Abstract
Background and Aims:
Transdermal buprenorphine patch (TDB) is increasingly used for chronic pain management because of non-invasive dosing, longer duration of action and minimal side effects. However its role in acute post-operative pain management for spinal instrumentation surgery is not well established. The aim of this study was to evaluate the analgesic efficacy of buprenorphine patch for postoperative pain relief in patients undergoing spinal instrumentation surgery.
Methods:
In this randomised, placebo-controlled, double-blinded, prospective study, 70 adult patients undergoing elective spinal instrumentation surgery were randomly allocated into two groups-TDB Group (buprenorphinepatch) and TDP Group (placebo patch). Time to first rescue analgesic requirement was the primary outcome. All patients also were monitored for total rescue analgesic requirement, drug-related adverse effect and haemodynamic status till 48 h after surgery. Statistical analysis was carried out using student independent t-test if normally distributed or with Mann–Whitney U-test if otherwise.
Results:
Time to first post-operative rescue analgesic (tramadol) requirement was much delayed in TDB Group than TDP Group (708.0 ± 6.98 min vs 54 ± 0.68 min, P < 0.001) and the total tramadol requirement was higher in TDB Group (490.60 ± 63.09 averagevs. 162.93 ± 63.91 mg, P < 0.001). Intra-and post-operative haemodynamic status was also stable in TDB Group without any adverse event.
Conclusion:
A TDB patch (10 μg/hour) applied 24 hours before surgery can be used as a postoperative analgesic for lumber fixation surgery without any drug-related adverse effect.
Key words: Buprenorphine patch, post-operative analgesia, rescue analgesia
INTRODUCTION
Spinal instrumentation surgery is associated with moderate-to-severe post-operative pain which can cause further discomfort and if persistent, may lead to chronic pain. Effective control of post-operative pain is a major challenge to the treating surgeon and the attending anaesthesiologist. Satisfactory perioperative analgesia improves the surgical outcome by reducing morbidity and organ dysfunction.[1] Nonsteroidal anti-inflammatory drugs and parenteral opioids are widely used drugs for post-operative analgesia in these patients.
Most of the parenteral opioids cause sedation, respiratory depression, nausea, vomiting and pruritus. The transdermal delivery systems (TDS) can overcome the adverse effect of oral and parenteral opioids.[2]
Buprenorphine is a semisynthetic derivative of thebaine, 75–100 times more potent than morphine and causes less respiratory depression.[3,4] It is also highly effective through transdermal route due to its high lipid solubility. Efficacy and safety of transdermal buprenorphine has been well established in chronic pain but data regarding acute post-operative pain relief in spinal surgery are still very limited.[5]
The aim of this study was to evaluate the analgesic efficacy of transdermal buprenorphine patch for postoperative pain relief in patients undergoing spinal instrumentation surgery.
METHODS
A prospective randomised, parallel, double-blind controlled study was performed after obtaining the approval of the Institutions Ethics Committee. This study was conducted in our neurosurgical unit (between January 2014 and October 2015) on seventy patients of either sex, in age group of 20–50 years. The study was registered with Clinical Trial Registry of India (CTRI) with trial number: CTRI/2017/03/008018. They were posted for elective lumbar spine instrumentation surgery under general anaesthesia and all of them belonged to the American Society of Anesthesiologists'(ASA) physical status I/II. Opioid-dependent and sensitive patients, patients on antidepressant drugs, haemodynamically unstable and morbidly obese patients were excluded from this study. Informed written consent was obtained from all patients.
All the patients were randomised into two equal groups (n = 35 in each group) using a computer-generated random number list and the allocation concealment was done by the serially numbered opaque sealed envelope technique. We recorded baseline pain score in both groups before application of patch. Each patient of TDB group received TDB patch 10 μg/h and TDP Group, received transdermal placebo patch (TDP group) 24 h before the operation. An anaesthesiologist who was not involved in the study, opened the envelope 24 h before surgery and applied the transdermal patch to hair-less sites, and refrained from further study participation. Both the patches were covered with similar sizes of Dynaplast™ so that the external appearance of both the patches could not be differentiated by a third person. The anaesthesiologist who recorded the study parameters and the patient were unaware about the group distribution. During pre-operative visit, all the patients were informed about the transdermal patch and were familiarised about visual analogue scale (VAS) based pain assessment.
All patients were pre-medicated on the night before surgery with tablet ranitidine 150 mg and alprazolam 0.25 mg orally. On the day of surgery, all the baseline parameters (heartrate [HR], blood pressure [BP], oxygen saturation [SpO2], sedation) were recorded and intravenous (IV) line was established. Before induction of anaesthesia, IV glycopyrrolate (0.2 mg) and midazolam 0.15 mg/kg were administered to all patients. After pre-oxygenation, anaesthesia was induced with IV propofol (2 mg/kg), lignocaine (1.5 mg/kg) and rocuronium (1 mg/kg) to facilitate intubation along with IV fentanyl (2 μg/kg). Anaesthesia was maintained with 60%N2 Oand 40%O2 along with isoflurane 1%–1.5% and atracurium infusion (0.005 mg/kg/min). Intraopertative bispectral index BIS was maintained within 50–60 through out the operation by adjusting the concentration of isoflurane. Ventilation was adjusted to maintain end-tidal CO2 between 35 and 40 mmHg.
The additional doses of IV fentanyl (1 μg/kg) was administered as and when required until 30 min prior to skin closure to maintain mean arterial pressure (MAP) and HR around 20% of pre-operative status. At the end of surgery, residual neuromuscular blockade was antagonised with IV neostigmine (0.05mg/kg) and glycopyrrolate (0.01 mg/kg). After adequate recovery (Aldrete score >8) all the patients were extubated and shifted to the post-anaesthetic care unit (PACU) for observation. Each patient was monitored in post-operative period for haemodynamic parameters (HR, BP), respiratory rate, sedation, SpO2 at 30 min intervals for the first 2h, then every one-hour interval for next 4h and every 4h interval for rest of the study periods (total 48 h). Postoperatively, diclofenac 75 mg intramuscular 12 hourly was administered as analgesic in both groups.
The degree of post-operative analgesia was assessed by VAS (from 0 to 100, where 0 = no pain, 100 = the worst pain) at same time intervals for 48 h and rescue analgesic (injection tramadol- 2 mg/kg maximum 100 mg) was administered when VAS ≥30.
The primary outcome of this study, the analgesic efficacy of TDB in post-operative period was evaluated by comparing the timing of first rescue analgesic and total doses of post-operative analgesic requirement in 48 h between the two groups. Associated haemodynamic changes and safety of TDB were also assessed by monitoring any untoward side effect such as nausea, vomiting, sedation and respiratory depression during the study as a secondary outcome.
It was planned that intraoperative hypotension (reduction of MAP >20% from baseline) will be treated with IV fluid and/or phenylephrine 50 mcg. Similarly, intraoperative bradycardia (HR <60 beats/min) will be treated with atropine 0.6 mg IV. Post-operative oxygen desaturation (SpO2<95% for >10 s) will be treated with oxygen supplementation through a nasal cannula.
Sedation was assessed by Ramsay sedation score (1 = awake, 2 = drowsy, 3 = sleepy but arousable to verbal commands, 4 = sleepy but arousable to moderate stimulus, 5 = unconscious) till the end of study period. Nausea and vomiting was assessed by nausea score (0 = none, 1 = mild, 2 = moderate, 3 = severe).
For the purpose of statistical analysis, timing of first rescue analgesic was considered as primary outcome of this study. It was estimated that 35 subjects were required per group to detect 30 min difference in this parameter with 80% power and 5% probability in Type I error. This calculation was based on the previous study investigating the analgesic property of TDB with standard deviation of 45 min.[6]
All raw data of study parameters were entered into a Microsoft Excel spread sheet and statistical assessment of the data was carried out using the statistical software Statistica 6.0 (Tulsa, Oklahoma: StatSoftInc.,2001) and Graph Padprism (version 5 SanDiego, California: GraphPadSoftware Inc.,2007). Numerical variables were compared between two groups using student independent t-test if normally distributed or with Mann–Whitney U-test if otherwise. Normality was tested using Kolmogorov–Smirnov goodness of fit test. Fisher's exact test has been used to compare categorical variables between two groups. All analyses were two-tailed and P < 0.05 was considered statistically significant.
RESULTS
In this study, eighty patients were screened, of which 10 patients were not included because of unwillingness and opioid sensitivity. Seventy patients were randomised for assessment, and none were lost during the follow-up [Figure 1].
Figure 1.
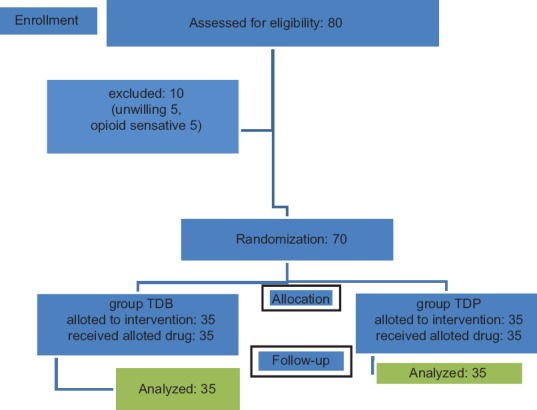
The CONSORT transparent reporting of trial - flow of patients in the trial. TDB – Transdermal buprenorphine; TDP – Transdermal placebo
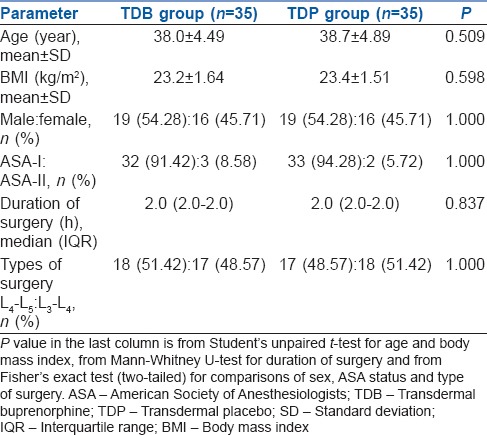
All the demographic characteristics such as age, height, weight, body mass index, sex, ASA status, duration of surgery were comparable between two groups [Table 1].
Table 1.
Summary of demographic and clinical characteristics in the two study groups
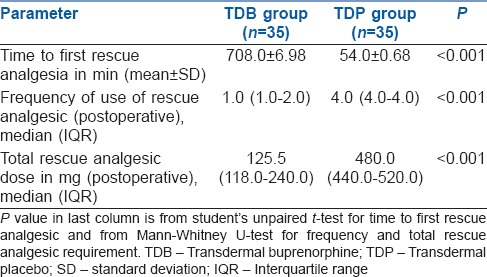
In the post-operative period, timing of first rescue analgesic requirement was significantly earlier in TDP Group patients [54.0 ± 0.68 min] in contrast to TDB Group [708.0 ± 6.98 min] (P < 0.01) [Table 2].
Table 2.
Comparison of first time, frequency and total recues analgesia requirement between the two study groups
All the patients of both groups were fully conscious, alert and haemodynamically stable before induction of general anaesthesia. There was no significant difference in the pre-operative VAS score between the two groups [Table 1].
All patients in TDB group maintained stable intraoperative haemodynamic status (HR, systolic blood pressure (SBP), diastolic blood pressure (DBP)) and did not require any additional IV fentanyl, whereas in TDP group most of the patients (60%) received additional doses of intraoperative fentanyl to control the haemodynamic surges. The intraoperative haemodynamic difference between two groups was statistically significant (P < 0.01).
The number of patients requiring post-operative rescue analgesic was higher in TDP Group than TDB group. Of the 35 patients in TDB Group, 5 (14.28%) did not require any rescue analgesics, 10 (28.56%) required twice and the rest (57.12%) required a single dose of rescue analgesic during the study, whereas all the patients (100%) in TDP Group required rescue analgesic (4 to 5 times during the study period).
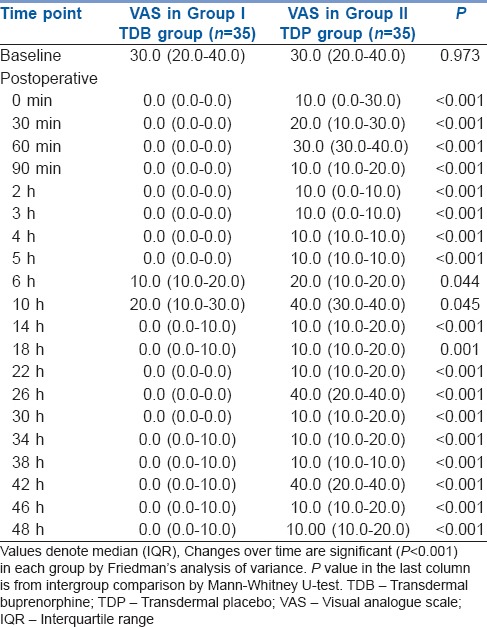
The frequency and total doses of post-operative tramadol requirement were also significantly higher in TDP Group than TDB Group (P < 0.001) [Table 2]. The postoperative VAS scores were always significantly less in TDB Group than TDP Group at all time intervals during the study [Table 3].
Table 3.
Changes in visual analogue scale score for pain over time in the two study groups
The post-operative mean HR, SBP and DBP in TDB Group were lower than TDP Group throughout the study. The descriptive statistics for these mean values between the two groups showed statistically significant differences (P < 0.05) during the study [Figures 2–4].
Figure 2.
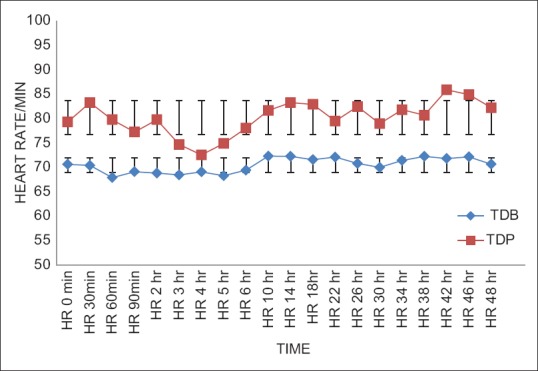
Comparison of postoperative heart rate/minute between two groups. Statistically significant change (P<0.05) in heart rate was found (Student's unpaired t test) among the groups of patients at all time intervals. TDB – Transdermal buprenorphine; TDP – Transdermal placebo
Figure 4.
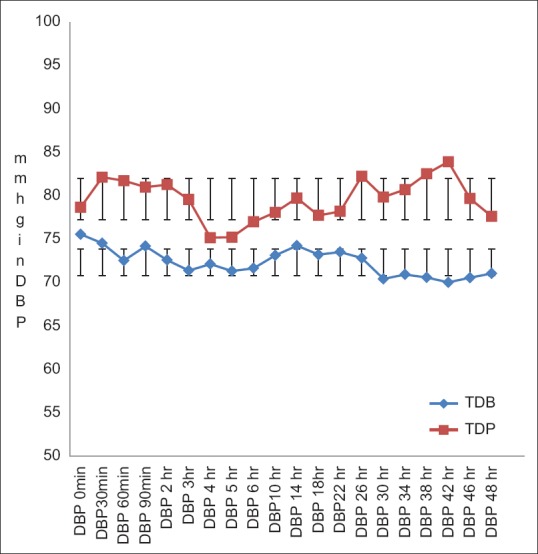
Comparison of postoperative diastolic blood pressure between two groups. Statistically significant change (P<0.05) in DBP was found (Student's unpaired t test) among the groups of patients. In TDB group -DBP was satisfactorily in normal range but lower than that of placebo group. DBP – Diastolic blood pressure, TDB – Transdermal buprenorphine; TDP – Transdermal placebo
Figure 3.
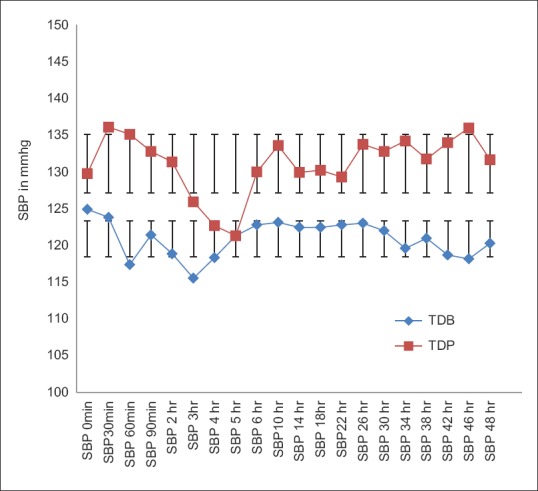
Comparison of postoperative systolic blood pressure between two groups. Statistically significant change (P<0.05) in SBP was found (Student's unpaired t test) among the groups of patients except in 5th hr. In TDB SBP was satisfactorily in normal range but lower than that of TDP group. SBP – Systolic blood pressure, TDB – Transdermal buprenorphine; TDP – Transdermal placebo
There was no incidence of post-operative hypoxia, respiratory depression, significant bradycardia or hypotension in either group. None of the patients in TDB Group complained of nausea, whereas three patients in TDP Group complained of nausea. None of the patients in both groups was excessively sedated in the study period.
DISCUSSION
Perioperative analgesic efficacy is frequently assessed by evaluating the variation in timing and total doses of post-operative analgesic requirement as an outcome measure.[7] In this study, we used the TDB for post-operative pain management after spinal fixation surgery.
We observed that the timing of post-operative first rescue analgesic requirement was significantly delayed in the TDB group compared to TDP group. The TDB group also maintained stable haemodynamics during the intra-and post-operative period. They had lower pain score and lower incidence of nausea. The total dose and frequency of post-operative analgesic requirement were also lower in the TDB group.
Central sensitisation and hyperexcitability develop after surgical incision and result in amplification of the post-operative pain. It raises the possibility of complications, increases cost of medical care and delays recovery. Pre-operative application of TDB patch alters this central processing by preemptive analgesia.[8]
TDS provides safe, convenient and reliable method of drug delivery. It is a preferable alternative to oral and parenteral drug delivery method as it avoids painful skin punctures and multiple dosing. It can bypass the first-pass metabolism and allows continuous drug delivery with a sustained plasma level. It also decreases the incidence of breakthrough pain and thereby decreasing the requirement of rescue analgesics. Drug-related adverse effects can be minimised with TDS, due to slow release and avoidance of sudden peak in drug plasma level.[9]
Buprenorphine is a partial agonist at the μ receptor and its analgesic efficacy is comparable with the usual doses of other opioids such as pentazocine, morphine and pethidine.[3,4] It can be administered by parenteral, sublingual or through intrathecal route as an adjuvant with local anaesthetic. These routes are not free from opioid-related adverse effects.[10,11,12]
The higher efficacy, tolerability and patient compliance with TDB patch in treatment of chronic malignant, non-malignant and neuropathic pain are well established.[13,14,15] When transdermal buprenorphine was compared to tramadol and co-codamol for non-cancer chronic osteoarthritic pain, it was noted to be superior.[16]
In India, buprenorphine patches are available in three different strengths as 5, 10, 20 μg/h and in our study, we selected 10μg/h TDB patch for post-operative pain relief.[17]
There are only few studies with transdermal buprenorphine for post-operative analgesia. All these studies were descriptive studies indicating the beneficial utility of transdermal buprenorphine for providing post-operative pain relief. Privetra and Guzzetta in two separate descriptive studies used 35 μg/h of TDB for patients undergoing shoulder and upper femur surgeries.[18,19] They concluded that TDB can be used safely for effective post-operative analgesia with higher patient satisfaction rates.
Similarly, in our study, the post-operative VAS scores were always lower in TDB group compared to TDP group, and this difference was significant at all post-operative time points.
As the peak effect of buprenorphine patch is seen at about 12–24 h after application, the TDB patches were placed 24 h before the surgery in this study, so that the desired drug concentration was achieved in the perioperative period.[20]
Arshad et al. compared transdermal fentanyl 25mcg/h to transdermal buprenorphine10mcg/h and noted that both fentanyl and buprenorphine are safe and effective for post-operative pain relief.[21]
Setti et al. used 17.5,35 and 52.5 μg/h of TDB patches in patients undergoing open gynaecologic surgeries, providing IV morphine and ketorolac as rescue analgesics. They found that the consumption of rescue analgesia was inversely correlated to the TDB dosage. Similarly, in our study, the first rescue analgesic requirement was delayed in TDB Group (708 min) as compared to TDP Group (54 min) due to the existing analgesic effect of TDB patch. Post-operative frequency and total doses of tramadol administration were higher in TDP group than TDB group.
Due to pre-existing analgesic effect and continuous steady plasma level of buprenorphine. the intra-and post-operative haemodynamic parameters were more stable in TDB Group.
Efficacy and tolerability of TDB patch for post-operative pain control in abdominal surgery and in the treatment of chronic pain have been well established in previous studies.[22,23,24]
It has been well documented that the analgesic effect of buprenorphine patch is prolonged (7 days) without any ceiling effect.[25] Unlike morphine and fentanyl, it has no immunosuppressive activity as well.[26] Some studies have reported that bradycardia and hypotension may occur with IV or intrathecal buprenorphine due to vagal stimulatory action.[27] In our study, no bradycardia or hypotension was noted with TDB patch. It has been documented that when buprenorphine patch was used in higher dose for cancer pain relief, it may cause post-operative nausea and vomiting (PONV), sedation or other adverse effects.[26] In our study, no such adverse effect occurred with 10 μg/h TDB patch. In contrast, PONV in TDP group was higher either due to increased post-operative pain or due to frequently administered IV tramadol for rescue analgesia.[28]
The minimal side effects observed in our study are not sufficient to justify conclusions on individual drug safety and further study in large and higher risk group patient population will be required to establish the safety profiles of pre-operative placement of TDB patch (10 μg/h).
CONCLUSION
A TDB patch (10 μg/hour) applied 24 hours before surgery can be used as a postoperative analgesic for lumber fixation surgery. It can reduce postoperative rescue analgesic consumption over 48 hours and maintain haemodynamic stability without serious complications like respiratory depression, sedation or PONV.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North America. 2005;23:21–36. doi: 10.1016/j.atc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–34. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 4.Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627–32. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 5.Sittl R, Griessinger N, Likar R. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: A multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2003;25:150–68. doi: 10.1016/s0149-2918(03)90019-1. [DOI] [PubMed] [Google Scholar]
- 6.Setti T, Sanfilippo F, Leykin Y. Transdermal buprenorphine for postoperative pain control in gynecological surgery: A prospective randomized study. Curr Med Res Opin. 2012;28:1597–608. doi: 10.1185/03007995.2012.719864. [DOI] [PubMed] [Google Scholar]
- 7.McQuay HJ, Poon KH, Derry S, Moore RA. Acute pain: Combination treatments and how we measure their efficacy. Br J Anaesth. 2008;101:69–76. doi: 10.1093/bja/aen108. [DOI] [PubMed] [Google Scholar]
- 8.Likar R. Transdermal buprenorphine in the management of persistent pain – Safety aspects. Ther Clin Risk Manag. 2006;2:115–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, et al. Gastrointestinal symptoms under opioid therapy: A prospective comparison of oral sustained-release hydromorphone, transdermal fentanyl, and transdermal buprenorphine. Eur J Pain. 2009;13:737–43. doi: 10.1016/j.ejpain.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Oifa S, Sydoruk T, White I, Ekstein MP, Marouani N, Chazan S, et al. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: A randomized, double-blind, four-arm trial in adults undergoing abdominal surgery. Clin Ther. 2009;31:527–41. doi: 10.1016/j.clinthera.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Candido KD, Winnie AP, Ghaleb AH, Fattouh MW, Franco CD. Buprenorphine added to the local anesthetic for axillary brachial plexus block prolongs postoperative analgesia. Reg Anesth Pain Med. 2002;27:162–7. doi: 10.1053/rapm.2002.30671. [DOI] [PubMed] [Google Scholar]
- 12.Dixit S. Post operative analgesia after caesarean section: An experienced with intrathecal buprenorphine. Indian J Anesth. 2007;51:515–8. [Google Scholar]
- 13.Plosker GL, Lyseng-Williamson KA. Buprenorphine 5, 10 and 20μg/h transdermal patch: A guide to its use in chronic non-malignant pain. CNS Drugs. 2012;26:367–73. doi: 10.2165/11208360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, Hanaoka K, Namiki A, Ogawa S, Kitajima T, Hosokawa T, et al. Efficacy, safety and pharmacokinetic study of a novel fentanyl-containing matrix transdermal patch system in japanese patients with cancer pain. Clin Drug Investig. 2008;28:313–25. doi: 10.2165/00044011-200828050-00005. [DOI] [PubMed] [Google Scholar]
- 15.Canneti A, Luzi M, Di Marco P, Cannata F, Pasqualitto F, Spinoglio A, et al. Safety and efficacy of transdermal buprenorphine and transdermal fentanyl in the treatment of neuropathic pain in AIDS patients. Minerva Anestesiol. 2013;79:871–83. [PubMed] [Google Scholar]
- 16.Karlsson M, Berggren AC. Efficacy and safety of low-dose transdermal buprenorphine patches (5, 10, and 20 microg/h) versus prolonged-release tramadol tablets (75, 100, 150, and 200 mg) in patients with chronic osteoarthritis pain: A 12-week, randomized, open-label, controlled, parallel-group noninferiority study. Clin Ther. 2009;31:510–13. doi: 10.1016/j.clinthera.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Gupta H, Babu RJ. Transdermal delivery: Product and patent update. Recent Pat Drug Deliv Formul. 2013;7:184–205. doi: 10.2174/187221130703131128121747. [DOI] [PubMed] [Google Scholar]
- 18.Privitera C, Guzzetta G. Postoperative pain management inshoulder surgery with transdermal (TDS) buprenorphinepatches. Reg Anesth Pain Med. 2008;33:e184. [Google Scholar]
- 19.Privitera C, Guzzetta G. Transdermal (TDS) buprenorphinepatches for postoperative pain management in orthopaedicsurgery in the elderly. Reg Anesth Pain Med. 2008;33:e187. [Google Scholar]
- 20.Koltzenburg M, Pokorny R, Gasser UE, Richarz U. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain. 2006;126:165–74. doi: 10.1016/j.pain.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Arshad Z, Prakash R, Gautam S, Kumar S. Comparison between transdermal buprenorphine and transdermal fentanyl for postoperative pain relief after major abdominal surgeries. J Clin Diagn Res. 2015;9:UC01–4. doi: 10.7860/JCDR/2015/16327.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böhme K, Likar R. Efficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain. A randomised, double-blind, placebo-controlled study. Pain Clinic. 2003;15:193–202. [Google Scholar]
- 23.Kumar S, Chaudhary AK, Singh PK, Verma R, Chandra G, Bhatia VK, et al. Buprenorphine patches for postoperative pain control in abdominal surgery. J Clin Diagn Res. 2016;10:UC05–8. doi: 10.7860/JCDR/2016/18152.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kress HG. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13:219–30. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Gordon A, Rashiq S, Moulin DE, Clark AJ, Beaulieu AD, Eisenhoffer J, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15:169–78. doi: 10.1155/2010/216725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tassinari D, Sartori S, Tamburini E, Scarpi E, Raffaeli W, Tombesi P, et al. Adverse effects of transdermal opiates treating moderate-severe cancer pain in comparison to long-acting morphine: A meta-analysis and systematic review of the literature. J Palliat Med. 2008;11:492–501. doi: 10.1089/jpm.2007.0200. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal K, Agarwal N, Agrawal V, Agarwal A, Sharma M, Agarwal K, et al. Comparative analgesic efficacy of buprenorphine or clonidine with bupivacaine in the caesarean section. Indian J Anaesth. 2010;54:453–7. doi: 10.4103/0019-5049.71046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai SN, Badiger SV, Tokur SB, Naik PA. Safety and efficacy of transdermal buprenorphine versus oral tramadol for the treatment of post-operative pain following surgery for fracture neck of femur: A prospective, randomised clinical study. Indian J Anaesth. 2017;61:225–9. doi: 10.4103/ija.IJA_208_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


