Abstract
Entrapment neuropathy is the result of pressure on a peripheral nerve as it passes through a narrow canal that is bounded by stiff tissues. In spite of their ubiquitous nature, they are underdiagnosed, underreported, and sometimes not properly managed, especially in developing countries. Entrapment neuropathies are of various types, but the most common type is carpal tunnel syndrome. Mechanisms involved in the pathophysiology of entrapment neuropathies include mechanical compression and nerve ischemia. A clear understanding of the various types and the underlying mechanisms of entrapment neuropathies are invaluable in the decision-making process involved in the management of every patient with the condition.
Keywords: Entrapment Neuropathies, Nerve Compression Syndromes, Carpal Tunnel Syndrome, Literature Review
Introduction
The original concept of entrapment neuropathy refers to "a peripheral nerve lesion presenting without evident external cause and localized in one of those anatomical zones where the nerve passes through a narrow path".1 These paths are not only narrow, they are also bounded by stiff tissues thus leading to confinements that may result in sustained tissue pressures.2 Some of the neuropathies are common while some are rare and some are even controversial. Since, the term has been used for other compression syndromes due to external pressure.
Millions of people are affected by entrapment neuropathies worldwide. It is especially common among individuals with predisposing occupations or in those with certain medical conditions.1,3 These neuropathies occur as a result of mechanical dynamic compression of a short segment of a nerve as it passes through a specific site, which is frequently a fibro-osseous tunnel or an opening in fibrous or muscular tissue.3 Symptoms usually begin insidiously and progress slowly, and these may include localized pain, sensory loss, and/or motor weakness with any of these three symptoms being more prominent than the other. Entrapment can occur in both the upper and lower limbs, and Table 1 gives a summary of some of the entrapment neuropathies including their sites of entrapment.4 Carpal tunnel syndrome (CTS) is discussed in some detail as an illustration.
Table 1. Entrapment neuropathies.4.
| Nerve | Site of entrapment |
|---|---|
| Suprascapular | Spinoglenoid notch |
| Lower trunk or medial cord of brachial plexus | Cervical rib or band at thoracic outlet (neurogenic thoracic outlet syndrome) |
| Median | |
| Wrist | Carpal tunnel |
| Elbow | Between heads of pronator teres (pronator teres syndrome) |
| Ulnar | |
| Wrist | Guyon’s canal (ulnar tunnel) |
| Elbow | Bicipital groove, cubital tunnel |
| Posterior interosseous | Radial tunnel- at point of entrance into supinator muscle (arcade of Frohse) |
| Lateral femoral cutaneous (meralgia paraesthetica) | Inguinal ligament |
| Obturator | Obturator canal |
| Posterior tibial | Tarsal tunnel, medial malleolus-flexor retinaculum |
| Interdigital plantar (Morton’s metatarsalgia) | Plantar fascia (heads of 3rd and 4th metatarsals) |
Adapted from: Adams and Victor’s Principles of Neurology; 9th Edition; 2009; McGraw-Hill.4
Mechanisms of entrapment neuropathies
For a good understanding of the mechanisms of entrapment neuropathies, a knowledge of the basic nerve injury types is necessary. There are three basic nerve injury types: stretch-related, laceration, and compression. Stretch-related injuries result from a stretch of the nerve as seen in avulsion of the brachial plexus (an example of this is the birth-related injury, Erb palsy). Laceration injuries can occur from knives, and compression injury is the third most common type. Entrapment neuropathies fall under the compression injury type. In an attempt to classify the physical and functional state of the damaged nerve trunk, these injuries have also been classified into three broad categories by Seddon5: neurapraxia, axonotmesis, and neurotmesis [Table 2].6 Sunderland later further stratified these into five categories according to severity.7 Most of the entrapment neuropathies belong to the category of neurapraxia.
Table 2. Classification of nerve injuries according to researchers Seddon and Sunderland.6.
| Seddon | Sunderland | Structural and functional processes |
|---|---|---|
| Neurapraxia | 1 | Myelin damage, conduction slowing, and blocking |
| Axonotmesis | 2 | Loss of axonal continuity; endoneurium intact; no conduction |
| Neurotmesis | 3 | Loss of axonal and endoneurial continuity; perineurium intact; no conduction |
| 4 | Loss of axonal, endoneurial, and perineurial continuity; epineurium intact; no conduction | |
| 5 | Entire nerve trunk separated; no conduction |
From: Stewart.6
Given the potential permanent morbidity that could result from harvesting human nerve tissue, it has been difficult studying the mechanisms of entrapment neuropathies in humans. Thus, many studies on the subject have been in animal models, and limited data exist from human studies.8 There are two basic pathological mechanisms involved in compression injuries: mechanical compression and ischemia.9
Mechanical compression
In contrast to Wallerian degeneration, which characterizes acute nerve injury, chronic nerve compression injuries have found associated with some of the changes discussed below. These are postulated to be evidence of mechanical compression of the nerve.
Demyelination and remyelination: This has been suggested to be the mechanism underlying the slowing in nerve conduction in chronic nerve compression as seen in entrapment neuropathies. Animal models of chronic nerve compression injuries have demonstrated features suggestive of demyelination followed by remyelination of the compressed fiber.10,11 Given the crucial role of myelin in saltatory conduction of action potential, it is plausible that this mechanism is responsible for the slowing of nerve conduction velocity seen in entrapment neuropathy due to thinner myelin and decreased internodal length.8
Concurrent proliferation and apoptosis of Schwann cells: Gupta and Steward demonstrated a proliferation of Schwann cells in the compressed segment and distal to the compression in an animal model.12 Electron microscopic analyses have shown that there is no axonal degeneration or swelling and these changes occur well before there is any detectable alteration in nerve conduction velocity. Decrease in intermodal length and myelin thickness underlie the disruption of the efficacy of nerve impulse propagation in chronic nerve compression like CTS.13
Downregulation of myelin-associated protein and axonal sprouting: Without any evidence of axonal injury, it has been demonstrated that there is sprouting of axons in the compressed nerve due to a downregulation of myelin-associated glycoprotein, which is known to inhibit axonal growth in adult humans.14
The response of the dorsal root ganglion: In response to chronic nerve compression, Chao et al,15 have shown that there is an upregulation of Growth Associated Protein 43 a growth cone molecule that is critical in the modulation of F-actin behavior to extracellular cues. This upregulation has been found to be localized to a portion of the small-caliber isolectin B4-binding molecule and calcitonin gene-related peptide-positive neurons. They concluded that chronic nerve compression induces a phenotypic change in the dorsal root ganglion, which appears to be associated with an increase in glial-derived neurotrophic factor at and near the compression site.
Ischemia
There is a well-developed microvascular system supplying the peripheral nerve, and this is important given the energy-dependent nature of action potentials in these nerves. It is thus plausible that compression of these blood vessels, as seen in entrapment neuropathies, will result in dysfunction of the affected nerves. A thickening of the walls of the microvessels in the endoneurium and perineurium has been demonstrated in a few case reports of patients who had nerve segment resection. These findings were also associated with perineurial edema, thickening, and fibrosis at the site of injury.16
There is usually a compression of part of a nerve with consequent disturbance of microcirculation earlier in the course of CTS, but this is restored immediately after transection of the flexor retinaculum. With this restoration of microcirculation, there is often an immediate and delayed return of nerve function which further buttresses the fact that ischemia plays a role in the development of the constellation of symptoms and signs seen in entrapment neuropathies.17 Further evidence for the role of ischemia in entrapment neuropathies was demonstrated in a report of three cases of hypertensive patients who developed CTS after the commencement of beta-blockers, but with complete resolution after the drug was discontinued.18 The patients were followed-up for up to 18 months without recurrence of the symptoms. Fluid retention occurring as a side effect of beta-blocker use has been postulated as the probable mechanism of CTS in these patients.
Carpal tunnel syndrome
CTS results from compression of the median nerve as it passes through the wrist within the carpal tunnel.2 It is the most prevalent entrapment neuropathy worldwide with significant negative effects on the quality of life of individuals suffering from the condition.19-21 The prevalence of the condition is not known in Nigeria. However, brachialgia paraesthetica nocturna (waking up at night due to unpleasant sensations in the fingers), which is one of the early symptoms of CTS, was found in up to 19.6% of the respondents in a questionnaire-based study in Ibadan.22 In population-based studies, a prevalence of 10–20% has been reported for symptoms of the disease while the prevalence of definite CTS ranges from 0.9% to 10% depending on the method of case ascertainment and the population studied.23-27 In a clinical surveillance of CTS, a bi-modal distribution was found in the age incidence with the first peak occurring in the sixth decade while the second peak occurs after the age of 70.28
Anatomy and pathophysiology of CTS
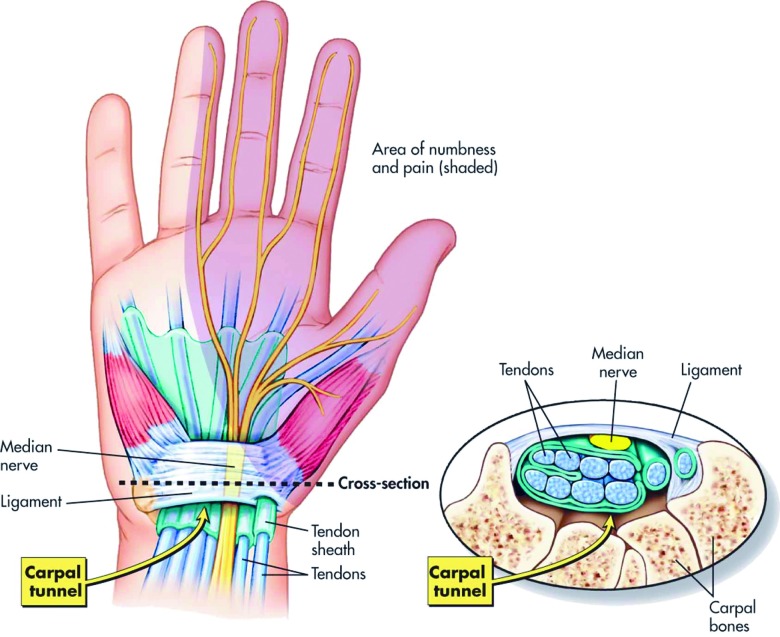
The carpal tunnel is a fibro-osseous outlet lying between the flexor retinaculum and the carpal bones [Figure 1]. The tunnel is tightly packed and contains the median nerve and the nine extrinsic flexor tendons of the thumb and fingers with its narrowest portion being about 2.5 cm distal to its entrance. In healthy individuals, the pressure within the tunnel ranges from 2–31 mmHg while in CTS this is as high as 32–110 mmHg depending on the position of the wrist.29 The pressure in the tunnel is increased up to 8-fold when the wrist is flexed and 10-fold when extended29; this could be the physiologic explanation for the Phalen’s test which is a clinical maneuver used in the diagnosis of the condition. Experimental studies have demonstrated a dose-response relationship between the duration and amount of pressure in the carpal tunnel and the extent of median nerve dysfunction.30
Figure 1.
The Carpal Tunnel. (Reproduced with permission from http://blog.corewalking.com/wp-content/uploads/2013/11/wrist.jpeg).
There are various risk factors for developing CTS including obesity, diabetes mellitus, hypothyroidism, acromegaly, flexor tenosynovitis, ganglion, and the physiological state of pregnancy. Every unit increase in the body mass index increases the risk of developing CTS by 7.4%.31 The condition has also been shown to be highly prevalent among those that do works requiring repetitive hand movement.32-34 It is idiopathic in up to 50% of cases, mainly premenopausal women.35
Clinical presentation of CTS
The usual presentation is that of paraesthesias affecting the thumb and the first two and a half fingers while some patients may complain of paraesthesias affecting the whole hand or pain radiating up the arm to the shoulder.36 In more than half of cases, the symptoms are bilateral at first presentation while most patients present with symptoms in the dominant hand first. In the history, occurrence of any of the following symptoms in the area of median nerve distribution suggests a high likelihood of CTS: dull aching discomfort in the hand, forearm or upper arm; hand paraesthesias; weakness or clumsiness of the hand; and dry skin, swelling or color changes in the hand.37 The provocative factors for these symptoms may include sleep, sustained hand or arm position, and repetitive movement of the wrist, and these symptoms can be mitigated by changes in hand posture or by simply shaking the affected hand.37
The provocative factors that are usually used in the evaluation are Phalen’s and Tinel’s signs. The former involves sustained flexion of the wrist at right angles for 60 seconds, and this usually brings on the symptoms of the patient. On the other hand, Tinel’s sign involves tapping over the carpal tunnel at the wrist, which usually elicits paraesthesias in the median innervated portion of the hand.3,36 Reverse Phalen’s maneuver (performed by having the patient maintain full wrist and finger extension for two minutes) changes the pressure within the carpal tunnel significantly more than Phalen’s sign and it has been shown to add to the sensitivity of the conventional screening methods.38 A combination of Phalen’s test and manual carpal compression test has been found to have a positive predictive value of 95% and a negative predictive value of 88% when compared to nerve conduction study and is encouraged in primary care setting for early diagnosis of CTS.39
Confirmation of diagnosis
For confirmation of diagnosis in most cases, electrodiagnostic testing together with clinical evaluation is accepted to be the standard means.40 Results considered to be abnormal include: an absolute sensory latency > 3.7 msec, a difference of3 0.4 msec between values obtained for the median nerve and those obtained for the radial or ulnar nerve, a motor conduction latency > 4.0 msec, and an incremental change of 0.4 msec in the palmar serial sensory study after controlling for the patient’s age and limb temperature.40 Electrophysiological evaluation also helps in grading the severity of CTS. Although the correlation between severity of nerve conduction studies (NCSs) and symptoms are not well established, application of a grading system can assist in prognosticating the outcome of surgery. Patients with middle-grade abnormalities on NCSs have a better surgical outcome compared to those with very severe or no abnormality.41,42 Other investigations include magnetic resonance imaging, ultrasound of the wrist and, where applicable, investigations to rule out a systemic disease.
Treatment
Treatment could be conservative or surgical. Conservative treatment is considered for patients with mild disease, and this includes wrist splinting and the use of anti-inflammatory medications (e.g., local steroid injection).3,37,43 Methylprednisolone injection was demonstrated in a randomized placebo-controlled trial to relieve symptoms and reduce the rate of surgical intervention.44 For those who fail to respond to conservative treatment or those with severe disease, carpal tunnel decompression is recommended.36,45 It has been shown that the outcome of carpal tunnel release is comparable in those with and without electrophysiologic confirmation, which means inability to do a neurophysiologic test should not preclude surgical intervention in patients with typical symptoms and signs of the condition.46 The use of radial extracorporeal shockwaves combined with wrist splinting is evolving as a treatment modality.47 Another novel treatment modality is the injection of platelet-rich plasma to which patients have shown significant short-term improvement although the studies were limited by a small sample size.48,49
Conclusion
Dysfunctions that are characteristic of entrapment neuropathies are associated with increased load as a result of increased pressure from the compression. This compression leads to focal demyelination and remyelination but without axonal injury. It is the focal demyelination followed by slow remyelination that results in the slow nerve conduction velocity seen in these conditions because of the loss of saltatory conduction by myelin. Ischemia of the nerve also leads to reduced microcirculation with consequent perineurial edema, thickening, and fibrosis at the site of compression.
In view of the ubiquitous nature of entrapment neuropathies, understanding the underlying mechanisms could be of use in the management of the patients with any of the conditions. This review has tried to dwell on a general overview of entrapment neuropathies with a short discussion on the commonest of them; CTS. A clear understanding of the various types and the underlying mechanisms of entrapment neuropathies are invaluable in the decision-making process involved in the management of every patient with the condition.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
References
- 1.Mumenthaler M. Clinical Aspects of Entrapment Neuropathies of Peripheral Nerves. In: Samii M, editor. Peripheral Nerve Lesions. Berlin, Heidelberg: Springer Berlin Heidelberg; 1990. p.258-269. [Google Scholar]
- 2.Rempel DM, Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. J Electromyogr Kinesiol 2004. Feb;14(1):71-75. 10.1016/j.jelekin.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Bayramoglu M. Entrapment neuropathies of the upper extremity. Neuroanat 2004;3:18-24. [Google Scholar]
- 4.Ropper AH, Samuels MA. Adams and Victor’s Principles of Neurology. 9th ed. McGraw-Hill Education; 2009. [Google Scholar]
- 5.Seddon HJ. A Classification of Nerve Injuries. Br Med J 1942. Aug;2(4260):237-239. 10.1136/bmj.2.4260.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JD. Focal Peripheral Neuropathies. 3rd ed. Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 7.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain 1951. Dec;74(4):491-516. 10.1093/brain/74.4.491 [DOI] [PubMed] [Google Scholar]
- 8.Pham K, Gupta R. Understanding the mechanisms of entrapment neuropathies. Review article. Neurosurg Focus 2009. Feb;26(2):E7. 10.3171/FOC.2009.26.2.E7 [DOI] [PubMed] [Google Scholar]
- 9.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus 2004. May;16(5):E1. 10.3171/foc.2004.16.5.2 [DOI] [PubMed] [Google Scholar]
- 10.Ludwin SK, Maitland M. Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J Neurol Sci 1984. May;64(2):193-198. 10.1016/0022-510X(84)90037-6 [DOI] [PubMed] [Google Scholar]
- 11.Berger BL, Gupta R. Demyelination secondary to chronic nerve compression injury alters Schmidt-Lanterman incisures. J Anat 2006. Jul;209(1):111-118. 10.1111/j.1469-7580.2006.00561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol 2003. Jun;461(2):174-186. 10.1002/cne.10692 [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Nassiri N, Hazel A, Bathen M, Mozaffar T. Chronic nerve compression alters Schwann cell myelin architecture in a murine model. Muscle Nerve 2012. Feb;45(2):231-241. 10.1002/mus.22276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R, Rummler LS, Palispis W, Truong L, Chao T, Rowshan K, et al. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp Neurol 2006. Aug;200(2):418-429. 10.1016/j.expneurol.2006.02.134 [DOI] [PubMed] [Google Scholar]
- 15.Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol 2008. Jan;506(2):180-193. 10.1002/cne.21537 [DOI] [PubMed] [Google Scholar]
- 16.Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Chronic nerve compression–an experimental model in the rat. Ann Plast Surg 1984. Aug;13(2):112-120. 10.1097/00000637-198408000-00004 [DOI] [PubMed] [Google Scholar]
- 17.Rempel D, Dahlin L, Lundborg G. Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg Am 1999. Nov;81(11):1600-1610. 10.2106/00004623-199911000-00013 [DOI] [PubMed] [Google Scholar]
- 18.Emara MK, Saadah AM. The carpal tunnel syndrome in hypertensive patients treated with beta-blockers. Postgrad Med J 1988. Mar;64(749):191-192. 10.1136/pgmj.64.749.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atroshi I, Gummesson C, Johnsson R, Sprinchorn A. Symptoms, disability, and quality of life in patients with carpal tunnel syndrome. J Hand Surg Am 1999. Mar;24(2):398-404. 10.1016/S0363-5023(99)70014-6 [DOI] [PubMed] [Google Scholar]
- 20.Thomsen NO, Cederlund R, Björk J, Dahlin LB. Health-related quality of life in diabetic patients with carpal tunnel syndrome. Diabet Med 2010. Apr;27(4):466-472. 10.1111/j.1464-5491.2010.02970.x [DOI] [PubMed] [Google Scholar]
- 21.Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 2016. Nov;15(12):1273-1284. 10.1016/S1474-4422(16)30231-9 [DOI] [PubMed] [Google Scholar]
- 22.Oyedele OO, Shokunbi MT, Malomo AO. The prevalence of hand pain in Ibadan–implications for the carpal tunnel syndrome. West Afr J Med 2002. Jul-Sep;21(3):204-207. [DOI] [PubMed] [Google Scholar]
- 23.Al Saleh J, El Sayed M, Monsef N, Darwish E. The prevalence and the determinants of musculoskeletal diseases in emiratis attending primary health care clinics in Dubai. Oman Med J 2016;31(2):117-123. [DOI] [PMC free article] [PubMed]
- 24.de Krom MC, Knipschild PG, Kester AD, Thijs CT, Boekkooi PF, Spaans F. Carpal tunnel syndrome: prevalence in the general population. J Clin Epidemiol 1992. Apr;45(4):373-376. 10.1016/0895-4356(92)90038-O [DOI] [PubMed] [Google Scholar]
- 25.Ferry S, Pritchard T, Keenan J, Croft P, Silman AJ. Estimating the prevalence of delayed median nerve conduction in the general population. Br J Rheumatol 1998. Jun;37(6):630-635. 10.1093/rheumatology/37.6.630 [DOI] [PubMed] [Google Scholar]
- 26.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999. Jul;282(2):153-158. 10.1001/jama.282.2.153 [DOI] [PubMed] [Google Scholar]
- 27.Khedr EM, Fawi G, Allah Abbas MA, El-Fetoh NA, Zaki AF, Gamea A. Prevalence of Common Types of Compression Neuropathies in Qena Governorate/Egypt: A Population-Based Survey. Neuroepidemiology 2016;46(4):253-260. 10.1159/000444641 [DOI] [PubMed] [Google Scholar]
- 28.Bland JD, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991-2001. J Neurol Neurosurg Psychiatry 2003. Dec;74(12):1674-1679. 10.1136/jnnp.74.12.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner RA, Andary M. Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol 2002. Sep;113(9):1373-1381. 10.1016/S1388-2457(02)00169-4 [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon SE. Pathophysiology of nerve compression. Hand Clin 2002. May;18(2):231-241. 10.1016/S0749-0712(01)00012-9 [DOI] [PubMed] [Google Scholar]
- 31.Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev 2015. Dec;16(12):1094-1104. 10.1111/obr.12324 [DOI] [PubMed] [Google Scholar]
- 32.England JD. Entrapment neuropathies. Curr Opin Neurol 1999. Oct;12(5):597-602. 10.1097/00019052-199910000-00014 [DOI] [PubMed] [Google Scholar]
- 33.Violante FS, Farioli A, Graziosi F, Marinelli F, Curti S, Armstrong TJ, et al. Carpal tunnel syndrome and manual work: the OCTOPUS cohort, results of a ten-year longitudinal study. Scand J Work Environ Health 2016. Jul;42(4):280-290. 10.5271/sjweh.3566 [DOI] [PubMed] [Google Scholar]
- 34.van Rijn RM, Huisstede BM, Koes BW, Burdorf A. Associations between work-related factors and the carpal tunnel syndrome–a systematic review. Scand J Work Environ Health 2009. Jan;35(1):19-36. 10.5271/sjweh.1306 [DOI] [PubMed] [Google Scholar]
- 35.Dekel S, Papaioannou T, Rushworth G, Coates R. Idiopathic carpal tunnel syndrome caused by carpal stenosis. Br Med J 1980. May;280(6227):1297-1299. 10.1136/bmj.280.6227.1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bland JD. Carpal tunnel syndrome. BMJ 2007. Aug;335(7615):343-346. 10.1136/bmj.39282.623553.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Practice parameter for carpal tunnel syndrome (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1993. Nov;43(11):2406-2409. 10.1212/WNL.43.11.2406 [DOI] [PubMed] [Google Scholar]
- 38.Werner RA, Bir C, Armstrong TJ. Reverse Phalen’s maneuver as an aid in diagnosing carpal tunnel syndrome. Arch Phys Med Rehabil 1994. Jul;75(7):783-786. [PubMed] [Google Scholar]
- 39.Fertl E, Wöber C, Zeitlhofer J. The serial use of two provocative tests in the clinical diagnosis of carpal tunel syndrome. Acta Neurol Scand 1998. Nov;98(5):328-332. 10.1111/j.1600-0404.1998.tb01743.x [DOI] [PubMed] [Google Scholar]
- 40.Phillips LH, II, Juel VC. The role of electrodiagnostic testing in carpal tunnel syndrome. Neurosurg Focus 1997. Jul;3(1):e2. 10.3171/foc.1997.3.1.5 [DOI] [PubMed] [Google Scholar]
- 41.Stevens JC. AAEE minimonograph #26: The electrodiagnosis of carpal tunnel syndrome. Muscle Nerve 1987. Feb;10(2):99-113. 10.1002/mus.880100202 [DOI] [PubMed] [Google Scholar]
- 42.Bland JD. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve 2001. Jul;24(7):935-940. 10.1002/mus.1091 [DOI] [PubMed] [Google Scholar]
- 43.Peters-Veluthamaningal C, Winters JC, Groenier KH, Meyboom-de Jong B. Randomised controlled trial of local corticosteroid injections for carpal tunnel syndrome in general practice. BMC Fam Pract 2010. Jul;11:54. 10.1186/1471-2296-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atroshi I, Flondell M, Hofer M, Ranstam J. Methylprednisolone injections for the carpal tunnel syndrome: a randomized, placebo-controlled trial. Ann Intern Med 2013. Sep;159(5):309-317. 10.7326/0003-4819-159-5-201309030-00004 [DOI] [PubMed] [Google Scholar]
- 45.Shi Q, MacDermid JC. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J Orthop Surg Res 2011. Apr;6:17. 10.1186/1749-799X-6-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zyluk A, Szlosser Z. The results of carpal tunnel release for carpal tunnel syndrome diagnosed on clinical grounds, with or without electrophysiological investigations: a randomized study. J Hand Surg Eur Vol 2013. Jan;38(1):44-49. 10.1177/1753193412445162 [DOI] [PubMed] [Google Scholar]
- 47.Raissi GR, Ghazaei F, Forogh B, Madani SP, Daghaghzadeh A, Ahadi T. The Effectiveness of Radial Extracorporeal Shock Waves for Treatment of Carpal Tunnel Syndrome: A Randomized Clinical Trial. Ultrasound Med Biol 2017. Feb;43(2):453-460. 10.1016/j.ultrasmedbio.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 48.Uzun H, Bitik O, Uzun Ö, Ersoy US, Aktaş E. Platelet-rich plasma versus corticosteroid injections for carpal tunnel syndrome. J Plast Surg Hand Surg 2017. Oct;51(5):301-305. [DOI] [PubMed] [Google Scholar]
- 49.Malahias MA, Johnson EO, Babis GC, Nikolaou VS. Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen Res 2015. Nov;10(11):1856-1859. 10.4103/1673-5374.165322 [DOI] [PMC free article] [PubMed] [Google Scholar]