Abstract
Background and Aims:
Apnoeic oxygenation during laryngoscopy has been emphasised in recent recommendations for airway management. We aimed to compare the effect of nasal oxygen supplementation on time for pulse oximeter oxygen saturation (SpO2) to fall from 100% to 92% (desaturation safety time), to assess the arterial oxygen partial pressures (PaO2) with and without nasal oxygen supplementation and the time for SpO2 to recover from 92% to 100% after initiation of ventilation.
Methods:
This is a prospective randomised placebo-controlled trial involving sixty patients, where nasal oxygen supplementation given at 10 L/min during apnoea of laryngoscopy in one group of patients (Group O2) was compared to no oxygen supplementation in other group (Group NoO2). Desaturation safety period and the PaO2 just after intubation were compared. Time for SpO2 to increase to 100% after initiation of ventilation was also assessed. Demographic details were compared using the Chi-square and t-tests. Student's t-test for independent variables was used to compare means of data obtained.
Results:
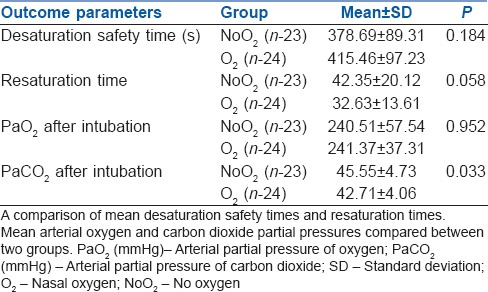
Desaturation safety period at 415.46 ± 97.23 seconds in group O2 versus 378.69 ± 89.31 seconds in group NoO2(P = 0.213) and PaO2(P = 0.952) and time to recovery of SpO2 (P = 0.058) were similar in both groups. Rise in arterial carbon dioxide secondary to apnoea was slower in oxygen supplementation group (P = 0.032).
Conclusion:
Apnoeic oxygen supplementation at 10 L/min flow by nasal prong did not significantly prolong the apnoea desaturation safety periods or the PaO2 in our study.
Key words: Insufflation, intubation, oximetry, oxygen
INTRODUCTION
Supplemental oxygenation during laryngoscopy has been advocated in almost all recent airway management guidelines.[1,2] Apnoeic oxygenation (AO) has been shown to improve the apnoea safety time when oxygen is delivered at high flows (>60 L/min) by special delivery systems, for example, in Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE).[3,4] Anaesthesia machines cannot deliver such high flows. All of them, however, do guarantee flows of 10 L/min. Adequate oxygenation of lung is important to maximise the time of non-hypoxaemic apnoea and this study tries to assess the effect of apnoeic ventilation at gas flows of 10 L/min.
Even in the absence of respiratory movements, oxygen insufflated into the upper airway diffuses down to the trachea. At the alveolar level, oxygen is being absorbed at 250 ml/min and the resultant negative pressure gradient creates a subatmospheric pressure in the alveolus. Oxygen from the oxygen-rich air is thus absorbed across the alveolar capillary membrane and dissolves in the blood.[5] This fact has been utilised during laryngoscopy in an apnoeic patient. We wanted to assess whether flows of 10 L/min delivered through nasal prongs to insufflate the upper airway are adequate to allow enough oxygen to be ‘drawn’ down the trachea and into the alveolus. In effect, this method of insufflation may afford protection from hypoxaemia during apnoea of intubation.
METHODS
This was a prospective randomised controlled trial conducted after obtaining institutional ethical committee clearance. The trial was registered at CTRI: Registry number – CTRI/2016/07/007087 and conducted over one year beginning at November 2015 at a tertiary level teaching hospital. Sixty patients with American Society of Anesthesiologists physical status Grade I or II, between 15 and 40 years of age and with a body mass index of <30 kg/m2 posted for surgery requiring general anaesthesia were included. The sample size was calculated based on a pilot study done on ten patients using the formula, n = (Z1-α/2+ Zβ)2 × 2 × σ2/d2) (where Z1-α/2, the critical value of the normal distribution at 1-α/2 is 1.96 and α is type one error kept at 5%, Zβ, the critical value of the normal distribution at β, for a power of 80%, β = 0.2 and the critical value is 0.84, σ2 is the pooled variance and d is the mean difference of time to desaturate between test and control groups in the pilot study). A difference of 10% was considered clinically significant.
Exclusion criteria included patients with any lung pathology or respiratory problem, history of any heart disease or cerebrovascular disease, any contraindication for nasal prong use, for example, tumours, fractures or trauma. Pregnant patients, patients with haemoglobin <10 g/dl and anticipated or known difficult airway were also excluded.
The primary aim of this study was to compare the effect of nasal oxygen supplementation on time for oxygenation desaturation, i.e. fall in oxygen saturation on pulse oxinmetry (SpO2) from 100% to 92% i.e. desaturation safety time. Secondary objectives were to assess the arterial oxygen partial pressures (PaO2) with and without nasal oxygen supplementation and to compare the time for recovery of SpO2 from 92% to 100%.
Airway evaluation was done at the pre-anaesthetic examniation one day before surgery. The study and possible complications arising thereof were explained to the patients. After a written informed consent, patients were randomly assigned into two groups using a computer-generated programme. One group received supplemental oxygen (Group O2, n = 30) and the other group did not (Group NoO2, n = 30). Concealment was done using sealed envelopes; similar in all respects, sequentially numbered and containing computer-generated number sequence inside. The seal was opened, and based on the randomisation, intervention was given by investigator not involved in the case. In the operating room after connecting routine pre-induction monitors i.e. pulse-oximeter, non-invasive blood pressure and electrocardiogram, baseline parameters were noted. Modified Allen's test was performed on left hand of the patient to confirm the collateral flow. Left radial artery cannulation was done under local anaesthesia.
Baseline arterial blood sample was taken while breathing room air. Pre-oxygenation was accomplished using a face mask with 3 min tidal volume technique. Magill's system with adjustable pressure-limiting valve fully open to the atmosphere was used. Fresh gas flow rate of 100% oxygen was kept at 10 L/min.
In patients in Group NoO2(no oxygen supplementation) i.e. those only pre-oxygenated in supine position, after induction of anaesthesia and administration of rocuronium, were left to air with nasal prongs attached but with no gases flowing through them. In Group O2(oxygen supplemented), after rocuronium administration, oxygen insufflation was done with oxygen flowing at 10 L/min via nasal prongs. A second arterial blood gas (ABG) sample was taken in all patients immediately after completing 3 min of pre-oxygenation.
Induction of anaesthesia was done with injection fentanyl 2 μg/kg, propofol 2 mg/kg and rocuronium 1.2 mg/kg. Intubation was initiated 120 s after injection of rocuronium to assume a ‘prolonged laryngoscopic attempt’ during which period in Group O2, nasal oxygen supplementation was done as described. In Group NoO2, no gas flows were given. Endotracheal intubation was confirmed using a flexible fibrescope before fixation of endotracheal tube. EtCO2 was not used to assess correct placement because ventilation was not allowed till SpO2 reached 92%, as per the study protocol. On confirmation of proper endotracheal intubation, nasal oxygen flow was stopped. A third ABG sample was taken once intubation was confirmed. After intubation, proximal end of endotracheal tube was left open to atmosphere in both groups with patient in apnoeic state. Anaesthesia during this period was maintained using infusion propofol 200 μg/kg/min. Pulse oximetry values were continuously monitored. Controlled desaturation was then allowed to occur till SpO2 decreased to 92%. ‘Desaturation safety period’ was monitored and recorded by an observer not involved with the study.
Once the SpO2 reached 92%, positive pressure ventilation was commenced with 1 minimum alveolar concentration (MAC) isoflurane in 100% oxygen till SpO2 reached 100%. This recovery time was noted by the blinded observer. Surgery and anaesthesia were then allowed to proceed in the usual manner.
Data were analysed using SPSS (Windows ver. 16.0, SPSS Inc., Chicago, IL, USA) and all results are presented as mean ± standard deviation. Statistical analysis was performed using t-test and Chi-square test to evaluate statistical significance between two groups for the demographic profile. t-test was used to compare variables when compared within the group and between the groups. Values with P < 0.05 have been considered to be statistically significant.
RESULTS
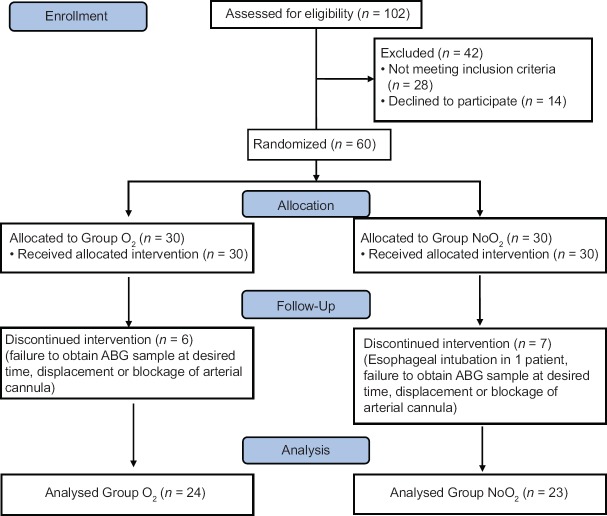
A total of 60 patients were divided into two groups. Patient flow through the study may be seen as a CONSORT diagram in Figure 1 The two groups were comparable with respect to age, weight, height and body mass index as shown in Table 1.
Figure 1.
Flow of patient through study protocol as a CONSORT diagram
Table 1.
Demographic profile
The ABG parameters such as pH, arterial carbon dioxide partial pressure (PaCO2), PaO2 and blood bicarbonate levels were similar in both groups preoperatively as well as after pre-oxygenation as shown in Table 2. Nasal oxygen insufflation marginally increased the desaturation safety period as compared to no oxygen insufflation (415.46 ± 97.23s vs. 378.69 ± 89.31 s) and it was not statistically significant. The PaO2 measured after intubation was not different in either group (241.37 ± 37.31 vs. 240.51 ± 57.54 mm hg) (P = 0.952). These patients recovered their saturation to 100%, faster after initiation of ventilation, although this was not statistically significant using the Student's t-test for independent samples (P = 0.058). We noted that arterial carbon dioxide accumulation due to apnoea was lesser in patients receiving oxygen supplementation by nasal prong during intubation than those who were not supplemented (P = 0.032) as shown in Table 3.
Table 2.
Arterial Blood Gas parameters in the two groups
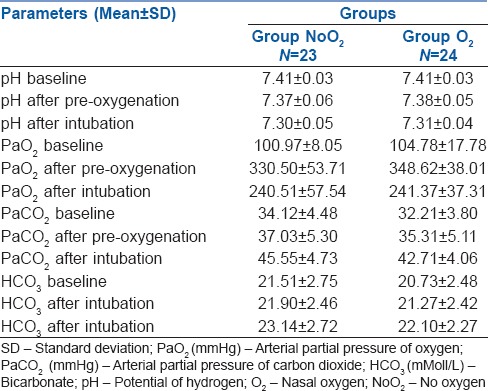
Table 3.
Effect of oxygen supplementation at 10 L/min compared between the two groups
No complication directly attributable to the study was encountered.
DISCUSSION
The concept of AO in humans by diffusion was first described by Holmdahl in 1956.[6] This technique has been used to prevent desaturation during bronchoscopies which allows liberty to work without a need for mechanical ventilation.[7] Of late, AO has been an area of interest for its promise during laryngoscopy to prolong the apnoea desaturation safety time. Recently, the All India Difficult Airway Association guidelines released last year have highlighted a need for supplemental oxygenation during airway manoeuvres.[1] In 2013, the updated recommendations of the Canadian Airway Focus Group also endorsed the use of continuous oxygen administration during the apnoeic period.[8] Evidence has shown that warm humidified oxygen at very high flows through the nose (~60 L/min) has definite advantages for prolonging apnoea desaturation time as in the THRIVE (Trans nasal Humidified Rapid-Insufflation Ventilatory Exchange) trial;[3] however, this is very costly for most hospitals even where it is available. On the other hand, all the anaesthesia machines can deliver oxygen at 10 L/min. The Difficult Airway Society and the Obstetric Anaesthetists' Association recommend the use of nasal prongs to insufflate oxygen at flows of 5 L/min to 15 L/min during the apnoeic period.[2,9] Nasal oxygen administration via nasal prongs at 5 L/min was compared with room air during simulated difficult laryngoscopy in obese patients.[10] Patients were kept apnoeic for 6 min and none of the patients who received nasal oxygen desaturated to <95% in these 6 min. They suggested that oxygen flows above 5 L/min may enhance nasal patency, improving the delivery of O2 to the pharynx.[10] We tried to objectively assess the utility of AO in a prolonged laryngoscopy situation.[11] In our study, the mean duration of apnoea after pre-oxygenation was 149.13 s in Group NoO2 and 145.87 s in the Group O2. Laryngoscopy took a further 29.1 s in Group NoO2 and 25.8 s in Group O2. To evaluate effectiveness, a study was done on nasopharyngeal oxygen insufflation at 5 L/min following pre-oxygenation on the onset of desaturation during apnoea for the subsequent 6 min. Authors have reported that all patients given nasal oxygen supplementation maintained saturation at 100% throughout the 6 min of apnoea compared to none in the control group which did not receive AO.[12] A randomised controlled trial was conducted on morbidly obese patients who received nasopharyngeal oxygen supplementation following pre-oxygenation versus pre-oxygenation alone.[13] Time from the onset of apnoea until SpO2 fell to 95% was compared between the two groups with a cut-off of 4 min. Sixteen of 17 patients receiving oxygen maintained saturation at 100% for 4 min. A similar beneficial effect of oxygen supplementation has been noted in a crossover study design.[14] In a prospective observational study of intubations outside of the operating theatre, no episodes of hypoxaemia were noted in the 31 patients who used AO with nasal prongs, whereas ten of the sixty patients who did not have AO developed hypoxaemia.[15]
A vast majority of even difficult laryngoscopies result in between 2 and 5 min of apnoea. In our study, AO for duration of this period did not offer any significant advantage probably because 10 L/min flow is not high enough to elicit a significant benefit and higher oxygen flows may be required to objectively highlight any significant differences, as in arterial oxygen content. This has been shown with the use of high-flow oxygen (>60 L/min flow). Also, for nasal oxygenation to be effective, patency up to the laryngeal inlet is an important consideration. During difficult laryngoscopies, the laryngoscope attempts to relieve airway obstruction. During ineffective mask ventilation secondary to airway obstruction as noted after a tongue fall, even nasal oxygen insufflation may prove to be of limited use. In our patients, no attempt was made to keep the airway patent by jaw thrust or chin lift during the apnoeic nasal insufflation. In some studies, a laryngoscope was placed inside the oral cavity to simulate difficult laryngoscopy with Cormack–Lehane grade 4. A difference in the results could be due to this difference in methodology.
There were benefits of nasal insufflation that we noted. There was a slowing down in carbon dioxide accumulation as well as faster recovery to full saturation on resumption of ventilation, in patients who were given nasal oxygen supplementation. Frumin et al. conducted a study on eight patients in whom AO was conducted for 15–55 min.[16] Here, the SpO2 never fell below 98%. However, ABG analysis showed raised PaCO2 levels of 130–160 mmHg and pH of 6.72–6.92. They observed that hypercarbia may be tolerable in an anaesthetised non-obese adult patient, with complete recovery of anaesthesia.[16] Authors also observed a moderate-to-severe arterial hypertension followed by a mild hypotension when artificial respiration was resumed; however, none of our patients had haemodynamic instability during the experiment. We observed that patients who were given nasal oxygen supplementation had lesser carbon dioxide retention during apnoea. Reduction of the dead space and lesser carbon dioxide rebreathing could be the reasons for this slower accumulation. After the induction of apnoea, nasal cannula diffuses oxygen down the trachea to the alveolus.[17] It is absorbed across the alveolar capillary membrane, despite the absence of respiratory movements at the time laryngoscopy is being performed. Carbon dioxide excretion into the alveolus diminishes during apnoea because carbon dioxide is approximately 25 times more soluble than oxygen in blood.[18] It is estimated that during apnoea CO2 is excreted into the alveolus at only 10 ml/min. Conversely, oxygen is absorbed at 250 ml/min. The resultant difference in flow rate of gases out of and into the alveolus (−240 ml/min) creates a gradient for flow of gases into the alveolus. The net result is that during apnoea, oxygen insufflated into the upper airway will be ‘drawn’ down the trachea and into the alveolus. Oxygenation can be maintained in non-breathing humans for 100 min through apnoeic diffusion. Heller et al. were the first to measure PaO2 in six apnoeic patients. Based on their observations on AO, they postulated that accumulating alveolar carbon dioxide and nitrogen can account for only a slight decline in PaO2 and that other factors were also operative in determining the total decrease in PaO2.[19]
Recovery after apnoea has been dealt with in this study. We noted that patients given nasal insufflation may recover their haemoglobin oxygen saturation faster. We are not sure about the mechanism for this, but it may be because oxygen from an oxygen-rich dead space is delivered to the alveoli faster when ventilation is resumed after apnoea.
The study had a relatively small sample size and thus underpowered for analysis with respect to our primary objective, found on post hoc analysis and this is a limitation of the study.
CONCLUSION
Nasal oxygen supplementation during apnoea of normal laryngoscopy does not prolong the desaturation safety times or help in increasing the PaO2. However, nasal oxygen supplementation during apnoea has a probable beneficial effect by slowing the increase in carbon dioxide accumulation, even at low flow rates. Recovery time to reach 100% saturation after apnoea was faster in those given nasal oxygen insufflation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Myatra SN, Shah A, Kundra P, Patwa A, Ramkumar V, Divatia JV, et al. All India Difficult Airway Association 2016 guidelines for the management of unanticipated difficult tracheal intubation in adults. Indian J Anaesth. 2016;60:885–898. doi: 10.4103/0019-5049.195481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult airway society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–48. doi: 10.1093/bja/aev371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): A physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–9. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med. 2012;59:165–750. doi: 10.1016/j.annemergmed.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Eger EI, Severinghaus JW. The rate of rise of paCO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419–25. doi: 10.1097/00000542-196105000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Holmdahl MH. Pulmonary uptake of oxygen, acid-base metabolism, and circulation during prolonged apnoea. Acta Chir Scand Suppl. 1956;212:1–28. [PubMed] [Google Scholar]
- 7.Lee SC. Improvement of gas exchange by apneic oxygenation with nasal prong during fiberoptic intubation in fully relaxed patients. J Korean Med Sci. 1998;13:582–6. doi: 10.3346/jkms.1998.13.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, et al. The difficult airway with recommendations for management – part 2 – the anticipated difficult airway. Can J Anaesth. 2013;60:1119–38. doi: 10.1007/s12630-013-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mushambi MC, Kinsella SM, Popat M, Swales H, Ramaswamy KK, Winton AL, et al. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia. 2015;70:1286–306. doi: 10.1111/anae.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: A randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22:164–8. doi: 10.1016/j.jclinane.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Stoelting RK. Blood pressure and heart rate changes during short-duration laryngoscopy for tracheal intubation: Influence of viscous or intravenous lidocaine. Anesth Analg. 1978;57:197–9. doi: 10.1213/00000539-197803000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Taha SK, Siddik-Sayyid SM, El-Khatib MF, Dagher CM, Hakki MA, Baraka AS, et al. Nasopharyngeal oxygen insufflation following pre-oxygenation using the four deep breath technique. Anaesthesia. 2006;61:427–30. doi: 10.1111/j.1365-2044.2006.04610.x. [DOI] [PubMed] [Google Scholar]
- 13.Baraka AS, Taha SK, Siddik-Sayyid SM, Kanazi GE, El-Khatib MF, Dagher CM, et al. Supplementation of pre-oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia. 2007;62:769–73. doi: 10.1111/j.1365-2044.2007.05104.x. [DOI] [PubMed] [Google Scholar]
- 14.Teller LE, Alexander CM, Frumin MJ, Gross JB. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988;69:980–2. doi: 10.1097/00000542-198812000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Dyett JF, Moser MS, Tobin AE. Prospective observational study of emergency airway management in the critical care environment of a tertiary hospital in Melbourne. Anaesth Intensive Care. 2015;43:577–86. doi: 10.1177/0310057X1504300505. [DOI] [PubMed] [Google Scholar]
- 16.Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789–98. doi: 10.1097/00000542-195911000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Barth L. Therapeutic use of diffusion breathing in bronchoscopy. Anaesthesist. 1954;3:227–9. [PubMed] [Google Scholar]
- 18.Nielsen ND, Kjaergaard B, Koefoed-Nielsen J, Steensen CO, Larsson A. Apneic oxygenation combined with extracorporeal arteriovenous carbon dioxide removal provides sufficient gas exchange in experimental lung injury. ASAIO J. 2008;54:401–5. doi: 10.1097/MAT.0b013e31817e2b5f. [DOI] [PubMed] [Google Scholar]
- 19.Heller ML, Watson TR, Jr, Imredy DS. Apneic oxygenation in man: Polarographic arterial oxygen tension study. Anesthesiology. 1964;25:25–30. doi: 10.1097/00000542-196401000-00005. [DOI] [PubMed] [Google Scholar]