing a second generation 8-OH quinoline (2-(dimethylamino) methyl-5, 7-dichloro-8-hydroxyquinoline [PBT2, Prana Biotechnology]) for targeting amyloid β (Aβ) in Alzheimer's disease (AD). In an earlier phase IIa, 3 month trial, PBT2 lowered cerebrospinal fluid Aβ by 13% and improved cognition (executive function) in a dose-related fashion in early AD. We, therefore, sought to learn whether PBT2 could alter the Aβ-PET signal in subjects with prodromal or mild AD, in an exploratory randomized study over a 12-month phase in a double-blind and a 12-month open label extension phase trial design.
Methods
For inclusion, the usual clinical criteria for prodromal or probable AD, Mini–Mental State Examination ≥20, and global Pittsburgh compound B (PiB)-PET standardized uptake volume ratio (SUVR) >1.7 were used. As this was an exploratory study, we included contemporaneous matched control data from the Australian Imaging Biomarker and Lifestyle Study (AIBL). Other measures included fluorodeoxyglucose-positron emission tomography, magnetic resonance imaging volumetrics, blood Aβ biomarkers, and cognition and function.
Results
Forty subjects completed the first 12-month double-blind phase (placebo = 15, PBT2 = 25), and 27 subjects completed the 12-month open label extension phase (placebo = 11, PBT2 = 16). Overall, PTB2 250 mg/day was safe and well tolerated. The mean PiB-PET SUVR at baseline was 2.51 ± 0.59. After adjusting for baseline SUVR, in the double-blind phase, the placebo group showed a nonsignificant decline in PiB-PET SUVR, whereas the PBT2 group declined significantly (P = .048). Subjects who did not enter or complete the extension study had a significantly higher 12-month Aβ-PET SUVR (2.68 ± 0.55) compared with those who completed (2.29 ± 0.48). Both groups differed significantly from the rate of change over 12 months in the AIBL control group. In the open label 12-month extension study, the PiB-SUVR stabilized. There were no significant differences between PBT2 and controls in fluorodeoxyglucose-positron emission tomography, magnetic resonance imaging volumetrics, blood Aβ biomarkers, or cognition/function over the course of the double-blind phase.
Discussion
There was no significant difference between PBT2 and controls at 12 months, likely due to the large individual variances over a relatively small number of subjects. PBT2 was associated with a significant 3% PiB-PET SUVR decline in the double-blind phase and a stabilization of SUVR in the open-label phase. From this exploratory study, we have learned that the entry criterion of SUVR should have been set at ≥ 1.5 and <2.0, where we know from the AIBL study that subjects in this band are accumulating Aβ in a linear fashion and that subjects who withdrew from this type of study have much higher SUVRs, which if not taken into account, could distort the final results. Because of large individual variations in SUVR, future studies of PBT2 will require larger numbers of subjects (n > 90 per arm) over a longer period (18 months or more). Further evaluation of higher doses of PBT2 in earlier stages of AD is warranted.
Trial Registration
ACTRN 12611001008910 and ACTRN 12613000777796.
Keywords: Randomised control trial, Alzheimer's disease, Aβ-amyloid PET molecular imaging, Novel 8-OH quinoline, Clinical trial design, Biomarkers for Alzheimer's disease
Highlights
-
•
Exploratory randomized controlled trial for Alzheimer's disease using novel 8-OH quinoline.
-
•
Use of Aβ positron emission tomography molecular imaging as intake criterion.
-
•
Small numbers, large variances, and higher than expected mean baseline standardized uptake volume ratio may have contributed to lack of demonstrable efficacy.
1. Background
The advent of a biomarker definition of sporadic Alzheimer's disease (AD) now allows determination of the efficacy of therapeutic interventions using the two validated biomarkers of amyloid β (Aβ): Aβ-PET and cerebrospinal fluid (CSF)-Aβ [1], [2], [3]. An abnormally high cortical signal from Aβ-PET reflects the amount of Aβ-amyloid closely associated with an insoluble pool in plaques and perivascular Aβ deposits [4], [5], whereas a decreasing CSF-Aβ level may reflect the interstitial diffusible pool of Aβ which feeds irreversibly the more insoluble fibrillary Aβ-amyloid plaque pool [6].
Despite the differing origins of these Aβ-biomarker signals, they are closely interrelated with similar predictive utility for diagnostic classification and progression of cognitive impairment [7], [8]. Longitudinal cohort studies demonstrate that the Aβ burden as measured by PET increases linearly at about 3% per year in the preclinical and prodromal stages of AD, which then slows after the full clinical syndrome of AD has developed [9], [10]. Similarly, CSF-Aβ levels decline over time during the evolution of AD [11].
We have been developing a second generation 8-OH quinoline (2-(dimethylamino) methyl-5, 7-dichloro-8-hydroxyquinoline [PBT2, Prana Biotechnology]) for targeting Aβ in AD. Originally developed as a metal-protein attenuating compound [12], [13], more recent results indicate that PBT2 can stabilize a non-toxic oligomeric (dimeric) conformer of Aβ [14]. In a phase IIa, 3-month trial, (n = 74) in mild/moderate AD, PBT2 lowered CSF Aβ by 13% and improved cognition (executive function) in a dose-related fashion [15]. We, therefore, sought to learn whether changes in the Aβ-PET signal could be detected after PBT2 administration in an exploratory study in a small number of subjects (n = 40; 15 placebo and 25 active) with prodromal or mild AD, over a 12-month phase in a placebo controlled double-blind study with a 12-month open label extension phase (the IMAGINE Study; Australian New Zealand Clinical Trials Registry Trial identifiers ACTRN 12611001008910 and ACTRN 12613000777796).
2. Methods
2.1. Study design and participants
The exploratory PBT2-204 IMAGINE study was a 12-month randomized, double-blind, placebo-controlled phase with an extension for another 12 months as an open-label phase. The primary objectives were to assess safety and tolerability of PBT2, and its effect on Aβ amyloid accumulation over these two 1-year intervals in subjects with prodromal or mild AD. The study was conducted at five sites in Melbourne from February 2012 through December 2014.
Eligible subjects were ≥55 years of age, met the criteria for prodromal or probable AD [16], [17], a Mini–Mental State Examination (MMSE) score ≥ 20, a score on the Hachinski Ischemic scale of four or lower, and a global Pittsburgh compound B (PiB)-PET standardized uptake volume ratio (SUVR) > 1.7. Concurrent use of standard anti-acetylcholinesterase inhibitor AD medications was permitted.
Exclusion criteria were other primary neurodegenerative disorders associated with dementia; major psychiatric disorders; a history of stroke or clinically significant cardiovascular disease; an abnormal screening brain magnetic resonance imaging (MRI) scan (evidence of ischemic necrosis or ≥2 lacunar lesions); and clinically significant retinal, optic nerve, or any ocular disease.
In addition to the primary objectives of this exploratory study (safety, tolerability, and efficacy of 250 mg/d of oral PBT2, in altering the global PiB SUVR from baseline to 12 months with an open-ended extension to 24 months), the secondary objectives included assessment of changes in regional PiB-PET SUVR, glucose metabolism as measured by fluorodeoxyglucose-positron emission tomography (FDG-PET), MRI volumetrics (hippocampal, cortical gray matter, and ventricular volumes), blood-based biomarkers (APOE [apolipoprotein E] haplotype, Aβ plasma levels, and Aβ cellular dimer/monomer levels), and cognition and daily function.
2.2. Study oversight and role of the sponsor and funding source
The study was approved by the institutional review board at each participating site. Written informed consent was obtained from the subjects or their legally authorized representatives or caregivers. The sponsor (Prana Biotechnology) assisted with the study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and were involved in the development and approval of the manuscript. The first (V.L.V.) and last (C.L.M.) authors had primary responsibilities for drafting the manuscript and the decision to submit for publication.
2.3. Randomization, masking, and open label extension
For the first 12-month phase, participants were assigned randomly (2:1) either to PBT2 250 mg once daily or to placebo. At the conclusion of this phase of the study, the double-blind results were analyzed and preliminary conclusions were drawn. All site investigators and coordinators were aware of these preliminary findings, but all participants and investigators/coordinators remained blinded to which arm (placebo/active) the participants had been in. All participants were then offered the opportunity to participate in a further 12-month open-label phase. All involved in this extension phase remained blinded to the treatment assignments in the double-blind phase until after the conclusion of the extension phase.
2.4. Procedures
Subjects underwent a screening visit to access eligibility followed by a baseline visit within one month, at which they were allocated to a treatment assignment. The overall schedule of visits and investigations is set out in Fig. 1.
Fig. 1.
Schedule of investigations and assessments. Abbreviations: Aβ, amyloid β; FDG-PET, fluorodeoxyglucose-positron emission tomography, MRI, magnetic resonance imaging.
The primary outcome measure, global PiB SUVR, was assessed at baseline and 6 and 12 months. Clinical assessment for safety and tolerability included vital signs; routine clinical laboratory parameters; 12-lead electrocardiogram; physical, neurological, and ophthalmological examination; neuropsychiatric inventory; and the Columbia Suicide Severity Rating Scale.
For the secondary outcome measures, participants underwent clinical and cognitive assessments at baseline and 6, 12, and 24 months.
Cerebral glucose metabolism was assessed through FDG-PET at baseline, 12, and 24 months by measuring change in the posterior cortical index from baseline to 12 and 24 months.
Brain volumetrics were assessed through MRI scanning at baseline, 12, and 24 months. The MRI volumetric outcomes were the changes in hippocampal, cortical gray matter, and ventricular volumes from baseline to 12 and 24 months.
Blood samples for Aβ biomarkers were taken at baseline, 6, and 12 months. Routine clinical biochemistry sampling occurred at screening, baseline, 6, 12, 18, and 24 months.
Cognition was assessed using a neuropsychological test battery (NTB) and MMSE. The NTB consisted of the Rey Auditory Verbal Learning Test Immediate and Delayed Tests; Wechsler Memory Scale Digit Span Digit Span; Controlled Oral Word Association Test; Category Naming Test; Trail Making Test Part A and Part B. Functional abilities were assessed using the Alzheimer's Disease Cooperative Study–Activities of Daily Living inventory (ADCS-ADL-23).
2.5. Image acquisition
2.5.1. PET
PiB-PET acquisitions have been described in detail previously. Briefly, a 30-min acquisition in 3D mode starting 40 minutes after injection of ∼370 MBq PiB was performed with a Phillips Allegro™ PET camera. A transmission scan was performed for attenuation correction. PET images were reconstructed using a 3D RAMLA algorithm.
For the FDG studies, all subjects were fasted for at least 6 hours and were normoglycemic at the time of FDG injection. A 20-minute static PET emission scan was acquired 30 minutes after injection of ∼200 MBq 18F-FDG on the same camera, and images were reconstructed using the same image reconstruction techniques.
2.5.2. MRI
Participants received an MRI using the Alzheimer's Disease Neuroimaging Initiative 3D Magnetization Prepared Rapid Gradient Echo sequence, with 1 × 1 mm in-plane resolution and 1.2 mm slice thickness, repetition time/echo time/relaxation time/VV = 2300/2.98/900, flip angle 9° and field of view 240 × 256, and 160 slices. T2 fast spin echo and fluid-attenuated inversion recovery sequences were also obtained.
2.6. Safety population and monitoring
The safety population included all participants who received at least one dose of PBT2, had a scan that was positive for amyloid at baseline (defined as an SUVR >1.7), and underwent at least one PET assessment after baseline. Safety was evaluated by means of reports of adverse events (AEs); clinical laboratory testing (hematologic and serum chemical testing and urinalysis); assessment of vital signs, physical, neurological, and ophthalmological examinations; and brain MRI. An independent, external Data and Safety Monitoring Committee, whose members were aware of the study assignments, reviewed the safety data.
2.7. Image analysis
2.7.1. PET
Standardized uptake values (SUVs) for PiB and FDG were calculated for all brain regions examined. The primary performance measure used for all the PET assessments was the SUVR, generated by dividing all regional PiB and FDG SUV by the respective cerebellar cortex SUV. The global PIB SUVR was calculated as the average SUVR of the anterior cingulate, posterior cingulate/precuneus, frontal, lateral temporal, parietal, and occipital cortex. The FDG posterior cortical index was calculated as the average SUVR of the lateral temporal, parietal, and posterior cingulate/precuneus cortices. All measurements were adjusted for baseline values. Regional SUVR of the individual regions described above were also examined.
PiB- and FDG-PET images were processed using a semi-automatic region of interest method as previously described [18]. Briefly, coregistration of each individual's MRI with the PET images was performed with SPM8 [19]. The narrow cortical region of interest template was placed on the coregistered MRI by an operator who was blind to the subject's clinical status and then transferred to the coregistered PiB- and FDG-PET images. Follow-up PET images were coregistered with the respective initial PiB and FDG images, and the same region of interest templates were applied.
2.7.2. MRI
Brain volumetrics were derived from T1 Magnetization Prepared Rapid Gradient Echo MRI images using a commercial software program, NeuroQuant®. The primary performance measures were the gray cortical matter, hippocampal and ventricular volumes normalized for head size using the total intracranial volume.
2.8. The AIBL comparator control group
Because of the relatively small number of controls in the double-blind phase of IMAGINE, we used a larger number of contemporary, well-matched, observational controls from the Australian Imaging Biomarker and Lifestyle (AIBL) longitudinal study, whose imaging and cognitive assessments had been collected using similar methods [20]. We identified 46 AIBL participants with at least two Aβ-PET images at 20-month intervals who were selected on the same inclusion criterion as IMAGINE and matched with an almost identical IMAGINE baseline SUVR. Forty AIBL participants were also matched to the IMAGINE 12-month SUVR.
2.9. Blood
2.9.1. Preparation of samples
Venesection was used to collect two 4-mL samples of whole blood in two ethylenediaminetetraacetic acid vacutainers 1.6 mg/mL (CEDTA, Greiner Bio-One) between the hours of 9.00 am and 3.00 pm. Blood processing commenced within 15 minutes of sample acquisition. Both tubes were centrifuged for 20 minutes at 4°C at 1900 × g before the upper plasma layers were removed and aliquoted. The remaining blood in tube A was titurated to produce a cellular fraction, which was subsequently aliquoted. For tube B, the buffy coat layer (found just below the plasma layer) was removed and added to 250-μL phosphate buffered saline (PBS). This mixture was titurated and added to 5-mL Ficoll-Paque PLUS (GE life Sciences) before being spun for 20 minutes at 20°C at 400 × g with soft break on. The supernatant was removed and added to 7 mL PBS while the pellet was collected and aliquoted as red blood cells. The supernatant/PBS mix was then centrifuged for 5 minutes at 20°C at 1900 × g before the resulting supernatant was discarded, and the pellet was resuspended in 500-μL Milli-Q H2O to give the white blood cell fraction. Multiple aliquots of each fraction were prepared in low binding polypropylene tubes, and all samples were stored at −80°C until required; this ensured that all samples only underwent a single freeze/thaw cycle.
2.9.2. Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry analysis
Mass spectrometric analysis of the blood fractions were carried out using ProteinChip® PS10 Arrays (Bio-Rad; CAT #C55-30044) loaded with WO2 (2 μL at 0.25 mg/mL). Chips were incubated for 2 hours at 20°C in a humidity chamber before excess antibodies were removed, and 10 μL blocking buffer (0.5 M ethanolamine in PBS) was added and incubated for 30 min. After the removal of the blocking buffer, arrays were washed three times for 5 minutes with 120 μL of 0.5 % Triton X-100/PBS (wash-buffer) followed by three 5-minute washes with 120 μL PBS.
Aliquots of the blood fractions (40 μL) were prepared with 40 μL of 8 M urea and 480 μL of wash-buffer and placed for 10 min in an ultrasound bath with ice. One hundred and fifty microliters of sample mix was added to each spot and incubated at room temperature for 3 hours.
Subsequent to incubation, samples were removed, and the arrays underwent three 10-second washes on a vigorous shaking table with 150-μL wash-buffer and 150 μL PBS, followed by two washes with 150-μL HEPES. The arrays were then dried before two 1-μL aliquots of a 50% saturated solution of SPA EAM were applied, with air-drying between treatments. The 50% saturated solution was prepared by suspending 5 mg ProteinChip sinapinic acid - energy absorbing molecule (Bio-Rad; CAT #C30-00002) in 0.5 % trifluoroacetic acid (Sigma-Aldrich; St Louis, Missouri), 50% acetonitrile (High performance liquid chromatography [HPLC] grade), 15 % isopropyl alcohol (HPLC grade), and 34.5 % HPLC grade H2O.
Each sample was analyzed in duplicate. All arrays were analyzed blind to diagnostic status using a ProteinChip surface-enhanced laser desorption/ionization System Enterprise Edition (BioRad). All spectra were internally normalized, and peak intensities were normalized using total ion current. Peak detection was carried out using the inbuilt BioRad ProteinChip Data Manager Biomarker Wizard, (Version 3.07.004). Aβ species, including potential oxidations, were matched to peaks within the resulting spectra using M/Z.
2.9.3. Plasma Aβ assay and APOE genotyping
Quantification of plasma Aβ (fragments 1-40, 1-42, n-40, and n-42) was achieved using the INNO-BIA plasma Aβ forms assay (Innogenetics NV, Ghent, Belgium) on the Luminex xMAP platform. Preparation of the methods follows the protocol as described [21]. The 96-well filter plates (Millipore Corporation, Bedford, Massachusetts) were drained by vacuum manifold. The mean interassay coefficients of variation, based on the included low and high kit standards, were 2.8% and 6.2% for Aβ1-40, 7.2% and 4.7% Aβ1-42, 4.4% and 3.8% for Aβn-40, and 1.0% and 1.3% for Aβn-42, respectively. The mean intraassay variations for duplicate samples were 2.7% for Aβ1-40, 1.4% for Aβ1-42, 3.3% for Aβn-40, and 1.9% for Aβn-42. APOE genotype was determined by direct sequencing.
2.10. Statistical analysis
The sample size was calculated so that the study would have 90% power, with the use of a two-sided test and an alpha level of 0.05, to detect a 20% reduction on global PiB SUVR between the PBT2 and the placebo group.
Normality of distribution was tested using the Shapiro–Wilk test and visual inspection of variable histograms. Categorical differences were evaluated using Fisher's exact test.
The changes from baseline in global PiB SUVR between the PBT2 and placebo groups were estimated both by using paired t-tests with adjustment for baseline SUVR and analysis of covariance (ANCOVA). In the ANCOVA, SUVR at the assessment of interest was treated as the dependent variable. Baseline SUVR, the interaction between treatment and visit, baseline MMSE score, APOE ε4 status, and age were added to the model as covariates.
For analyses of cognitive change, the prespecified analyses for the 12-month double-blind placebo-controlled clinical trial defined that the primary performance measure from each NTB test was compared with the placebo and PBT2 groups at the 12-month assessment using ANCOVA, where performance at the baseline assessment, age at entry, APOE ε4 status, and MMSE score were treated as covariates. To examine performance in the open label component, these same ANCOVAs were re-run to compare differences between groups at the 24-month assessment, with performance at the 12-month baseline assessment, age at entry, APOE ε4 status, and MMSE at entry were treated as covariates. Finally, to explore the extent to which performance in the NTB changed over the 24-month period, irrespective of treatment, ANCOVAs were used to compare performance at the 24-month assessment to that at baseline. In these models, performance at the baseline assessment age at entry, APOE ε4 status, and MMSE at entry were treated as covariates.
Data are presented as mean ± standard deviation unless otherwise stated. No adjustments for multiple comparisons were performed.
3. Results
The IMAGINE Study ran between February 2012 and December 2014, with 75 subjects screened, 42 enrolled; 40 subjects completed the first 12-month double-blind phase (placebo = 15, PBT2 = 25), and 27 subjects (placebo = 11, PBT2 = 15) completed the open label extension phase (Fig. 2). The baseline characteristics and demographics are summarized in Table 1. In both phases, withdrawals were considered to be unrelated to drug administration. In the double-blind phase, two subjects in the PBT2 group withdrew (one because of acute myeloid leukemia and one because of a photosensitive rash), and one subject in the placebo group was lost to follow-up (but was included in the data analysis). In the extension study phase, 33 accepted the invitation to participate, 6 withdrew, and 27 subjects completed the extension study. The 13 subjects who did not enter or complete the extension study for whatever reason had a 12-month mean PiB-PET SUVR of 2.68 ± 0.55, a value significantly (P = .0008) higher than those who continued in the extension study (SUVR of 2.30 ± 0.47).
Fig. 2.
Flow diagram for the IMAGINE study (PBT2-204).
Table 1.
Baseline characteristics and demographics
| Characteristics and demographics | Double blind |
Extension |
AIBL |
||
|---|---|---|---|---|---|
| Placebo (n = 15) | PBT2 (n = 25) | Completers at 12 months (n = 27) | Withdrawals at 12 months (n = 13) | (n = 46) | |
| Age (years) | 72.1 (6.9) | 71.4 (10.1) | 71.1 (8.2) | 74.2 (11.0) | 75.0 (7.5) |
| Gender (M/F) | 11/4 | 11/14 | 15/12 | 7/6 | 28/18 |
| APOE ε4+ (%) | 10 (67) | 19 (76) | 21 (78) | 8 (62) | 37 (80) |
| Global SUVR | |||||
| Unadjusted | 2.48 (0.41) | 2.43 (0.36) | 2.26 (0.31)∗ | 2.64 (0.33)† | 2.46 (0.30) |
| Adjusted | 2.54 (0.65) | 2.48 (0.56) | 2.30 (0.47) | 2.68 (0.55)‡ | 2.50 (0.49) |
| FDG-PET§ | 0.90 (0.09) | 0.93 (0.07) | 0.89 (0.08) | 0.87 (0.07) | − |
| Hippocampal volume§ | 3.76 (0.53) | 3.88 (0.41) | 3.69 (0.48) | 3.75 (0.33) | 3.57 (0.44) |
| Gray matter volume§ | 244.9 (19.0) | 246.3 (19.7) | 242.4 (24.3) | 237.9 (13.3) | N/A |
| Ventricular volume§ | 29.4 (10.0) | 29.0 (12.2) | 31.0 (10.8) | 34.3 (15.4) | 29.7 (11.0) |
| Blood plasma§ Aβ40 | 182.4 (23.5) | 192.1 (35.0) | 174.7 (32.2) | 178.3 (30.6) | N/A |
| Aβ42 | 38.4 (8.4) | 40.7 (10.3) | 39.5 (10.3) | 40.4 (6.4) | N/A |
| Aβ monomer | 27.6 (12.6) | 25.6 (17.3) | 14.7 (7.2) | 17.0 (8.6) | N/A |
| Aβ dimer | 135.8 (81.8) | 131.5 (110.3) | 69.1 (38.3) | 90.6 (49.1) | N/A |
| Cognition | |||||
| MMSE | 25.2 (2.2) | 23.6 (2.5) | 24.4 (2.4) | 23.8 (2.8) | 24.6 (2.8) |
| Main composite | 0.19 (0.7) | −0.07 (0.7) | 0.05 (0.8) | −0.32 (0.7) | N/A |
| Memory composite | 0.40 (1.1) | −0.18 (0.9) | −0.02 (1.1) | −0.34 (0.9) | N/A |
| Executive function composite | 0.08 (0.7) | −0.02 (0.8) | 0.08 (0.7) | −0.33 (90.8) | N/A |
| ADCS-ADL-23 | 64.4 (8.4) | 67.4 (8.7) | 64.5 (9.4) | 62.6 (13.0) | N/A |
NOTE. Data are mean (standard deviation) or N (%).
Abbreviations: AIBL, Australian Imaging Biomarker and Lifestyle Study; APOE, apolipoprotein E; SUVR, standardized uptake volume ratio; FDG-PET, fluorodeoxyglucose-positron emission tomography; Aβ, amyloid β; MMSE, Mini–Mental State Examination; ADCS-ADL, Alzheimer's Disease Cooperative Study–Activities of Daily Living; N/A, data not available.
P < .05.
P < .001 (compared to completers at 12 months).
P < .0001 (compared to completers at 12 months).
All values adjusted for baseline.
3.1. Safety and tolerability
A total of 199 AEs were reported in the double-blind phase (118 in the PBT2 group and 81 in the placebo group), of which 150 were mild, 36 were moderate, and 13 were severe. A summary of AEs is presented in Table 2. Overall, there was no statistically significant difference in the proportions of AEs in the placebo versus PBT2 groups. Similarly, there were no significant differences in the routine clinical chemistry, hematology tests, electrocardiograms, color vision, visual acuity, and visual fields throughout the double-blind phase.
Table 2.
Summary of adverse events in the double-blind phase
| Adverse events | Placebo group (n = 15) | PBT2 group (n = 27, includes 2 withdrawals) |
|---|---|---|
| One of more AE | 15 | 26 |
| Related AE | 10 | 23 |
| Serious AE | 4 | 3 |
| Death | 0 | 1 |
| Nature of AE reported by ≥ 2 participants | ||
| Cardiac | 2 | 1 |
| Ear | 2 | 0 |
| Eye | 5 | 10 |
| Gastrointestinal | 8 | 7 |
| General | 0 | 5 |
| Immune system | 1 | 3 |
| Infection | 11 | 14 |
| Injury | 1 | 2 |
| Investigational | 1 | 4 |
| Metabolic | 3 | 2 |
| Musculoskeletal | 4 | 6 |
| Neoplasia | 0 | 2 |
| Nervous system | 6 | 17 |
| Respiratory | 1 | 4 |
| Skin | 2 | 8 |
Abbreviation: AE, adverse event.
AEs in the open label study showed a similar pattern as seen in the double-blind phase. There were no significant differences between the placebo-PBT2 group and the PBT2-PBT2 group. Two serious AEs were reported in each group in the open-label phase.
Overall, the longer term treatment (up to 24 months) with PBT2 was well tolerated and safe. The safety findings from this study are consistent with those that would be expected in a population of elderly adults with prodromal or mild AD.
3.2. The effect of APOE haplotype
Because of small numbers and the large proportion of APOE ε4+ subjects in this study, it was not possible to analyze data for ε4+ and ε4- subjects separately.
3.3. The effect of PBT2 on Aβ-PET
In planning this study, we set the intake criterion of a PiB-PET SUVR of >1.7. After enrollment, the mean of the whole group baseline values of 2.51 ± 0.59 (n = 40) was higher than expected. For comparison with this baseline SUVR, a matched group of AIBL participants (SUVR 2.53 ± 0.47, n = 46, P = .81) was selected.
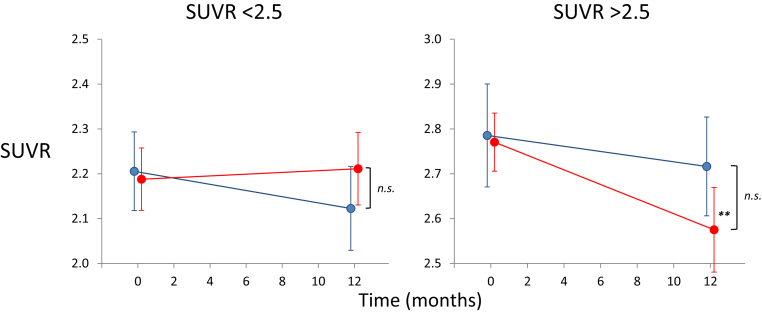
Reviewing the PiB-PET values for all subjects over both phases of this study, the variance of scores between and within individuals was quite large (Fig. 3). We first examined the effect of baseline SUVR and found a significant difference between the SUVR change at 12 months between the placebo group (no effect of baseline SUVR r = −0.14, P = .63) and the PBT2 group (larger decrease with higher baseline SUVR, r = −0.042, P = .035) (Fig. 4). Dividing the groups into those above and below the mean baseline level (<or >2.5 SUVR), there were no significant differences seen between the placebo and PBT2 groups on those <2.5 SUVR, but there was a significant decrease from baseline only in the PBT2 > 2.5 group (P = .0017) (Fig. 5). On this basis, we concluded that it was appropriate to adjust for baseline SUVR in subsequent analyses. The changes from adjusted baseline for the various groups are given in Table 3.
Fig. 3.
Longitudinal PiB data by placebo or active group. Line graphs of individual neocortical PiB SUVR in the different groups, over the double blind (0–12 months) and open label (12–24 months) phases of the study. Placebo group in blue; PBT2 group in red. Dotted line indicates the SUVR threshold of 1.7 used as inclusion criteria. Abbreviation: SUVR, standardized uptake volume ratio.
Fig. 4.
Relationship between the change (Δ) in PiB SUVR and baseline PiB SUVR in the different groups, over the double-blind (0–12 months) phase of the study. While there was a significant correlation between change in PiB SUVR and baseline PiB SUVR in the PBT2 group, this was not observed in the placebo group. Placebo group in blue; PBT2 group in red. Filled symbol denotes APOE ε4 carriers, while open symbol denotes APOE ε4 noncarriers. Abbreviation: SUVR, standardized uptake volume ratio.
Fig. 5.
Longitudinal PiB data by baseline PiB SUVR. Line graphs showing the mean (±SEM) PiB SUVR at baseline and at 12 months for the placebo (in blue) and PBT2 (in red) groups, in those participants with an adjusted baseline PiB SUVR of either <2.5 (left) or >2.5 (right). While there were no significant differences between placebo and PBT2 in either group, there was a significant decrease from baseline in the >2.5 PiB SUVR PBT2 group (P = .0023). Abbreviation: SUVR, standardized uptake volume ratio; n.s., not significant. ∗∗ P < .001.
Table 3.
Neuroimaging changes from baseline (adjusted) by group
| Neuroimaging changes | Double blind |
Open label |
AIBL Δ/yr | ||||
|---|---|---|---|---|---|---|---|
| 0–12 Mo |
12–24 Mo |
0–24 Mo |
|||||
| Placebo | PBT2 | Placebo | PBT2 | All | PBT2 | ||
| PiB SUVR Δ/yr (n) | −0.08 (15) | −0.09∗ (25) | −0.0034 (11) | 0.058 (16) | 0.033 (27) | −0.04 (16) | 0.06† (40) |
| FDG PCI SUVR Δ/yr (n) | −0.034∗ (15) | −0.041∗ (24) | −0.005 (11) | −0.002 (14) | −0.045‡ (25) | −0.046‡ (14) | N/A |
| Hippocampal volume Δ/yr (n) | −0.21† (15) | −0.11† (25) | −0.12∗ (11) | −0.27‡ (15) | −0.33‡ (26) | −0.18‡ (15) | −0.20† (30) |
| Gray matter volume Δ/yr | −4.15∗ | −5.86† | −0.62 | −7.50† | −4.58† | −5.92‡ | −7.22† |
| Ventricular volume Δ/yr | 2.80‡ | 3.15‡ | 3.07‡ | 3.22‡ | 3.16‡ | 2.76‡ | 3.81‡ |
Abbreviations: AIBL, Australian Imaging Biomarker and Lifestyle Study; PiB, Pittsburgh compound B; SUVR, standardized uptake volume ratio; FDQ, fluorodeoxyglucose; PCI, posterior cortical index; N/A, Not available.
P < .05.
P < .001.
P < .0001.
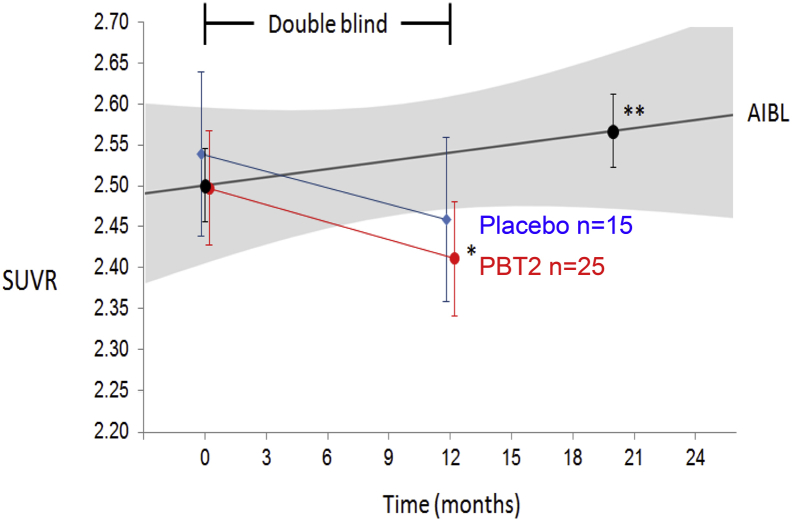
Fig. 6 shows the baseline-adjusted mean SUVR values at baseline and 12 months of the double-blind phase. There is no significant difference between the groups with a very large overlap in standard error of the mean at each time point. Unexpectedly, the placebo group showed a nonsignificant decline from baseline at 12 months (P = .06), whereas the PBT2 group showed a significant decline from their baseline levels (P = .048) (Fig. 6). Comparing the 12-month differences in slopes in SUVR with the matched AIBL historical controls (n = 46), there is a significant difference in the placebo group (P = .013) and PBT2 group (P = .0018).
Fig. 6.
Longitudinal PiB SUVR data from the IMAGINE double-blind phase. Line graphs showing the mean (±SEM) PiB SUVR at baseline and at 12 months for the placebo (blue circles) and PBT2 (red circles) groups. As a contemporaneous control group, a group of 46 AIBL participants (black circles) meeting the IMAGINE inclusion criteria and matched for IMAGINE baseline PiB SUVR. While there were no significant differences between placebo and PBT2 at 12 months, there was a significant decrease of 0.09 PiB SUVR/yr in the PBT2 group (P = .048). While the IMAGINE placebo group showed a nonsignificant decrease of 0.08 PiB SUVR/yr, the AIBL participants showed a significant increase of 0.04 PiB SUVR/yr (P = .0014), and an even greater increase (0.07 PiB SUVR/yr) was observed in the APOE ε4 placebo group of the bapineuzumab trial [22]. Abbreviations: AIBL, Australian Imaging Biomarker and Lifestyle Study; SUVR, standardized uptake volume ratio. ∗∗P < .001.
In the 12-month extension phase, 11 placebo and 16 PBT2 subjects accepted the invitation to participate. Their SUVR values at baseline, 12, and 24 months are shown in Fig. 7. At 12 months, the 13 subjects who declined to participate or who withdrew during the extension phase had a mean SUVR of 2.68 ± 0.55, which was significantly (P = .0014) higher than the mean SUVR of 2.30 ± 0.47 of those 27 subjects who completed the full 24-month study (Fig. 6). The mean SUVR of the 11 placebo subjects who entered the extension study did not decline significantly in the double-blind phase (P = .09), but the mean SUVR of the 16 PBT2 subjects in the double-blind phase had a very significant decline from baseline (P = .009). Both groups combined in the extension study showed no significant change over the 12 months of open label treatment (P = .22). However, the baseline mean SUVR of 2.4 ± 0.48 in this combined group was significantly different from the mean at 24 months (2.33 ± 0.49, paired t-test, P = .05) (Fig. 7). The slopes of change in these subjects over 24 months were significantly (P = .001) different from those in the AIBL comparator group (Fig. 6).
Fig. 7.
Longitudinal PiB SUVR data from participants who completed the two phases of the IMAGINE study. Line graphs showing the mean (±SEM) PiB SUVR at baseline and at 12 months for 11 placebo (blue circles) and 16 PBT2 (red circles) participants in the IMAGINE double-blind phase of the study. In purple triangles, the 27 participants who completed the open label extension study. As comparison group, a group of 40 AIBL participants (black squares) meeting the IMAGINE inclusion criteria and matched for the baseline PiB SUVR of the 27 participants who completed the two phases of the IMAGINE study. While there were no significant differences between the 11 placebo and 16 PBT2 participants at 12 months, there was a significant decrease of 0.14 PiB SUVR/yr in the PBT2 group (P = .005). While the 11 IMAGINE placebo participants showed a nonsignificant decrease of 0.07 PiB SUVR/yr, the AIBL participants showed a significant increase of 0.04 PiB SUVR/yr (P = .0014). While no significant changes in PiB SUVR from 12 to 24 months were observed on the 27 participants who completed the IMAGINE open-label phase, a significant decrease of 0.04 PiB SUVR/yr (P = .05) was observed when changes in PiB SUVR from 0 to 24 months were considered. Abbreviations: AIBL, Australian Imaging Biomarker and Lifestyle Study; SUVR, standardized uptake volume ratio. ∗P < .05; ∗∗P < .001.
No additional significant effects were observed when the different regions were separately examined.
3.4. The effect of PBT2 on glucose metabolism
The FDG-SUVR in both placebo and PBT2 groups declined significantly (P = .05, .008 respectively) in the double-blind phase and then stabilized in those who participated in the open label study over the ensuing 12 months (Table 2).
3.5. The effect of PBT2 on brain MRI volumetrics
There was a nonsignificant trend for a slower rate of change in hippocampal atrophy to be slower in the PBT2 group (−0.029 cc/yr) compared to the placebo group (−0.057 cc/yr) over the double-blind phase. This trend was not maintained in the subjects who went on to open label, with continued hippocampal atrophy in both groups (Table 2). Gray matter volumes decreased significantly on both groups in the double-blind phase and in the extension phase (Table 2). Similarly, significant increases in ventricular volumes were observed over the double-blind and open-label phases (Table 2).
3.6. The effect of PBT2 on blood Aβ biomarkers
There was no significant effect of PBT2 on any of the Aβ blood biomarkers at 6 or 12 months in the double-blind phase of this study.
3.7. The effect of PBT2 on cognition and function
For the double-blind part of the clinical trial, no difference between the placebo and PBT treatment conditions was identified for any NTB performance measure. For the open label part of the trial, performance did not improve generally at the 24-month assessment relative to the 12-month assessment. When these data were separated into those who had and had not been randomized to PBT2 in the double-blind stage of the trial, no differences between double-blind treatment groups were observed. Finally, compared to performance at the baseline assessment, NTB performance measures at the 24-month assessment had not changed significantly from baseline. These cognitive results are summarized in Table 4. Assessment of function (ADCS-ADL-23) similarly showed no differences between groups (Table 1).
Table 4.
Cognitive scores: adjusted group means (+SE) for the placebo and PBT groups for the double blind and open-label phases of the IMAGINE trial
| Cognitive scores | Double blind |
Open label |
||||
|---|---|---|---|---|---|---|
| 0–12 Mo Δ/yr |
12–24 Mo Δ/yr |
0–24 Mo Δ/yr |
||||
| Placebo N = 12 | PBT2 N = 21 | Placebo N = 11 | PBT2 N = 16 | All N = 27 | PBT2 N = 27 | |
| Category fluency | 10.95 (0.86) | 9.74 (0.63) | 7.77 (1.16) | 10.9 (0.94) | 9.34 (0.70) | 9.38 (0.69) |
| Verbal fluency | 25.37 (2.60) | 30.46 (1.89) | 28.38 (2.28) | 27.43 (1.84) | 27.90 (1.35) | 27.23 (1.78) |
| RAVLT acquisition | 34.98 (3.13) | 37.1 (2.27) | 27.86 (2.29) | 35.04 (2.29) | 31.45 (1.70) | 30.99 (2.30) |
| RAVLT delay | 2.44 (0.82) | 2.65 (0.57) | 1.98 (0.46) | 1.77 (0.37) | 1.87 (0.28) | 1.83 (0.49) |
| Trial making B | 160.24 (12.69) | 167.36 (9.48) | 106.73 (21.01) | 99.48 (17.14) | 103.10 (12.10) | 102.27 (12.59) |
| Digit span | 13.38 (0.98) | 14.79 (0.71) | 13.71 (0.82) | 13.45 (0.65) | 13.58 (0.48) | 13.49 (0.57) |
Abbreviation: RAVLT, Rey Auditory Verbal Learning Test.
4. Discussion
There is a pressing need for disease modifying therapeutic strategies for AD, and Aβ-amyloid remains high on the list of targets. Recent encouraging results with immuno-modulating or -clearing effects of antibodies directed at Aβ [23], [24], [25] point strongly to intervention at the earliest stages of AD, as defined by specific imaging and CSF biomarkers. The failure to date of inhibition or modulation of γ-secretase [26] underscores the hopes held for the efficacy of β-secretase (BACE1) inhibitors either alone or in combination [27]. Other approaches which directly or indirectly target Aβ are in varying stages of development [28], [29], [30], including PBT2 representing the class of 8-OH quinolones [31], [32].
PBT2 has completed early phase IIa studies in AD [15] and Huntington's disease [33] and, together with this present exploratory study, has proven to be generally safe and well tolerated. The lack of cognitive or functional benefits or any significant effects on glucose metabolism, MRI volumetrics, or blood biomarkers is not surprising, given the small numbers in this exploratory trial. The previous phase IIa study involving PBT2 [15] had 75 subjects over 3 months. A 13% fall in CSF Aβ42 was observed in that study. In the present study, we focussed attention on the utility of PiB-PET readouts.
The molecular imaging results in this trial indicate that PBT2 could lower the PiB-PET Aβ signal by 3% over 12 months, in contrast to the 3% increase observed in a closely matched group taken from the AIBL longitudinal study. The smaller placebo group showed large variability and an unexpected nonsignificant decline over the first 12 months, which precluded reaching the primary outcome measure of the trial (Fig. 3). Future studies will need to be sufficiently powered to take this variability into account (see below). The inclusion criterion of SUVR >1.7 resulted in a group selected with a mean SUVR of 2.51 ± 0.59 which is well within the plateau phase of fully developed AD [9], [10]. Inadvertently, we may have selected a group of AD subjects who had high PiB-PET SUVR yet only relatively mild cognitive impairments (MMSE > 20). In this mild AD range, there is little or no correlation of cognitive status with PiB-PET SUVR [34]. A similar phenomenon may also have occurred in the recently completed trial of solanezumab in mild AD [35]. Interestingly, we observed a significant negative correlation of baseline SUVR and change over 12 months in the PBT2 group but not in the placebo group (Fig. 4), and that the drug-effect was more evident in the subjects with higher baseline SUVR (Fig. 5). Much larger studies would be required to confirm this effect. However, conducting this type of study at the higher (nonlinear) end of the SUVR spectrum may be counter-productive.
We noted that the subjects who did not enter or who dropped out of the extension phase had significantly higher SUVRs than those who completed. This probably reflects on the poorer physical well-being of those subjects who are carrying higher Aβ burdens and should be a warning for clinical trialists to take these drop-outs into consideration when interpreting trial results. In the 27 subjects who did complete both phases of this study, the PBT2 group showed a highly significant fall in the first 12 months (Fig. 7), coming off a baseline value of 2.42 ± 0.51. This would suggest that even lower starting baselines should have been employed.
Comparing the present study with others which have used PiB-PET as a biomarker, we note that the bapineuzumab APOE ε4 carrier subgroup had a mean baseline SUVR of 2.11 ± 0.33 and showed a drug effect of 4.8% over 18 months [22], [36] (Fig. 8). The recently reported aducanumab phase 1 study had intake criteria of PiB-equivalent of ∼1.9-2.1 [25] (see also Fig. 8), and the AZD 3293 trial of a BACE 1 inhibitor has intake criteria of PiB-equivalent ∼1.6-2.0 [38]. Based on Aβ-CSF levels, we infer that the intake PiB SUVR of the PBT2 Euro study was ∼2.15 [39]. Taken together, these observations suggest that the IMAGINE PiB baseline was exceptionally high, and that future studies should aim for intake PiB SUVR between 1.5 and 2.0, a value which is well-within the linear rates of change as seen in AIBL and other longitudinal studies [9], [10]. This will necessitate a trade-off between milder levels of cognitive impairment on the preclinical/prodromal stages of AD (with consequent loss of power in detecting cognitive change) and improving the signal: noise rations in the linear-change region of the SUVR. If the SUVR results of the present study are to be confirmed, we calculate that a minimum of 90 subjects per arm will be required, over a longer period (18 months). Finally, having a lower number of subjects randomized to placebo than to the active treatment might reduce the chances to see a drug effect, due to a larger variance in the placebo group as observed in this study. A more balanced approach should be taken [40].
Fig. 8.
Comparison of the IMAGINE study PiB results with the reported PiB results of the bapineuzumab trial [22], [36], and the florbetapir results from the aducanumab trial [25]. To allow comparison between the different Aβ tracers, florbetapir SUVRWCb are expressed in PiBCb-like units called BeCKeTs [37]. A striking difference between the IMAGINE and the bapineuzumab and aducanumab trials is the Aβ burden at baseline. Overall, IMAGINE participants not only had significantly higher baseline Aβ burden, these baseline values were at levels for which we have demonstrated the rates of Aβ accumulation slow [9], making it difficult to discriminate between a potential drug effect that slows Aβ accumulation and the natural slowing that occurs at those SUVR levels. These comparisons highlight the importance of not only establishing a SUVR floor, but also the need of establishing a SUVR ceiling to ensure all participants are accumulating Aβ at a similar rate. Abbreviations: AIBL, Australian Imaging Biomarker and Lifestyle Study; SUVR, standardized uptake volume ratio. ∗ P < .05; ∗∗ P < .001.
Future progress in achieving disease modification in AD is dependent on the three strategic arms of determining maximal tolerated dose, exploring rational combinations of therapeutics, and design of “super-adaptive” trials with frequent interim biomarker analyses [41]. Data from this exploratory IMAGINE study and the further clinical development of PBT2 could be useful in developing each of these strategic arms.
Research in Context.
-
1.
Systematic review: We reviewed all publications and disclosures of clinical trials employing amyloid β-PET molecular imaging as a biomarker readout for Aβ amyloid–targeted modifying therapeutic strategies in Alzheimer's disease (AD).
-
2.
Interpretation: This exploratory trial did not show efficacy of PBT2 in a small double-blinded study. However, subjects recruited had a mean baseline PiB-PET standardized uptake volume ratio of 2.51, much higher than anticipated, and this may have contributed to the failure.
-
3.
Future directions: Setting an upper level on the PET-standardized uptake volume ratio at the time of recruitment might help ensure that subjects fall into the linear accumulation phase of AD. Further evaluation of PBT2 in earlier stages of AD is warranted.
Acknowledgment
This clinical trial was sponsored by Prana Biotechnology. Additional research support was provided in part by grants from the National Health and Medical Research Council (NHMRC), Australia. The Australian Imaging, Biomarker and Lifestyle (AIBL) study is supported in part by NHMRC: other commercial and philanthropic sources are acknowledged at https://aibl.csiro.au.
Footnotes
Conflict of interest declaration: K.J.B. is a paid consultant and R.C. was a part-time employee of Prana Biotechnology. C.R. was, at the time, a retained consultant with Prana Biotechnology Ltd as part of their standing scientific advisory group and performed the duties of the medical monitor during the course of the study. R.T. is a paid consultant and shareholder in Prana Biotechnology Ltd. R.T. had no input into the drafting or review of any portion of the manuscript other than those portions pertaining to study concept and design and consultation on biochemistry, and no input into the analysis of the data from other portions. C.L.M. was a Founding Director and shareholder of Prana Biotechnology but has no current appointment with the company.
References
- 1.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Blennow K., Mattsson N., Scholl M., Hansson O., Zetterberg H. Amyloid biomarkers in Alzheimer's disease. Trends Pharmacol Sci. 2015;36:297–309. doi: 10.1016/j.tips.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Coleman R.E., Doraiswamy P.M. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 5.Matveev S.V., Spielmann H.P., Metts B.M., Chen J., Onono F., Zhu H. A distinct subfraction of Aβ is responsible for the high-affinity Pittsburgh compound B-binding site in Alzheimer's disease brain. J Neurochem. 2014;131:356–368. doi: 10.1111/jnc.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson B.W., Elbert D.L., Mawuenyega K.G., Kasten T., Ovod V., Ma S. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78:439–453. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmqvist S., Zetterberg H., Blennow K., Vestberg S., Andreasson U., Brooks D.J. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 8.Li Q.X., Villemagne V.L., Doecke J.D., Rembach A., Sarros S., Varghese S. Alzheimer's disease normative cerebrospinal fluid biomarkers validated in PET amyloid-β characterized subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Alzheimers Dis. 2015;48:175–187. doi: 10.3233/JAD-150247. [DOI] [PubMed] [Google Scholar]
- 9.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 10.Jack C.R., Wiste H.J., Lesnick T.G., Weigand S.D., Knopman D.S., Vemuri P. Brain β-amyloid load approaches a plateau. Neurology. 2013;80:890–896. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toledo J.B., Xie S.X., Trojanowski J.Q., Shaw L.M. Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013;126:659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adlard P.A., Cherny R.A., Finkelstein D.I., Gautier E., Robb E., Cortes M. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Cherny R.A., Atwood C.S., Xilinas M.E., Gray D.N., Jones W.D., McLean C.A. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 14.Ryan T.M., Roberts B.R., McColl G., Hare D.J., Doble P.A., Li Q.X. Stabilization of nontoxic Aβ-oligomers: insights into the mechanism of action of hydroxyquinolines in Alzheimer's disease. J Neurosci. 2015;35:2871–2884. doi: 10.1523/JNEUROSCI.2912-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lannfelt L., Blennow K., Zetterberg H., Batsman S., Ames D., Harrison J. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7:779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Dubois B., Feldman H.H., Jacova C., Cummings J.L., DeKosky S.T., Barberger-Gateau P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 18.Villemagne V.L., Pike K.E., Chetelat G., Ellis K.A., Mulligan R.S., Bourgeat P. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 20.Ellis K.A., Szoeke C., Bush A.I., Darby D., Graham P.L., Lautenschlager N.T. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) Int Psychogeriatr. 2014;26:543–554. doi: 10.1017/S1041610213001956. [DOI] [PubMed] [Google Scholar]
- 21.Rembach A., Faux N.G., Watt A.D., Pertile K.K., Rumble R.L., Trounson B.O. Changes in plasma amyloid beta in a longitudinal study of aging and Alzheimer's disease. Alzheimers Dement. 2014;10:53–61. doi: 10.1016/j.jalz.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu-Seifert H., Siemers E., Holdridge K.C., Andersen S.W., Lipkovich I., Carlson C. Delayed-start analysis: Mild Alzheimer's disease patients in solanezumab trials, 3.5 years. Alzheimers Dement. 2015;1:111–121. doi: 10.1016/j.trci.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2015;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 25.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [Addendum. Nature. 2017;2546:2564] [DOI] [PubMed] [Google Scholar]
- 26.De Strooper B. Lessons from a failed γ-secretase Alzheimer trial. Cell. 2014;159:721–726. doi: 10.1016/j.cell.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Strömberg K., Eketjäll S., Georgievska B., Tunblad K., Eliason K., Olsson F. Combining an amyloid-beta (Aβ) cleaving enzyme inhibitor with a γ-secretase modulator results in an additive reduction of Aβ production. FEBS J. 2015;282:65–73. doi: 10.1111/febs.13103. [DOI] [PubMed] [Google Scholar]
- 28.Karran E., Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014;76:185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jawhar S., Wirths O., Schilling S., Graubner S., Demuth H.U., Bayer T.A. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem. 2011;286:4454–4460. doi: 10.1074/jbc.M110.185819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izzo N.J., Xu J., Zeng C., Kirk M.J., Mozzoni K., Silky C. Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers II: sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS ONE. 2014;9:e111899. doi: 10.1371/journal.pone.0111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeVine H., 3rd, Ding Q., Walker J.A., Voss R.S., Augelli-Szafran C.E. Clioquinol and other hydroxyquinoline derivatives inhibit Aβ(1-42) oligomer assembly. Neurosci Lett. 2009;465:99–103. doi: 10.1016/j.neulet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matlack K.E., Tardiff D.F., Narayan P., Hamamichi S., Caldwell K.A., Caldwell G.A. Clioquinol promotes the degradation of metal-dependent amyloid-β (Aβ) oligomers to restore endocytosis and ameliorate Aβ toxicity. Proc Natl Acad Sci U S A. 2014;111:4013–4018. doi: 10.1073/pnas.1402228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huntington Study Group Reach2HD Investigators Safety, tolerability, and efficacy of PBT2 in Huntington's disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:39–47. doi: 10.1016/S1474-4422(14)70262-5. [DOI] [PubMed] [Google Scholar]
- 34.Rowe C.C., Ellis K.A., Rimajova M., Bourgeat P., Pike K.E., Jones G. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Strobel G. 2017. Solanezumab: Did Aβ 'reflux' from blood confound target engagement in CSF? [Internet] Available at: http://www.alzforum.org/print-series/745106. Accessed October 11, 2017. [Google Scholar]
- 36.Liu E., Schmidt M.E., Margolin R., Sperling R., Koeppe R., Mason N.S. Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85:692–700. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villemagne V.L., Doré V., Yates P., Brown B., Mulligan R., Bourgeat P. En attendant centiloid. 2014. Adv Res. 2014;2:723–729. [Google Scholar]
- 38.Clinical Trials.gov [Internet]. Registration number: NCT02245737. An efficacy and safety study of LY3314814 in early Alzheimer's disease (AMARANTH); 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02245737?term=AZD3293&rank=8. Accessed October 11, 2017
- 39.Weigand S.D., Vemuri P., Wiste H.J., Senjem M.L., Pankratz V.S., Aisen P.S. Transforming cerebrospinal fluid Aβ42 measures into calculated Pittsburgh Compound B units of brain Aβ amyloid. Alzheimers Dement. 2011;7:133–141. doi: 10.1016/j.jalz.2010.08.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider L.S., Lahiri D.K. The perils of Alzheimer's drug development. Curr Alzheimer Res. 2009;6:77–78. doi: 10.2174/156720509787313871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strobel G. 2015. As DIAN plans trial number two, the goal is to go big. ALZFORUM [Internet] Available at: http://www.alzforum.org/news/conference-coverage/dian-plans-trial-number-two-goal-go-big. Accessed October 11, 2017. [Google Scholar]