Abstract
Aims
The impact of refractory angina pectoris (AP) in patients with ischaemic cardiomyopathy (ICM) is unknown. We investigated the characteristics and outcomes of ICM patients with persistent AP following cardiac catheterization.
Methods and results
Patients who underwent coronary angiography at Duke from 2000 to 2009 with an EF <40% and ICM with persistent AP were compared with similar patients without persistent AP. Persistent AP was defined by patient report of ischaemic symptoms within 1 year of index catheterization. Time-to-event was examined using Kaplan–Meier or cumulative incidence and Cox proportional hazards modelling methods for death/myocardial infarction (MI)/revascularization [i.e. major adverse cardiac events (MACE)], death/MI, death, and cardiovascular death/hospitalization. Of 965 ICM patients, 298 (31%) had persistent AP. These patients were younger and had more previous revascularization than patients without persistent AP. Both groups had high use of aspirin, beta-blockers, ACE inhibitors, and statins, but modest nitrate use. Over a median follow-up of > 5 years, patients with persistent AP had increased rates of MACE, and cardiovascular death/hospitalization compared with patients without persistent AP [5-year cumulative event rates of 53% vs. 46% (p = 0.013) and 73% vs. 60% (p < 0.0001), respectively], but similar rates of death (p = 0.59) and death/MI (p = 0.50). After multivariable adjustment, persistent AP remained associated with increased MACE [hazard ratio (HR) 1.30; 95% confidence interval (CI) 1.08–1.57], and cardiovascular death/hospitalization (HR 1.36; 95% CI 1.14–1.62).
Conclusion
Persistent AP is common despite medical therapy in patients with ICM and is independently associated with increased long-term MACE and rehospitalization. Future prospective studies of persistent AP in ICM patients are warranted.
Keywords: Heart failure, Persistent angina pectoris, Outcomes
Introduction
Angina pectoris (AP) is the symptomatic condition related to myocardial ischaemia.1,2 We have previously shown that AP is present in >50% of patients with ischaemic cardiomyopathy (ICM) and is associated with increased cardiovascular death or cardiovascular rehospitalization.3 However, baseline AP was not associated with increased all-cause death, major adverse cardiac events (MACE), or all-cause hospitalization in ICM patients. In contrast, AP was associated with increased MACE due to more revascularization in a cohort of heart failure (HF) patients with preserved EF (i.e. HFpEF).4 Thus, AP has different prognostic implications in various patient populations. The impact of AP that persists or recurs following intervention in ICM patients is unknown. Given the high event rate in the ICM population and the neutral results of most recent trials of novel agents in HF patients,5,6 there is a need to identify potential therapeutic targets and patients at higher risk. We investigated the clinical characteristics and outcomes of ICM patients with persistent AP in the year following index cardiac catheterization.
Methods
Data source and patient population
Patient data were obtained from the Duke Databank for Cardiovascular Disease (DDCD), an ongoing databank of all patients who undergo diagnostic cardiac catheterization at Duke University Medical Center. Patients were included in the study population if they underwent coronary angiography from January 2000 to December 2009, had ICM with an LVEF <40%, and histories of ≥50% stenosis in ≥1 epicardial coronary vessel (only those patients with histories of significant CAD receive DDCD follow-up). Coronary stenoses were graded by visual consensus of ≥2 experienced observers. Patients were excluded from analysis if they did not have AP at baseline or did not have follow-up to 1 year after cardiac catheterization. Additionally, patients were excluded if they had primary valvular heart disease (defined as severe aortic or mitral insufficiency or severe stenosis of any heart valve), congenital heart disease, acquired immunodeficiency syndrome, or metastatic cancer. Data from the index catheterization were prospectively collected as part of routine patient care. Baseline clinical variables taken at the time of the index catheterization for each patient were stored in the DDCD using methods previously described.7 The baseline AP characteristics were documented, including the severity, frequency, and pattern of occurrence, by the clinician. Follow-up was obtained through self-administered questionnaires, with telephone follow-up to non-responders. Patients not contacted through this mechanism had vital status determined through a search of the National Death Index.8
Definitions
Similar to our previous work,3 we used a revision of the standardized research definition of ICM previously shown to be the most prognostically powerful:9 (i) a history of myocardial infarction (MI) or revascularization [coronary artery bypass grafting (CABG) or PC1]; (ii) ≥50% stenosis of the left main or proximal left anterior descending coronary artery; or (iii) ≥50% stenosis of ≥2 epicardial vessels. As in previous studies,3,4 baseline AP classification was based on physician-obtained patient history just before cardiac catheterization and was defined as chest pain within the previous 6 weeks. Persistent AP was defined by patient report of ischaemic symptoms on a follow-up survey within 1 year of index catheterization. Specifically, patients were asked whether they had ‘angina symptoms in the previous week’. Angina was presented as the following: ‘A symptom of pain or discomfort that is due to the heart and is usually located in the chest, but may also be felt in the arms, back, neck, or jaw. Angina may feel like pain, pressure, soreness, or indigestion. It is not the soreness or discomfort felt around the chest incision after heart surgery.’
Revascularization was defined as treatment with PCI or CABG. Death, MI, and cardiovascular rehospitalization were determined using methods previously described.7,10
Statistical analysis
Baseline characteristics are described with medians and interquartile ranges for continuous variables and percentages for discrete variables in ICM patients with vs. without persistent AP. These characteristics were compared using theWilcoxon rank-sum test for continuous variables, and χ2 tests for categorical variables unless otherwise noted. Unadjusted time-to-event results were assessed using Kaplan–Meier methods, and comparisons were made using the log-rank test for three endpoints: the primary endpoint of death/MI/revascularization (i.e. MACE) and secondary endpoints of death/MI, death/rehospitalization and death. To account for the competing risk of non-cardiovascular deaths, unadjusted time-to-event results were assessed using Cumulative Incidence, and a comparison was made using the Grey Test for the additional secondary endpoint of cardiovascular death/cardiovascular hospitalization. Multivariable Cox proportional hazards regression analysis was used to adjust for baseline differences between groups. A comprehensive set of covariates was used for the adjustment analysis (see Table 3 footnote) based on clinical relevance and data from previous investigations.3,4 The adjustment covariates included revascularization within 1 year after the index catheterization. All available follow-up information was used in all time-to-event analyses. The follow-up period starts at 1 year following the index catheterization procedure. A p-value <0.05 was used to indicate statistical significance for all comparisons. The protocol was approved by the institutional review board at Duke University. Statistical analyses were performed by Duke Clinical Research Institute (Durham, NC, USA) using SAS system version 9.2 (Cary, NC, USA).
Table 3.
Persistent angina pectoris as a predictor of outcome on adjusted analysis
| Endpoint | Adjusteda hazard ratio (95% confidence interval) | P-value |
|---|---|---|
| Death/myocardial infarction/revascularization | 1.30 (1.08–1.57) | 0.006 |
| Death/myocardial infarction | 1.17 (0.95–1.44) | 0.14 |
| Death | 1.14 (0.92–1.41) | 0.23 |
| Cardiovascular death/cardiovascular hospitalization | 1.36 (1.14–1.62) | 0.0006 |
| All-cause death/all-cause hospitalization | 1.33 (1.13–1.56) | 0.0006 |
Adjusted for age, sex, race, hypertension, diabetes, prior myocardial infarction, hyperlipidaemia, NYHA class, cerebrovascular disease, peripheral vascular disease, previous smoking history, ventricular gallop, Charlson Index, body mass index, heart rate, systolic blood pressure, use of beta-blocker, ACE inhibitor, or ARB, hydralazine, nitrates, calcium channel blocker, aspirin, clopidogrel, statin, diuretic, and serum creatinine, sodium, blood urea nitrogen, haemoglobin, percutaneous coronary intervention or coronary artery bypass grafting within 1 year of index catheterization.
Results
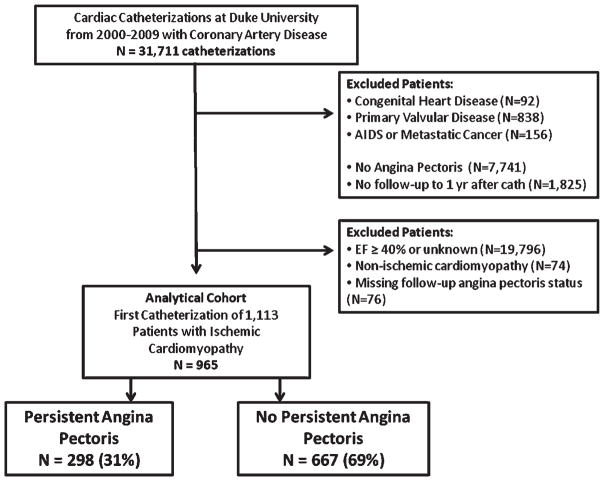
A total of 965 patients met the criteria for the study (Figure 1) and 298 (31%) had persistent AP. Baseline characteristics for the study groups are provided in Table 1. The two groups were fairly similar, with several notable exceptions. Patients with persistent AP were younger with more prior revascularization (i.e. revascularizations occurring prior to the index catheterization) than those without persistent AP. In addition, baseline AP tended to be more severe (i.e. a higher percentage of patients with symptoms with any exertion or at rest) and more frequent in the persistent AP group. Patients without persistent AP at follow-up tended to have unstable symptoms at the index catheterization compared to the patients with persistent AP who tended to have progressive symptoms at the index catheterization. Both groups had high use of aspirin, beta-blockers, and ACE inhibitors/ARBs, but modest nitrate use. In terms of anti-anginal medications, 93% of patients in the persistent AP group received a beta-blocker, calcium channel blocker, or nitrate at baseline compared with 92% in the non-AP group. At the time of index catheterization or within the next 30 days, the percentage of patients that underwent coronary revascularization was similar between patients experiencing persistent AP and those not experiencing persistent AP. Specifically, PCI was performed within 30 days in 35.2% of persistent AP patients and in 36.0% of non-persistent AP patients, and CABG was performed in 23.5% of persistent AP patients and in 26.1% of non-persistent AP patients (both p-values for comparison >0.05). Also, a similar percentage of patients in both groups did not have a revascularization within the first 30 days, but then underwent at least one revascularization during the period from 30 days up to 1 year following index catheterization: PCI in 4.0% of persistent AP patients and 2.1% of non-persistent AP patients, and CABG in 2.0% of persistent AP patients and 3.4% of non-persistent AP patients (both p-values >0.05).
Figure 1.
Patients included in this analysis. AIDS, acquired immunodeficiency syndrome; yr, year.
Table 1.
Patient characteristics at the index catheterization
| Variable | Persistent angina pectoris
|
P-value | |
|---|---|---|---|
| No (n =667) | Yes (n =298) | ||
| Age (years) | 64 (55–73) | 61 (52–70) | <0.001a |
| Men | 77% | 74% | 0.38 |
| Race | 0.64 | ||
| White | 74% | 76% | |
| Black | 19% | 18% | |
| Hypertension | 71% | 73% | 0.69 |
| Diabetes mellitus | 34% | 36% | 0.57 |
| Prior myocardial infarction | 72% | 67% | 0.14 |
| Hyperlipidaemia | 65% | 71% | 0.07 |
| Three-vessel coronary disease | 57% | 60% | 0.71 |
| Ejection fraction (%) | 33 (27–36) | 33 (26–37) | 0.46 |
| NYHA class III–IV | 21% | 22% | 0.64 |
| Cerebrovascular disease | 10% | 13% | 0.16 |
| Peripheral vascular disease | 12% | 15% | 0.26 |
| Previous smoking | 62% | 65% | 0.28 |
| Previous percutaneous coronary intervention | 33% | 40% | 0.03 |
| Previous coronary bypass | 36% | 44% | 0.01 |
| Charlson Index ≥2 | 20% | 22% | 0.50 |
| Baseline AP characteristics | |||
| Canadian Cardiovascular Society severity | <0.001 | ||
| No symptoms with ordinary activity | 0% | 1% | |
| Symptoms with moderate exertion | 11% | 11% | |
| Symptoms with ordinary exertion | 14% | 13% | |
| Symptoms with any exertion or at rest | 35% | 48% | |
| Symptoms unrelated to exertion | 40% | 27% | |
| AP episodes per week | 3 (2–5) | 4 (2–7) | <0.001 |
| Body mass index (kg/m2) | 28 (25–32) | 28 (25–32) | 0.38 |
| Heart rate (b.p.m.) | 73 (64–84) | 72 (63–83) | 0.15 |
| Systolic blood pressure (mmHg) | 136 (121–154) | 131 (118–150) | 0.08 |
| Rales | 14% | 13% | 0.57 |
| S3 gallop | 8% | 10% | 0.36 |
| LVEDP, mmHg | 20 (13–27) | 20 (14–25) | 0.52 |
| Serum sodium (mmol/L) | 139 (137–141) | 140 (138–141) | 0.22 |
| Blood urea nitrogen (mg/dL) | 17 (13–23) | 16 (12–21) | 0.09 |
| Serum creatinine (mg/dL) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 0.46 |
| Haemoglobin (g/dL) | 13.7 (12.5–14.8) | 13.6 (12.1–14.8) | 0.21 |
| Beta-blocker use | 91% | 93% | 0.30 |
| ACE inhibitor/ARB | 86% | 86% | 0.90 |
| Hydralazine use | 5% | 4% | 0.93 |
| Nitrate use | 18% | 23% | 0.08 |
| Calcium channel blocker use | 35% | 34% | 0.73 |
| Ranolazine useb | 0.4% | 0% | 0.56c |
| Aspirin use | 92% | 93% | 0.74 |
| Clopidogrel use | 57% | 53% | 0.31 |
| Statin use | 78% | 80% | 0.43 |
| Diuretic use | 74% | 78% | 0.29 |
Values are expressed as %, or median (Q1–Q3).
AP, angina pectoris; LVEDP, left ventricular end-diastolic presssure.
Result from Student’s t-test.
Ranolazine was FDA (Food and Drug Administration) approved in 2006.
Result from Fisher’s exact test.
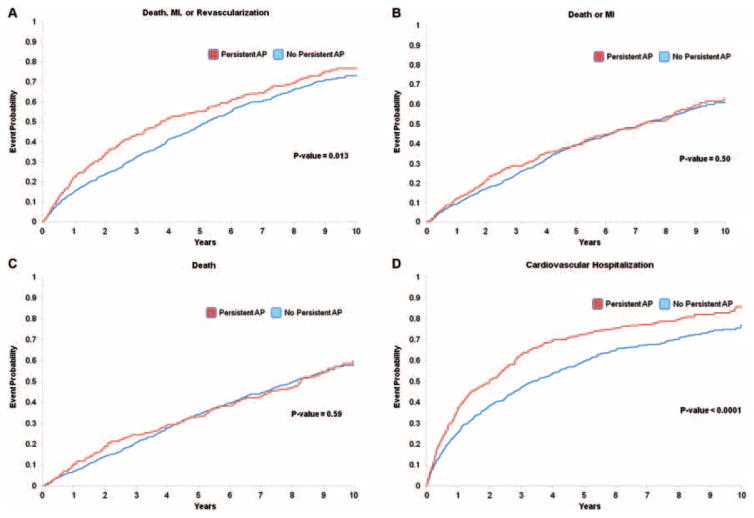
The median follow-up time for all patients was 5.6 years (interquartile range: 2.9–8.3). The 5-year unadjusted Kaplan–Meier death rate for the study population was 33.7%. Persistent AP patients were observed to have a significantly increased event rate for the primary endpoint of MACE (5-year Kaplan–Meier event rates of 52.9% vs. 45.7%, p =0.013) as well as cardiovascular death or hospitalization (5-year Cumulative Incidence of 72.7% vs. 59.6%, p <0.0001] (Table 2). There were no significant differences between the event rates in those with and without persistent AP for the endpoints of death/MI and death (Table 2). The time-to-event plots in patients with vs. without persistent AP are presented in Figure 2. Following risk adjustment, persistent AP was associated with a significantly higher risk of MACE and cardiovascular death/hospitalization over the follow-up period compared with no AP (both p <0.05) (Table 3). Persistent AP was an independent predictor of MACE [hazard ratio (HR) 1.30; 95% confidence interval (CI) 1.08–1.57] and cardiovascular death/hospitalization (HR 1.36; 95% CI 1.14–1.62). Patients with and without persistent AP had similar risk for death/MI and death over the follow-up period (both p >0.06) (Table 3). Results for the composite endpoint of all-cause death/hospitalization in those with persistent AP (adjusted HR 1.33; 95% CI 1.13–1.56; p =0.0006) were similar to the results for cardiovascular death/cardiovascular hospitalization. The proportional hazards assumption was assessed and not violated for AP in all multivariable Cox proportional hazards regression analyses.
Table 2.
Five- and ten-year unadjusted event rates for those with and without persistent angina pectoris*Time 0 is one year after the index catheterization
| Endpoint | 5 Yeara Angina pectoris |
10 Yeara Angina pectoris |
P-valueb | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| Death/myocardial infarction/revascularization | |||||
| Events for composite (first events) | 289 | 155 | 379 | 201 | |
| Death | 257 | 135 | 346 | 180 | |
| Myocardial infarction | 22 | 13 | 22 | 14 | |
| Revascularization | 10 | 7 | 11 | 7 | |
| KM rate (95% CI) | 45.7 (41.9–49.8) | 52.9 (47.3–58.7) | 68.8 (64.3–73.3) | 74.6 (68.6–80.3) | 0.013 |
| Death/myocardial infarction | |||||
| Events for composite (first events) | 247 | 115 | 330 | 163 | |
| Death | 203 | 85 | 280 | 127 | |
| Myocardial infarction | 44 | 30 | 50 | 36 | |
| KM rate for composite (95% CI) | 39.3 (35.6–43.3) | 39.3 (34.0–45.2) | 60.9 (56.2–65.6) | 62.8 (56.2–69.3) | 0.50 |
| Death | |||||
| Events | 214 | 97 | 306 | 151 | 0.59 |
| KM rate for composite (95% CI) | 34.1 (30.5–38.0) | 33.2 (28.1–38.9) | 57.5 (52.8, 62.4) | 59.4 (52.7–66.2) | |
| Cardiovascular death/cardiovascular hospitalization | |||||
| Events for composite (first events) | 382 | 213 | 443 | 237 | |
| Cardiovascular death | 72 | 26 | 90 | 32 | |
| Cardiovascular hospitalization | 310 | 187 | 353 | 205 | |
| Cumulative Incidence rate for composite (95% CI) | 59.6 (55.8–63.6) | 72.7 (67.7–78.1) | 76.7 (72.5–81.1) | 85.6 (80.7–90.8) | <0.0001 |
| Death/rehospitalization | |||||
| Events for composite (first events) | 505 | 263 | 551 | 276 | |
| Death | 74 | 27 | 83 | 31 | |
| Rehospitalization | 431 | 236 | 468 | 245 | |
| KM rate for composite (95% CI) | 78.2 (74.8–81.4) | 89.4 (85.5–92.6) | 91.3 (87.8–94.1) | 97.2 (92.7–99.3) | <0.0001 |
Time 0 is one year after the index catheterization.
P-value is from a Log-Rank test (or a Gray test for Cumulative Incidence) over all follow up between the strata of whether or not a patient had persistent angina.
CI, confidence interval; KM, Kaplan–Meier.
Figure 2.
Unadjusted event plots for (A) death, myocardial infarction, or revascularization [i.e. major adverse cardiac events (MACE)], (B) death or myocardial infarction, (C) death, and (D) cardiovascular death, or cardiovascular hospitalization in ischaemic cardiomyopathy patients with and without persistent angina pectoris. ‘Time 0’ corresponds to 1 year after the index catheterization. AP, angina pectoris; MI, myocardial infarction.
Discussion
Persistent AP was common in this ICM cohort (31%) despite medical therapy and previous revascularization. ICM patients with persistent AP had similar baseline characteristics compared with those without persistent AP symptoms. Nonetheless, those with persistent AP were at significantly increased risk for long-term MACE and rehospitalization. Specifically, we found that persistent AP was independently associated with a 30% increased risk for MACE and a 36% increased risk for cardiovascular death or hospitalization during follow-up. Similar to previous analyses of AP, we found that persistent AP was not associated with increased risk for death or death/MI. Thus, persistent AP identifies an ICM patient population at high risk for subsequent morbidity.
We found that nearly a third of patients with AP at baseline continued to have AP within 1 year following index catheterization. These patients had persistent AP despite high usage of anti-anginal therapies such as beta-blockers. Interestingly, patients who went on to experience persistent angina had similar revascularization rates at index catheterization and within the following year compared with those who did not experience persistent angina. It is also notable that 34% of the patients with persistent AP received calcium channel blockers, despite the contraindication to non-dihydropyridine calcium channel blockers in the setting of HF with reduced EF.1,2 Furthermore, the modest use of nitrates and ranolazine in these patients despite ongoing symptoms of angina suggests that there is room for significant improvement in the use of medical therapies to reduce AP in these patients.1
The persistent AP patients in this cohort were overall similar to those without persistent symptoms, yet several between-group differences were present that may have clinical implications. For instance, patients who developed persistent angina had more severe and more frequent AP at baseline. In addition, those who went on to have persistent AP tended to have progressive symptoms in the weeks prior to the baseline catheterization compared with those without persistent symptoms. In other words, patients with an insidious course of angina rather than rapidly worsening symptoms may represent the population most likely to continue to have persistent symptoms. With early identification of the patients who are more likely to have persistent AP, clinicians may be able to target medical therapies to these individuals in order to reduce the associated morbidity. Future studies will need to explore whether early intensification of anti-anginal therapy can reduce subsequent events.
Persistent AP was a strong independent predictor of long-term MACE. These findings, along with the lack of association between AP and death or death/MI, suggest that the implications for persistent AP are strongly correlated with increased revascularization. We have previously shown that baseline AP in HFpEF patients was associated with a 30% increased risk for death/MI/revascularization/stoke due to differences in revascularization.4 In the present study, we extend these findings to the ICM population with reduced EF who experience persistent AP. Thus, persistent AP appears to identify an ICM subgroup at substantially increased risk for long-term morbidity. These results have important clinical applications given the procedural costs and quality of life implications for revascularization procedures. Future studies are needed to investigate if improved management of AP, particularly during the first year following index catheterization, may reduce long-term revascularization rates.
Persistent AP was also independently associated with a 36% increased risk for cardiovascular death or hospitalization in ICM patients. Given that baseline AP was previously shown to be independently associated with a 12% increased risk for cardiovascular death/hospitalization in ICM patients compared with ICM patients without any AP,3 the present findings demonstrate the substantial increased risk for rehospitalization in those with persistent AP. In other words, baseline AP is associated with increased risk, but the magnitude of the risk increases further in the setting of refractory AP. These results have important clinical applications, since hospitalizations have increased over time in HF patients, and costs related to hospitalization account for the majority of the total cost of HF care.11,12 Whether improved management of AP reduces cardiovascular hospitalizations requires further study.
Our finding that persistent AP did not portend increased death or death/MI is concordant with previous studies of stable AP in the non-HF population as well as in HF populations with either reduced3 or preserved EF.4 Follow-up of the Angina Prognosis Study in Stockholm (APSIS) demonstrated that patients with stable AP had similar all-cause mortality compared with patients without AP over a median follow-up of 9 years.13 These results support previous data that revascularization may be performed to relieve anginal symptoms, but may not improve prognosis14 unless the patient demonstrates other high-risk features.1,2,15
Limitations
Our findings should be considered in the context of several limitations. The DDCD captures a subset of cardiac patients undergoing cardiac catheterization, which limits the population studied and may not reflect event rates in a broader population. However, the robust representation of both women and minorities in the DDCD provides important insight regarding frequently under-represented patient groups. Our use of a persistent AP classification at a single time point (follow-up survey within 1 year) is another potential limitation. Moreover, while the report of angina at follow-up was assessed with the same questionnaire for all study participants, the follow-up evaluation was different from the initial in-hospital evaluation and does not conform to a specific standard definition such as the devised by the Canadian Cardiovascular Society. However, the strengths of our follow-up angina definition were the consistent use across all patients and the relatively straightforward questioning involved which simplifies its incorporation into routine clinical practice. There was no specific ischaemia evaluation performed at follow-up. Nonetheless, this is the largest study to date to explore outcomes associated with persistent or recurrent angina following initial cardiac catheterization. Given the multiple analyses conducted, these results should be viewed as exploratory. Our study provides the foundation for future studies of AP in ICM patients in an attempt to improve patients’ symptoms and reduce the associated morbidity.
Conclusion
Persistent AP is common in patients with ICM despite medical therapy and previous revascularization, and is independently associated with increased long-term MACE and rehospitalization. Future prospective studies of persistent AP in ICM patients are warranted given the increased risk of adverse outcomes in this population.
Acknowledgments
Funding
The National Institute of General Medical Sciences (grant no. T32GM086330 to R.J.M.). Funding for this work was provided by Gilead Sciences.
Footnotes
Conflict of interest: R.J.M., M.F., and C.M.O have received research funding from Gilead Sciences, Inc. The remaining authors report no relevant conflicts of interest.
References
- 1.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van derWall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Mentz RJ, Fiuzat M, Shaw LK, Phillips HR, Borges-Neto S, Felker GM, O’Connor CM. Comparison of clinical characteristics and long-term outcomes of patients with ischemic cardiomyopathy with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease) Am J Cardiol. 2012;109:1272–1277. doi: 10.1016/j.amjcard.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Mentz RJ, Broderick S, Shaw LK, Fiuzat M, O’Connor CM. Heart failure with preserved ejection fraction: comparison of patients with and without angina pectoris (from the Duke Databank for Cardiovascular Disease) J Am Coll Cardiol. 2014;63:251–258. doi: 10.1016/j.jacc.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 6.Mentz RJ, Felker GM, Ahmad T, Peacock WF, Pitt B, Fiuzat M, Maggioni AP, Gheorghiade M, Ando Y, Pocock SJ, Zannad F, O’Connor CM. Learning from recent trials and shaping the future of acute heart failure trials. Am Heart J. 2013;166:629–635. doi: 10.1016/j.ahj.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PJ, Lee KL, Harrell FE, Jr, Behar VS, Rosati RA. Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation. 1980;62:718–726. doi: 10.1161/01.cir.62.4.718. [DOI] [PubMed] [Google Scholar]
- 8.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–168. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 9.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 10.Mark DB, Nelson CL, Califf RM, Harrell FE, Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 11.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S. 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 13.Hjemdahl P, Eriksson SV, Held C, Forslund L, Nasman P, Rehnqvist N. Favourable long term prognosis in stable angina pectoris: an extended follow up of the angina prognosis study in Stockholm (APSIS) Heart. 2006;92:177–182. doi: 10.1136/hrt.2004.057703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleland JG, Calvert M, Freemantle N, Arrow Y, Ball SG, Bonser RS, Chattopadhyay S, Norell MS, Pennell DJ, Senior R. The Heart Failure Revascularisation Trial (HEART) Eur J Heart Fail. 2011;13:227–233. doi: 10.1093/eurjhf/hfq230. [DOI] [PubMed] [Google Scholar]
- 15.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Gardin JM, O’Rourke RA, Williams SV, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50:2264–2274. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]