Despite the advent of bortezomib, thalidomide and lenalidomide, relapse of multiple myeloma (MM) is common, and novel therapies are needed urgently.1 Interactions of MM cells with bone marrow (BM) accessory and immune effector cells inhibit antitumor immunity as well as induce MM growth, survival and drug resistance.1 For example, we showed that plasmacytoid dendritic cells (pDCs) are increased in the BM of MM patients compared with normal BM, and these contribute to immune dysfunction, as well as promote tumor cell growth and survival.2 Aberrant pDCs’ function in MM is evidenced by their interaction not only with MM cells but also with immune effector T cells: MM BM pDCs have decreased ability to trigger T-cell proliferation compared with normal pDCs.2 Dysfunctional T cells and natural killer (NK) cells in MM3,4 together with functionally defective pDCs2 confer immune suppression in MM. To date, the mechanism(s) and the role of immunoregulatory molecules mediating pDC–T cell and pDC–NK cell interactions in MM remain undefined. Here we extended our previous studies2,5 to examine the role of immune checkpoint receptor programmed cell death protein 1 (PD1) and its ligand PDL1 in pDC–T cell and pDC–NK cell interactions in the MM BM milieu, and to determine whether this interaction represents a therapeutic target to restore antitumor immunity and cytotoxicity.
PD1 (CD279), a member of the CD28 family of receptors, is expressed on the surface of antigen-activated and -exhausted T cells.4 PD1 has two ligands, PDL1 (B7-H1; CD274) and PDL2 (B7-DC; CD273). Although PDL1 expression has not been observed in normal epithelial cells, it is highly expressed on many solid tumors.6 PDL2 is more broadly expressed on normal healthy tissues than PDL1. The physiological role of PD1 is to maintain T-cell homeostasis by restricting T-cell activation and proliferation, thereby preventing autoimmunity. Importantly, the interaction of PD1+ T cells with PDL1-expressing cells inhibits T-cell responses.7–9 In the context of MM, studies have demonstrated PD1-expressing T cells and NK cells in the MM BM milieu, as well as PDL1 on MM cells.3,10–13 However, the expression of PDL1–PD1 on MM patient-derived pDCs and its functional significance during pDC–MM–T–NK cell interactions remain undefined.
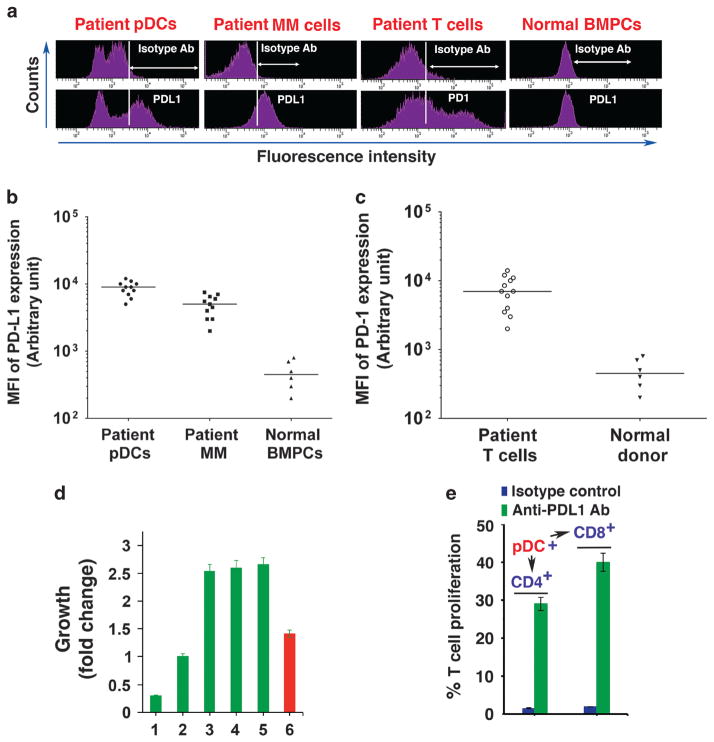
We first analyzed freshly isolated MM cells, pDCs and T cells from MM patient BM samples (n = 11) for PDL1 and PD1 expression using flow cytometry (fluorescence-activated cell sorter (FACS)). Both MM cells and pDCs expressed high surface levels of PDL1, whereas T cells showed high PD1 levels (Figures 1a–c). No significant PDL1 expression was noted on normal BM plasma cells. Our findings are consistent with previous reports showing that MM cells, but not normal plasma cells, express PDL1.3,10–13 These data indicate that the interactions between PDL1-expressing MM cells and pDCs with PD1-positive T cells may contribute to both T-cell and pDC immune dysfunction in MM, and MM cells may escape antitumor immunity by virtue of PDL1 expression.
Figure 1.
(a) PDL1 and PD1 expression analysis. pDCs, MM cells and T cells were isolated from patient BM samples using immunomagnetic cell separation kits specific for each cell type, followed by FACS. pDCs, MM cells and normal BM plasma cells (BMPCs) were stained with brilliant violet 421-conjugated PDL1 Ab, and PDL1 levels were analyzed using FACS. MM patient T cells were stained with Alexa-647-conjugated PD1 Ab and were examined for PD1 levels. A representative FACS analysis from 11 MM patients and 6 normal BM donors is shown. (b) Frequency of PDL1 expression on patient pDCs and tumor cells. Data are presented as mean fluorescence intensity (MFI) of PDL1 expression on pDCs and MM cells isolated from patient BM samples (n =11). BMPCs from normal healthy donors served as controls (n =6). Median MFI is shown for each cohort; P<0.0001 for both pDCs and patient MM cells versus normal BMPCs. (c) Frequency of PD1 expression on patient T cells. Data are presented as MFI of PD1 expression on T cells isolated from MM patient BM samples (n =12). BM mononuclear cells from normal healthy donors (n =6) served as controls. Median MFI values are presented for both patient and control groups; P<0.0001 for patient versus normal T-cell population. (d) Effect of anti-PDL1 Ab on pDC-induced patient MM cell growth. pDCs and autologous MM cells from patient BM samples (n =8) were cocultured in the presence of isotype control Ab or anti-PDL1 Ab for 72 h, and then analyzed for growth. pDCs (lane 1), MM cells (lane 2) and pDCs plus MM cells (lane 3) were cultured with isotype control Ab for 72 h, followed by growth analysis. pDCs and MM cells were also cocultured in the presence of anti-PDL1 Ab (lane 4: 5 μg/ml; lane 5: 10 μg/ml) and analyzed for growth (mean ± s.d.; P<0.005; n =3). CpG oligodeoxynucleotides-treated (1 μg/ml) cocultures of pDCs and MM cells (lane 6) served as a positive control for MM cell growth inhibition. Cocultures of pDCs and MM cells were performed at 1:5 (pDC:MM) ratio. Growth assays were performed using 1 × 104 pDCs and 5 × 104 MM cells in 200 μl media in 96 well plates. Error bars indicate s.d. (e) PDL1–PD1 blockade triggers T-cell proliferation. pDCs from MM patients (n =10) were cocultured with autologous T cells using 1:10 (pDC:T) ratio in the presence of isotype-matched control Ab or anti-PDL1 Ab (5 μg/ ml) for 5 days, and CD4+ or CD8+ T cells were quantified using CellTrace Violet-Cell proliferation Kit by FACS (mean ±s.d.; P<0.05, n =3).
We next examined whether blockade of PDL1–PD1 restores anti-MM immune response and/or affects pDC-induced MM cell growth, using a monoclonal antibody (Ab) specifically directed against PDL1. A recent study analyzed the expression of PD1 and PD1-ligands in the tumor immune microenvironment and demonstrated clinical responses to anti-PD1 Ab therapy in PDL1-positive tumors.8 PDL1 is expressed in both pDCs and MM cells, including relapsed or refractory MM,13 and we hypothesize that blockade of PDL1 will alleviate T-cell immune suppression conferred by both MM cells and pDCs during pDC–MM–T cell interactions. Moreover, as PDL1 binds not only to PD1 but also to CD80, on T cells to induce T-cell inhibition,14 anti-PDL1 Ab may block both co-inhibitory signals on T cells. Preclinical and clinical studies have begun to examine the utility of anti-PDL1 monoclonal Ab in MM.10,11,15 Here we targeted PDL1 rather than PDL2 for the following reasons: (1) PDL1 is more restricted in its expression on normal tissues than PDL2, and targeting PDL1 may therefore cause less on-target off-tissue toxicity;9 (2) a recent report correlated PDL1, but not PDL2, expression with response to anti-PD1 therapy;8 and (3) we found that both pDCs and MM cells express variable and low levels of PDL2 versus PDL1.
We first examined whether blockade of PDL1 affects the ability of pDC to induce MM cell growth. The patient MM cells or MM cell lines (MM.1S, MM.1R and RPMI-8226) were cultured either alone or together with MM–pDCs in the presence or absence of anti-PDL1 Ab for 72 h, followed by analysis of growth. pDCs triggered proliferation of autologous MM cells and MM cell lines, as in our previous studies.2,5 Importantly, anti-PDL1 Ab did not significantly inhibit pDC-triggered growth of MM cells (Figure 1d and Supplementary Figure 1). Our recent study showed that targeting toll-like receptor-9 blocks pDC-induced MM cell growth,2,5 which served as a positive control in these studies (Figure 1d and Supplementary Figure 1). Although blocking PDL1 does not affect pDC-induced MM cell growth, pDC–MM cell interactions upregulate PDL1 expression on both cell types, consistent with earlier observations that BM stromal cells induce PDL1 expression on MM cells.13 Such interactive mechanisms enhancing PDL1 expression in the MM BM milieu further abrogate PD1-expressing T-cell responses. Nonetheless, our findings show that targeting PDL1 alone does not either affect the viability of pDCs and MM cells or modulate pDC-induced growth of MM cells.
We next examined whether blockade of PDL1–PD1 affects the biologic sequelae of pDC–T cell interactions. pDCs from MM patients (n = 10) were cocultured with autologous T cells in the presence of anti-PDL1 Ab or isotype control Ab for 5 days, and CD4+/CD8+ T cells were quantified with CellTrace Violet-Cell Proliferation Kit (Life Technologies, Grand Island, NY, USA) using FACS. Blockade of PDL1–PD1 with anti-PDL1 Ab triggered a significant increase in both CD4+ and CD8+ T-cell populations: 29 ± 2.1% and 40 ± 2.7% increases in CD4+ and CD8+ T cells, respectively, compared with isotype controls (P<0.005; Figure 1e). A similar increase in CD4+ and CD8+ T-cell population was observed in response to anti-PDL1 Ab in cocultures of pDCs, T cells and MM cells (data not shown). Together, these results indicate that blockade of PDL1–PD1 using anti-PDL1 Ab enhances T-cell response to autologous pDCs.
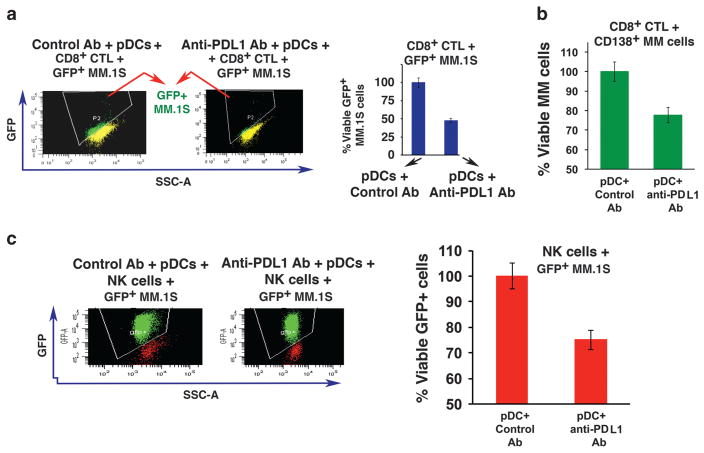
We next asked whether blockade of PDL1–PD1 triggers the generation of MM-specific cytotoxic T lymphocytes (CTLs) ex vivo. Freshly isolated CD8+ T cells from MM patient BM (n = 10) were cultured with autologous pDCs in the presence of isotype control or anti-PDL1 Ab for 5 days; then green fluorescent protein-positive (GFP+) MM.1S cells were added for 3 days, followed by quantification of viable GFP+ MM.1S cells. Anti-PDL1 Ab triggers robust MM-specific CD8+ CTL activity, evidenced by decreased numbers of viable GFP+ MM.1S cells (Figure 2a). Importantly, pDC-induced CD8+ CTLs show marked cytolytic activity against autologous MM cells (n = 8) (Figure 2b). Of note, 5 of the 8 patients in whom pDCs, T cells and MM cells were studied had MM resistant to bortezomib, dexamethasone and lenalidomide therapy.
Figure 2.
Anti-PDL1 Ab induces MM-specific CD8+ CTLs and NK cell-mediated cytotoxicity. (a) Freshly isolated CD8+ T cells from MM patient BM (n =10) were cocultured with autologous pDCs at 1:10 (pDC:T) ratio in the presence of isotype-matched control Ab or anti-PDL1 Ab (5 μg/ml) for 5 days; then GFP+ MM.1S cells were added for 3 days (E:T ratio 20:1 for CD8+ T:GFP+ MM.1S), followed by quantification of viable GFP+ MM.1S cells by FACS (right panel, bar graph) (mean ±s.d.; P<0.03). The loss of viable GFP signal is shown in a representative histogram (left panel), indicating MM cell lysis by CD8+ CTLs. (b) CD8+ T cells from MM patients (n =8) were prestained with CellTrace Violet and cultured with autologous pDCs at 1:10 (pDC:T) ratio in the presence of isotype-matched control Ab or anti-PDL1 Ab (5 μg/ml) for 5 days; then autologous CD138+ MM cells were added for another 2 days (E:T ratio 20:1, CD8+ T:MM), followed by staining with 7-AAD and fluorescein isothiocyanate-conjugated anti-CD138 Ab for quantitative analysis of CTL-mediated cytotoxicity against MM cells using FACS. MM cells were also incubated without CD8+ T cells to measure baseline apoptosis. The loss of viable CD138+ MM cells indicates MM cell lysis by anti-PDL1-activated CTLs (mean ±s.d.; P<0.05). (c) Freshly isolated NK cells from MM patient BM (n =5) were cocultured with autologous pDCs at 1:10 (pDC:NK) ratio in the presence of anti-PDL1 Ab or isotype-matched control Ab (5 μg/ml) for 5 days; then GFP+ MM.1S cells were added for another 3 days (E:T ratio 10:1, NK:GFP+ MM.1S), followed by quantification of viable GFP+ MM.1S cells by FACS (right panel, bar graph) (mean ±s.d.; P<0.05). The loss of viable GFP signal is shown in a representative histogram (left panel), indicating NK cell-mediated MM cell lysis.
Besides CD8+ T cells, blockade of PDL1–PD1 also triggers proliferation of CD4+ T cells in pDC–T cell cocultures (Figure 1e), and we therefore next examined whether pDC-induced CD4+ T cells are cytolytic against MM cells. MM patient CD4+ T cells were cultured with autologous pDCs in the presence of isotype control or anti-PDL1 Ab for 5 days; then GFP+ MM.1S cells were added for 3 days, followed by quantification of viable GFP+ MM.1S cells. Anti-PDL1 Ab increased MM-specific CD4+ CTL activity, albeit to a lesser extent than CD8+ T cells (Supplementary Figure 2). Taken together, our findings show that blockade of PDL1–PD1 with anti-PDL1 Ab in pDC–T cell interactions triggers broad anti-MM immunity.
A previous study showed that NK cells from MM patients, but not normal healthy donors, express PD1.3 We therefore next examined whether blockade of PDL1–PD1 in pDC–NK cell interactions using anti-PDL1 Ab alters NK cell-mediated MM cell cytotoxic activity. NK cells isolated from MM patient samples (n = 5) were cocultured with autologous pDCs in the presence of anti-PDL1 Ab or isotype control for 5 days; then GFP+ MM.1S cells were added to the activated NK cells for 3 days, followed by quantification of viable GFP+ MM.1S cells using FACS. Anti-PDL1 Ab triggered a marked increase in NK cell cytolytic activity against MM cells (Figure 2c). These findings suggest that PDL1–PD1 signaling axis between pDCs and NK cells abrogates normal NK cell function in MM and, conversely, that anti-PDL1 Ab restores NK cell-mediated anti-MM activity.
Our study therefore shows that pDCs confer T-cell and NK cell immune suppression in the MM BM milieu by engaging immune checkpoints via PDL1–PD1 signaling axis. We show that PDL1 is highly expressed on pDCs and MM cells, implicating a two-pronged suppression of PD1-expressing T cell and NK cell immune function, and blockade of PDL1–PD1 using anti-PDL1 Ab generates robust MM-specific CD8+ CTL activity, as well as enhances NK cell-mediated MM cell cytolytic activity. As PDL1-expressing pDCs are increased in MM BM and localize with PDL1-positive MM cells,2 PDL1 expression may correlate with progression of disease, with highest levels in relapsed or relapsed/refractory MM. Indeed, a recent study showed that PDL1 expression positively correlates with increased proliferative potential of tumor cells and resistance to therapies in MM.13 Finally, current anti-MM therapies, that is, immunomodulatory agents such as thalidomide, lenalidomide or pomalidomide, modulate MM-host immune responses, suggesting that a combination treatment with anti-PDL1 Ab may enhance both host anti-MM immunity and clinical response.
Supplementary Material
Acknowledgments
This investigation was supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) grant P50100707, PO1-CA078378 and RO1 CA050947. KCA is an American Cancer Society Clinical Research Professor.
Footnotes
AUTHOR CONTRIBUTIONS
DC designed the research, analyzed the data and wrote the manuscript; AR performed the experiments, analyzed the data and wrote the manuscript; YS and DSD helped in flow cytometry; PR and NCM provided clinical samples; and KCA analyzed the data and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–452. doi: 10.1200/JCO.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atanackovic D, Luetkens T, Kroger N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia. 2014;28:993–1000. doi: 10.1038/leu.2013.310. [DOI] [PubMed] [Google Scholar]
- 5.Ray A, Tian Z, Das DS, Coffman RL, Richardson P, Chauhan D, et al. A novel TLR-9 agonist C792 inhibits plasmacytoid dendritic cell-induced myeloma cell growth and enhance cytotoxicity of bortezomib. Leukemia. 2014;28:1716–1724. doi: 10.1038/leu.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 7.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12:489–492. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-gamma and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 11.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17:1133–1145. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblatt J, Avivi I, Vasir B, Uhl L, Munshi NC, Katz T, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19:3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 14.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/ programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190:5620–5628. doi: 10.4049/jimmunol.1202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.