Abstract
Population incidence of Guillain-Barré syndrome (GBS) is required to assess changes in GBS epidemiology, but published estimates of GBS incidence vary greatly depending on case ascertainment, definitions, and sample size. We performed a meta-analysis of articles on GBS incidence by searching Medline (1966–2009), Embase (1988–2009), Cinahl (1981–2009) and CABI (1973–2009) as well as article bibliographies. We included studies from North America and Europe with at least 20 cases, and used population-based data, subject matter experts to confirm GBS diagnosis, and an accepted GBS case definition. With these data, we fitted a random-effects negative binomial regression model to estimate age-specific GBS incidence. Of 1,683 nonduplicate citations, 16 met the inclusion criteria, which produced 1,643 cases and 152.7 million person-years of follow-up. GBS incidence increased by 20% for every 10-year increase in age; the risk of GBS was higher for males than females. The regression equation for calculating the average GBS rate per 100,000 person-years as a function of age in years was exp[−12.0771 + 0.01813(age in years)] × 100,000. Our findings provide a robust estimate of background GBS incidence in Western countries. Our regression model may be used in comparable populations to estimate the background age-specific rate of GBS incidence for future studies.
Keywords: Guillain-Barré syndrome; Guillain-Barré syndrome, incidence; Guillain-Barré syndrome, meta-analysis
Introduction
Guillain-Barré syndrome (GBS) is a condition characterized by the acute or subacute onset of varying degrees of weakness in limbs or cranial nerve-innervated muscles, associated decreased or absent deep tendon reflexes, and a characteristic profile in the cerebrospinal fluid and electrodiagnostic studies [1]. The underlying etiology and pathophysiology of GBS are not completely understood [2], but it is thought to be an immune-mediated process, resulting from the generation of autoimmune antibodies and inflammatory cells that cross-react with epitopes on peripheral nerves and roots, leading to demyelination, axonal damage or both [3]. This immune response is thought to be initiated in response to a variety of antigenic stimuli, such as viral or bacterial infection, particularly Campylobacter jejuni [4, 5]. Vaccines are another antigenic stimulus for which potential associations with GBS have been reported, including formulations of Semple rabies vaccine, tetanus toxoid vaccine, and some formulations of influenza vaccine [6–8]. With rare exceptions, the biological or epidemiological evidence for a causal association between GBS and antecedent infections or vaccination is equivocal.
A firm measure of the incidence of GBS is increasingly important. GBS appears to be the most frequent cause of nonpoliovirus acute flaccid paralysis worldwide; however, accurate estimates of GBS incidence are unknown for many countries. Additionally, the rare association of various vaccines with GBS has made this syndrome an important focus of vaccine safety monitoring [9]. Assessing the presence, magnitude, and attributable risk of vaccine-associated GBS requires reliable age-specific incidence estimates. However, reported estimates of GBS incidence for all ages combined vary from 0.16 to 3.0 per 100,000 person-years [10]. Some of the variability may be due to true differences in GBS incidence; for example, GBS incidence is thought to be higher in parts of Asia [11]. However, even in Europe and North America where most studies have been conducted, reported GBS incidence varies considerably [10]. Some variability is likely artifactual resulting from different case ascertainment methods, case definitions, and case inclusion criteria. A recent comprehensive systematic literature review summarized data from articles worldwide describing the epidemiology of GBS, including trends in incidence [10]. However, the expansive nature of this review included all articles irrespective of methodology, precluding direct comparisons of incidence estimates.
Here we present findings of a systematic review and meta-analysis of published studies reporting GBS incidence to obtain the most reliable estimates of population-based age-specific incidence of GBS in North America and Europe.
Materials and Methods
Search Strategy
We searched for published work in any language recorded in Medline (January 1, 1966 to December 28, 2009), Embase (1988 to December 28, 2009), Cinahl (1981 to December 28, 2009) and CABI (1973 to December 28, 2009). For searching databases, we used the following key words: ‘Polyradiculoneuropathy’, ‘Incidence’, ‘Epidemiology’, ‘Guillain-Barré Syndrome’, ‘Immunization’, ‘Vaccination’, ‘Campylobacter’, and ‘Respiratory Tract Infections’ (Appendix). We also searched the reference lists of articles selected for full-text review for additional references.
Selection Criteria
We selected studies based upon the following criteria: population based (cases were identified from a well-defined enumerated population); case finding was either prospective, retrospective, or a combination of both; at least 20 cases were identified; GBS cases were confirmed by subject matter experts (neurologists) from prospective patient evaluation, medical chart review, or both, and a clear and widely accepted case definition for GBS was used [e.g. National Institute of Neurological and Communicative Disorders and Stroke definition (NINCDS) [12], the case definition developed by Asbury and Cornblath [13], or the Brighton Collaboration [14] criteria]. We excluded studies that were not population based, or which depended upon administrative medical codes only (e.g. International Classification of Diseases codes) to identify cases. We limited the assessment to studies conducted in North America and Europe, because incidence of GBS in many parts of the world is not known, and some evidence suggests that the epidemiology of GBS may be substantially different in other regions.
Study Selection and Data Collection
Two investigators (J.J.S., O.W.M.) independently reviewed the title and abstract of all citations identified by the initial search strategy and excluded citations that clearly did not meet the inclusion criteria. We retrieved the full text of the remaining studies and both investigators reviewed each study to assess whether it met the inclusion criteria. When reviewers disagreed or were uncertain about the suitability of a study, a third investigator (M.W.) reviewed the paper and all investigators arrived at a consensus by discussion. One investigator, a board-certified neurologist (J.J.S.), extracted the following data from studies that met the inclusion criteria: study design, case ascertainment method, case definition used, study period, number of GBS cases identified (crude and age-specific), denominators (crude and age-specific), reported GBS incidence, and perceived study limitations. These data were verified by a second investigator (M.W.). When papers did not report the numerator and denominator used to calculate rates or only presented age-specific rates graphically, we attempted to contact the study authors for this information.
Statistical Analysis
For each study that reported age-specific incidence rates of GBS, we plotted the rate versus the midpoint of the reported age group and superimposed the plots on one graph for comparison. Because the oldest age group was open-ended in all of the studies, we assigned the median age for these groups using publicly available vital statistics data from the country in which the assessment was performed. For these assignments, we used data from the geographic area and time period that most closely matched each study population.
We fit random-effects Poisson and negative binomial regression models to the age-specific data. Models that included age as a continuous variable with a random effect for the intercept, slope, or both were explored [15]. Six of the 13 studies reported information to calculate age-specific rates of GBS by sex. For these 6 studies, we fit the same regression model used for the 13 studies overall, with the addition of the effect of sex.
All regression models were fit using the NLMIXED procedure in SAS version 9.2. We used the results from the negative binomial regression model to derive an average rate of GBS for 9 successive 10-year age groups (0–9, 10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89 years). For each estimated rate, we calculated a 95% prediction interval, which measures the uncertainty of the estimated rate for a randomly selected study by incorporating the between-study variability assumed by the model [16].
Results
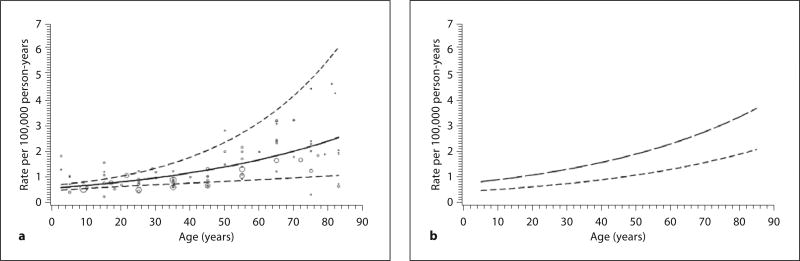
We identified 1,879 citations from the database search, of which 1,683 citations were unique (i.e. nonduplicate) (fig. 1). After reviewing the titles and abstracts, we discarded 1,637 citations (97%) that clearly did not meet the inclusion criteria for this review. We examined the full text of the remaining 46 articles in detail, of which 30 did not meet the inclusion criteria: 15 did not use a clear and widely accepted case definition or did not use subject matter experts to confirm the diagnosis of GBS; 8 were not population based, and 7 reported data that were substantially or wholly reported by other articles also selected for review. We did not identify additional studies that met the inclusion criteria from searching reference lists. Our final selection included 16 articles that met the inclusion criteria for this review [8, 17–31], of which 13 have sufficient data to be included in the meta-analysis [8, 17, 18, 20–27, 29, 31].
Fig. 1.
Study selection.
The selected articles reported data from Canada (n = 1), England (n = 2), Italy (n = 5), The Netherlands (n = 1), Spain (n = 4), Sweden (n = 1), and the United States (n = 2) (table 1). The mean study duration was 15 months (range, 4 months to 45 years) and the study period ranged from 1935 to 2002. Eight studies used prospective case identification, 6 retrospective case identification, and 2 studies both prospective and retrospective case identification. For GBS case definition, 12 studies (75%) applied the NINCDS criteria [12], 2 the criteria by Asbury and Cornblath [13], and 1 the Brighton Collaboration definition [14] (table 1). The study by Schonberger et al. [8] did not use a well-defined case definition; however, these data were rigorously reviewed several years later by Langmuir et al. [32], who found that 91% of cases had sufficient data to be classified as having GBS. We included the article by Schonberger et al. [8] in preference to the article by Langmuir et al. [32] because it reported age-specific rates of GBS in the US population that did not receive the 1976 swine influenza vaccine, which we considered to be the background rate of GBS.
Table 1.
Summary of studies included in the review, ordered by location and study period
| No. | Authors | Location | Study period | Case ascertainment | Case definition |
|---|---|---|---|---|---|
| 1 | Deceuninck et al. [26] | Province of Quebec, Montreal, Canada | November 1, 2000 to December 31, 2002 | Retrospective review of medical discharge records | Brighton criteria |
| 2 | Winner and Evans [31] | Oxfordshire, England | January 1, 1974 to December 31, 1986 | Retrospective review of medical discharge records | NINCDS with Asbury addendum |
| 3 | Rees et al. [28] | South East England | July 1, 1993 to June 30, 1994 | Prospective case reporting, and review of hospital admission and death data | Asbury and Cornblath criteria |
| 4 | Govoni et al. [27] | Ferrara, Italy | 1981–2001 | Prospective and retrospective review of medical discharge records | NINCDS |
| 5 | Emilia-Romagna Study Group [17] | Emilia-Romagna region, Italy | January 1, 1992 to December 31, 1993 | Prospective case reporting | NINCDS |
| 6 | Beghi and Bogliun [19] | Lombardy, Italy | February 1, 1994 to May 31, 1995 | Prospective case reporting | NINCDS |
| 7 | Bogliun and Beghi [21] | Lombardy, Italy | January 1 to December 31, 1996 | Prospective case reporting | NINCDS |
| 8 | Chio et al. [23] | Piemonte and Valle d’Aosta regions, Italy | January 1, 1995 to December 31, 1996 | Prospective case reporting | NINCDS |
| 9 | van Koningsveld et al. [30] | South west Netherlands | January 1, 1987 to December 31, 1996 | Retrospective review of medical discharge records | NINCDS |
| 10 | Sedano et al. [29] | Cantabria, Spain | January 1975 to December 1988 | Retrospective review of medical discharge records | NINCDS |
| 11 | Aladro-Benito et al. [18] | Canary Islands, Spain | 1983–1998 | Retrospective review of medical discharge records | NINCDS |
| 12 | Cuadrado et al. [24] | 11 study centers, Spain | 1985–1997 | Prospective case reporting | NINCDS |
| 13 | Cuadrado et al. [25] | 11 study centers, Spain | 1998–1999 | Prospective case reporting | NINCDS |
| 14 | Cheng et al. [22] | Sweden | January 1 to December 31, 1996 | Prospective case identification by neurologist network and inpatient registries | NINCDS |
| 15 | Beghi et al. [20] | Olmsted County, Minn., USA | 1935–1980 | Retrospective review of Mayo Clinic neurology records | NINCDS |
| 16 | Schonberger et al. [8] | United States | October 1, 1976 to January 31, 1977 | Prospective case reporting | Motor weakness in both lower extremities, areflexia, autonomic dysfunction, fever, and recoverya |
Of the 13 studies included in the meta-analysis, the number of cases ranged from 33 to 418 (median, 81) (table 2). The number of age groups for which rates were reported ranged from 3 to 9 (median, 7). Five articles presented both case counts and denominators [17, 18, 20, 22, 25]; 5 articles presented case counts and rates [8, 24, 27, 29, 31]; 1 study provided only rates and we obtained case counts from the authors [21]; 1 study published only the rates in a line graph and we obtained publicly available census data to generate case counts and denominators [23], and 1 study published only the rates in a histogram and we obtained case counts and denominators from the authors [26].
Table 2.
Number of GBS cases, person-years, and rate of GBS, by study and age group, total for 13 published studies and by sex for 6 studies
| No. | Study | Age group years |
Midpointa years |
Total | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| cases | person- years |
rate per 100,000 PY |
cases | person- years |
rate per 100,000 PY |
cases | person- years |
rate per 100,000 PY |
||||
| 1 | Deceuninck et al. [26]b | 0–4 | 2.5 | 14 | 761,945 | 1.84 | ||||||
| 5–14 | 10 | 12 | 1,999,647 | 0.60 | ||||||||
| 15–20 | 18 | 7 | 1,313,873 | 0.53 | ||||||||
|
|
||||||||||||
| all | 33 | 4,075,465 | 0.81 | |||||||||
|
| ||||||||||||
| 2 | Winner and Evans [31]c | 0–4 | 2.5 | 5 | 384,615 | 1.3 | ||||||
| 5–14 | 10 | 1 | 1,000,000 | 0.1 | ||||||||
| 15–24 | 20 | 9 | 1,285,714 | 0.7 | ||||||||
| 25–34 | 30 | 12 | 1,000,000 | 1.2 | ||||||||
| 35–44 | 40 | 8 | 800,000 | 1.0 | ||||||||
| 45–54 | 50 | 10 | 666,667 | 1.5 | ||||||||
| 55–64 | 60 | 12 | 600,000 | 2.0 | ||||||||
| 65–74 | 70 | 9 | 500,000 | 1.8 | ||||||||
| 75+ | 79 | 6 | 315,789 | 1.9 | ||||||||
|
|
||||||||||||
| all | 72 | 6,552,785 | 1.1 | |||||||||
|
| ||||||||||||
| 4 | Govoni et al. [27] | 0–19 | 10 | 3 | 566,038 | 0.53 | 1 | 285,714 | 0.35 | 2 | 280,324 | 0.72 |
| 20–39 | 30 | 10 | 1,020,408 | 0.98 | 6 | 517,241 | 1.16 | 4 | 503,167 | 0.80 | ||
| 40–59 | 50 | 21 | 1,044,776 | 2.01 | 14 | 498,221 | 2.81 | 7 | 546,555 | 1.28 | ||
| 60–79 | 70 | 28 | 864,198 | 3.24 | 17 | 367,965 | 4.62 | 11 | 496,233 | 2.21 | ||
| 80+ | 82 (82, 83) | 7 | 162,791 | 4.30 | 1 | 50,505 | 1.98 | 6 | 112,286 | 5.34 | ||
|
|
||||||||||||
| all | 69 | 3,658,211 | 1.89 | |||||||||
|
| ||||||||||||
| 5 | ERSG [17]d | 0–9 | 5 | 4 | 549,420 | 0.73 | ||||||
| 10–19 | 15 | 2 | 835,806 | 0.24 | ||||||||
| 20–29 | 25 | 10 | 1,168,726 | 0.86 | ||||||||
| 30–39 | 35 | 9 | 1,077,682 | 0.84 | ||||||||
| 40–49 | 45 | 7 | 1,062,634 | 0.66 | ||||||||
| 50–59 | 55 | 12 | 1,075,628 | 1.12 | ||||||||
| 60–69 | 65 | 24 | 1,023,894 | 2.34 | ||||||||
| 70+ | 77 | 19 | 1,025,234 | 1.85 | ||||||||
|
|
||||||||||||
| all | 87 | 7,819,024 | 1.11 | |||||||||
|
| ||||||||||||
| 7 | Bogliun and Beghi [21] | 0–34 | 17 | 35 | 4,430,380 | 0.79 | ||||||
| 35–54 | 45 | 33 | 2,481,203 | 1.33 | ||||||||
| 55–74 | 65 | 52 | 1,614,907 | 3.22 | ||||||||
| 75+ | 81 | 18 | 385,439 | 4.67 | ||||||||
|
|
||||||||||||
| all | 138 | 8,911,929 | 1.55 | |||||||||
|
| ||||||||||||
| 8 | Chio et al. [23]e | 0–9 | 5 | 7 | 669,315 | 1.05 | 4 | 343,988 | 1.16 | 3 | 325,327 | 0.92 |
| 10–19 | 15 | 9 | 830,775 | 1.08 | 6 | 424,862 | 1.41 | 3 | 405,913 | 0.74 | ||
| 20–29 | 25 | 12 | 1,297,499 | 0.92 | 7 | 666,317 | 1.05 | 5 | 631,182 | 0.79 | ||
| 30–39 | 35 | 11 | 1,293,563 | 0.85 | 8 | 655,635 | 1.22 | 3 | 637,928 | 0.47 | ||
| 40–49 | 45 | 11 | 1,235,793 | 0.89 | 8 | 617,501 | 1.30 | 3 | 618,292 | 0.49 | ||
| 50–59 | 55 | 26 | 1,195,441 | 2.17 | 18 | 589,758 | 3.05 | 8 | 605,683 | 1.32 | ||
| 60–69 | 65 | 27 | 1,097,141 | 2.46 | 16 | 513,336 | 3.12 | 11 | 583,805 | 1.88 | ||
| 70–79 | 75 | 14 | 689,356 | 2.03 | 5 | 282,018 | 1.77 | 9 | 407,338 | 2.21 | ||
| 80+ | 83 (83, 83) | 9 | 436,576 | 2.06 | 3 | 140,752 | 2.13 | 6 | 295,824 | 2.03 | ||
|
|
||||||||||||
| all | 126 | 8,745,459 | 1.44 | |||||||||
|
| ||||||||||||
| 10 | Sedano et al. [29]f | 10–19 | 15 | 18 | 1,146,497 | 1.57 | 10 | 595,238 | 1.68 | 8 | 551,259 | 1.46 |
| 20–29 | 25 | 9 | 1,139,241 | 0.79 | 3 | 576,923 | 0.52 | 6 | 562,318 | 1.10 | ||
| 30–39 | 35 | 6 | 937,500 | 0.64 | 5 | 480,769 | 1.04 | 1 | 456,731 | 0.26 | ||
| 40–49 | 45 | 8 | 761,905 | 1.05 | 6 | 389,610 | 1.54 | 2 | 372,295 | 0.55 | ||
| 50–59 | 55 | 12 | 851,064 | 1.41 | 7 | 426,829 | 1.64 | 5 | 424,235 | 1.19 | ||
| 60–69 | 65 (F 60+: 71) | 8 | 650,407 | 1.23 | 5 | 297,619 | 1.68 | 3 | 745,230 | 0.40 | ||
| 70+ | 75 (M 70+: 75) | 2 | 625,000 | 0.32 | 2 | 232,558 | 0.86 | 0 | ||||
|
|
||||||||||||
| all | 63 | 6,111,614 | 1.03 | |||||||||
|
| ||||||||||||
| 11 | Aladro-Benito et al. [18] | 0–9 | 5 | 6 | 1,466,272 | 0.41 | 3 | 729,760 | 0.41 | 3 | 736,512 | 0.40 |
| 10–19 | 15 | 12 | 1,562,144 | 0.77 | 4 | 777,472 | 0.51 | 8 | 784,672 | 1.02 | ||
| 20–29 | 25 | 10 | 1,366,864 | 0.73 | 6 | 680,288 | 0.88 | 4 | 686,576 | 0.58 | ||
| 30–39 | 35 (F: 40) | 9 | 954,160 | 0.94 | 8 | 474,880 | 1.68 | 1 | 867,568 | 0.12 | ||
| 40–49 | 45 | 5 | 773,008 | 0.65 | 5 | 384,720 | 1.30 | 0 | ||||
| 50–59 | 55 | 15 | 750,080 | 2.00 | 9 | 373,328 | 2.40 | 6 | 376,752 | 1.59 | ||
| 60–69 | 65 | 16 | 500,752 | 3.19 | 11 | 249,248 | 4.40 | 5 | 251,504 | 1.99 | ||
| 70–79 | 75 (F 70+: 76) | 7 | 289,728 | 2.41 | 6 | 144,192 | 4.16 | 1 | 212,480 | 0.47 | ||
| 80+ | 83 (M 80+: 83) | 1 | 133,264 | 0.75 | 1 | 66,320 | 1.51 | 0 | ||||
|
|
||||||||||||
| all | 81 | 7,796,272 | 1.04 | |||||||||
|
| ||||||||||||
| 12 | Cuadrado et al. [24] | 20–29 | 25 | 45 | 9,000,000 | 0.50 | ||||||
| 30–39 | 35 | 48 | 7,868,852 | 0.61 | ||||||||
| 40–49 | 45 | 46 | 6,865,672 | 0.67 | ||||||||
| 50–59 | 55 | 62 | 5,904,762 | 1.05 | ||||||||
| 60–69 | 65 | 86 | 5,180,723 | 1.66 | ||||||||
| 70–79 | 75 | 40 | 3,200,000 | 1.25 | ||||||||
| 80+ | 83 | 10 | 1,538,462 | 0.65 | ||||||||
|
|
||||||||||||
| all | 337 | 39,558,471 | 0.85 | |||||||||
|
| ||||||||||||
| 13 | Cuadrado et al. [25] | 20–29 | 25 | 8 | 1,777,672 | 0.45 | 5 | 901,000 | 0.55 | 3 | 876,672 | 0.34 |
| 30–39 | 35 | 10 | 1,555,670 | 0.64 | 8 | 775,402 | 1.03 | 2 | 780,268 | 0.26 | ||
| 40–49 | 45 | 14 | 1,361,720 | 1.03 | 8 | 671,698 | 1.19 | 6 | 690,022 | 0.87 | ||
| 50–59 | 55 | 20 | 1,161,958 | 1.72 | 13 | 574,586 | 2.26 | 7 | 587,372 | 1.19 | ||
| 60–69 | 65 | 25 | 1,033,412 | 2.42 | 19 | 487,866 | 3.89 | 6 | 545,546 | 1.10 | ||
| 70–79 | 75 | 15 | 646,548 | 2.32 | 11 | 265,006 | 4.15 | 4 | 381,542 | 1.05 | ||
| 80+ | 83 (83, 83) | 6 | 313,564 | 1.91 | 3 | 105,070 | 2.86 | 3 | 208,494 | 1.44 | ||
|
|
||||||||||||
| all | 98 | 7,850,544 | 1.25 | |||||||||
|
| ||||||||||||
| 14 | Cheng et al. [22] | 0–9 | 5 | 6 | 590,004 | 1.02 | 3 | 302,615 | 0.99 | 3 | 287,389 | 1.04 |
| 10–19 | 15 | 6 | 494,913 | 1.21 | 3 | 253,872 | 1.18 | 3 | 241,041 | 1.24 | ||
| 20–29 | 25 | 8 | 642,109 | 1.25 | 5 | 324,229 | 1.54 | 3 | 317,880 | 0.94 | ||
| 30–39 | 35 | 8 | 645,327 | 1.24 | 6 | 330,664 | 1.81 | 2 | 314,663 | 0.63 | ||
| 40–49 | 45 | 8 | 628,943 | 1.27 | 5 | 318,341 | 1.57 | 3 | 310,602 | 0.96 | ||
| 50–59 | 55 | 5 | 533,947 | 0.94 | 4 | 268,975 | 1.49 | 1 | 264,972 | 0.38 | ||
| 60–69 | 65 | 12 | 386,727 | 3.10 | 11 | 183,505 | 5.99 | 1 | 203,222 | 0.49 | ||
| 70–79 | 75 | 16 | 356,935 | 4.48 | 6 | 154,422 | 3.89 | 10 | 202,513 | 4.94 | ||
| 80+ | 83 (83, 84) | 4 | 202,328 | 1.98 | 1 | 68,155 | 1.47 | 3 | 134,173 | 2.24 | ||
|
|
||||||||||||
| all | 73 | 4,481,233 | 1.63 | |||||||||
|
| ||||||||||||
| 15 | Beghi et al. [20] | 0–17 | 9 | 8 | 991,669 | 0.81 | ||||||
| 18–39 | 29 | 13 | 970,235 | 1.34 | ||||||||
| 40–59 | 50 | 16 | 563,286 | 2.84 | ||||||||
| 60+ | 70 | 11 | 338,087 | 3.25 | ||||||||
|
|
||||||||||||
| all | 48 | 2,863,277 | 1.68 | |||||||||
|
| ||||||||||||
| 16 | Schonberger et al. [8] | 0–17 | 9 | 86 | 15,579,710 | 0.55 | ||||||
| 18–24 | 21.5 | 60 | 5,617,978 | 1.07 | ||||||||
| 25–44 | 35 | 96 | 10,810,811 | 0.89 | ||||||||
| 45–64 | 55 | 108 | 8,181,818 | 1.32 | ||||||||
| 65+ | 72 | 68 | 4,047,619 | 1.68 | ||||||||
|
|
||||||||||||
| all | 418 | 44,237,936 | 0.94 | |||||||||
PY = Person-years.
For studies that provided rates by sex, the midpoint for the last age group is indicated for each sex. In a few instances, an age group with a zero number of GBS cases was combined with an adjacent age group.
The authors provided data by single-year age groups, which we combined into three age groups.
Rates in this article were presented with only one number after the decimal point.
Emilia-Romagna Study Group.
Rates were estimated by age and sex from a graph presented in the article. The overall rate in the article was 1.36 cases per 100,000 person-years.
Data for the first age group in this study (0–9 years) were deemed unreliable based on comparison with other information in the article and were therefore not included.
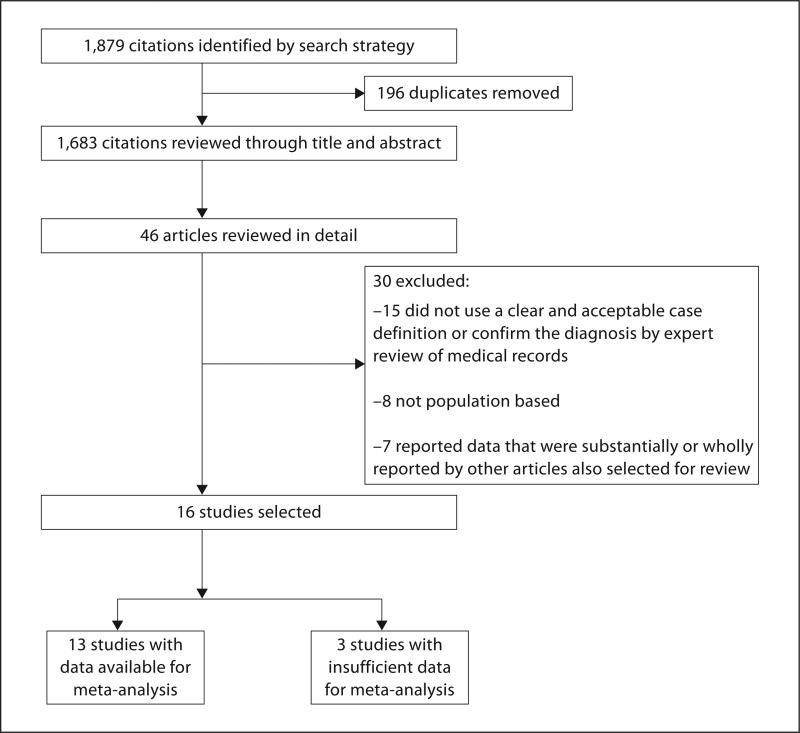
The reported crude incidence ranged from 0.81 to 1.89 (median, 1.11) cases per 100,000 person-years (table 2). Among the 13 studies, the rate of GBS increased exponentially with age, with increasing variation in the rates from the younger to the older age groups (fig. 2a). The range of age-specific incidence rates increased from roughly 3-fold differences between studies in the younger age groups to as much as 10-fold differences in the older age groups.
Fig. 2.
a Plot of age-specific incidence rate of GBS per 100,000 person-years versus age in years, for 13 published studies. b Plot of age-specific incidence rate of GBS per 100,000 person-years versus age in years, for 6 published studies that provided rates in males. c Plot of age-specific incidence rate of GBS per 100,000 person-years versus age in years, for 6 published studies that provided rates in females.
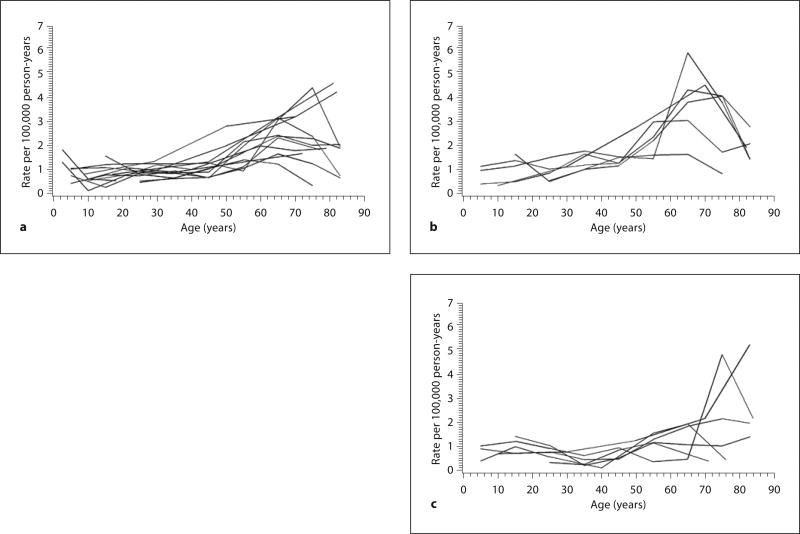
The meta-analysis included 1,643 cases and 152.7 million person-years of follow-up. The random-effects negative binomial regression model fit the data better than the random-effects Poisson model (likelihood ratio test, p < 0.01). The best-fitting negative binomial regression model included age as a continuous variable with a single random effect for the slope parameter to represent deviation of each study’s true effect from the overall mean effect. Results from this model suggested a 20% increase in the average GBS rate for every 10-year increase in age (fig. 3a). For persons aged 0–90 years in North America and Europe, the regression equation for calculating the average GBS rate per 100,000 person-years as a function of age in years was exp[−12.0771 + 0.01813(age in years)] × 100,000.
Fig. 3.
a Plot of average age-specific incidence rate of GBS per 100,000 person-years versus age in years based on regression analysis of 13 published studies, with pointwise 95% prediction intervals (dashed lines) and observed rates (bubbles proportional to the number of person-years). b Plot of average age-specific incidence rate of GBS per 100,000 person-years versus age in years based on regression analysis of 6 published studies that provided rates by sex (males: long dashed lines, females: short dashed lines).
The age-specific GBS rate increased from 0.62 cases per 100,000 person-years among 0- to 9-year-olds to 2.66 cases per 100,000 person-years among 80- to 89-year-olds (table 3). The prediction intervals became wider with increasing age, especially after about age 70 years (fig. 3a).
Table 3.
Estimated rate of GBS by age group based on regression analysis of 13 studies, and estimated rate of GBS by age group and sex based on regression analysis of 6 studies
| Age group years |
Mid-point years |
R ate per 100,000 person-years (95% PI) | ||
|---|---|---|---|---|
|
| ||||
| total (n = 13) | males (n = 6) | females (n = 6) | ||
| 0–9 | 5 | 0.62 (0.52–0.75) | 0.80 (0.59–1.10) | 0.45 (0.32–0.64) |
| 10–19 | 15 | 0.75 (0.60–0.92) | 0.97 (0.72–1.31) | 0.55 (0.39–0.76) |
| 20–29 | 25 | 0.90 (0.67–1.19) | 1.18 (0.86–1.61) | 0.66 (0.47–0.93) |
| 30–39 | 35 | 1.07 (0.74–1.56) | 1.43 (0.99–2.06) | 0.80 (0.54–1.18) |
| 40–49 | 45 | 1.29 (0.80–2.06) | 1.73 (1.12–2.68) | 0.97 (0.62–1.53) |
| 50–59 | 55 | 1.54 (0.87–2.74) | 2.09 (1.24–3.54) | 1.18 (0.69–2.01) |
| 60–69 | 65 | 1.85 (0.94–3.64) | 2.54 (1.37–4.70) | 1.42 (0.76–2.66) |
| 70–79 | 75 | 2.22 (1.01–4.86) | 3.07 (1.50–6.27) | 1.72 (0.84–3.54) |
| 80–89 | 85 | 2.66 (1.09–6.48) | 3.72 (1.65–8.40) | 2.09 (0.92–4.74) |
PI = Prediction interval, based on the t distribution with 12 degrees of freedom (t0.975 = 2.1788) for total rates and with 5 degrees of freedom (t0.975 = 2.5706) for sex-specific rates.
Age-specific rates of GBS by sex revealed higher rates for males than females (fig. 2b, c). This pattern was confirmed by the model-based estimates (fig. 3b), which suggested a relative risk for males of 1.78 (95% CI, 1.36–2.33). For the calculation of age-specific rates of GBS by sex, the regression equations were exp[−12.4038 + 0.01914(age in years) + 0.5777] × 100,000 for males and exp[−12.4038 + 0.01914(age in years)] × 100,000 for females.
Discussion
GBS is an uncommon disease and individual studies frequently lack sufficient numbers of cases to make reliable age-specific incidence estimates. Our meta-analysis of high-quality population-based published studies provides a robust estimate of average age-specific GBS incidence in North America and Europe. A regression model based on data combined from the studies showed an exponential increase in GBS incidence from 0.62 to 2.66 per 100,000 person-years across all age groups. The prediction intervals for the estimated age-specific GBS incidence rates suggested that there was increasing uncertainty in the rates as age increased. This increasing variability in the GBS rate with age assumed by the regression model was consistent with the pattern of increased variation in observed incidence rates with age.
Differences in age-specific incidence rates across different study areas may be due to the application of case definitions rather than a true difference in the epidemiology of GBS. Although most of the studies included in our review used the same NINCDS criteria, GBS case definitions are syndrome-based, and their application depends on interpretation of clinical observations. Even though invasive tests such as lumbar puncture and electrodiagnostic studies can increase the level of diagnostic certainty, there is no biological marker to reliably diagnose GBS. Nevertheless, the application of syndrome-based case definitions utilizing expert neurologist chart review is superior to relying on administrative data such as hospital discharge (International Classification of Diseases) codes, which are less specific and often overestimate true incidence [33, 34].
Observed rates in the studies were generally close to predicted rates derived from our regression model. In several studies, however, observed rates for the youngest or oldest age groups deviated significantly from predicted rates. There may be several reasons for these discrepancies. GBS is more difficult to diagnose in younger age groups, especially in pediatric patients, and varying rates in some study areas may reflect diagnostic uncertainty and either over- or underdiagnosis of GBS in younger patients [35, 36]. The lower incidence of GBS in older age groups may reflect a survivor bias, in which individuals surviving into their 80s and 90s are less likely to develop GBS, although there is no substantiated biological basis for this hypothesis.
Our assessment found a significantly higher risk of GBS among males, a finding that has been consistently demonstrated in published studies. The male predominance in GBS differs from that of most other autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus, which frequently demonstrate higher rates in females [37, 38]. The reason for the higher risk of GBS in males is unknown.
Our study has several limitations. We only included data from published studies. However, unpublished sources of GBS incidence tend to be from administrative databases (International Classification of Diseases codes) and so would not be eligible for inclusion in the review. We focused our review on populations from North America and Europe, for which the largest number and most carefully conducted studies are available. However, the epidemiology of GBS may vary globally and our regression model for calculating age-specific rates may not be applicable to all regions. Of the 13 studies included in our meta-analysis, 8 were from Italy or Spain [17, 18, 21, 23–25, 27, 29]. However, we did not find any striking difference in GBS incidence between these and other countries that we included. We were unable to contact authors of 3 articles that met our eligibility criteria but for which we had insufficient data to be included in the meta-analysis [19, 28, 30]. Exclusion of these articles was unlikely to have changed our modeled incidence estimates as their reported crude incidence was 0.92 (n = 109), 1.2 (n = 79), and 1.18 (n = 476) per 100,000 person-years, which fell within the range of the crude incidence in the 13 studies included in our meta-analysis.
Our findings provide a robust estimate of background GBS incidence. In light of the increasing variability in the background GBS incidence with age, future studies assessing the effects of potential risk factors need to provide carefully determined background rates, particularly in the oldest age groups. Investigators can use our model of the increase of GBS incidence across age groups for assessing changes of GBS incidence following immunizations, infections, or putative causal exposures.
Acknowledgments
The authors would like thank Dr. Maria José Sedano, Dr. Ettore Beghi, and Dr. Geneviève Deceuninck for providing unpublished data from their studies. We also thank Dr. Lawrence B. Schonberger for helpful discussions about GBS in the United States as well as use of data reported in the article authored by him. We are grateful to Gail Bang at the Centers for Disease Control and Prevention who helped us with the database search.
Appendix
Database Search Strategies
Medline
exp Polyradiculoneuropathy/
exp Incidence/
1 and 2
exp Epidemiology/
1 and 4
exp Guillain-Barre Syndrome/
exp Immunization/
6 and 7
exp Vaccination/
6 and 9
exp Campylobacter/
6 and 11 and incidence.ti,ab.
exp Respiratory Tract Infections/
6 and 13
6 and rate.ti,ab.
6 and influenza.ti,ab. 3 or 5 or 8 or 10 or 12 or 14 or 16
Embase
exp Polyradiculoneuropathy/
exp Incidence/
1 and 2
exp Epidemiology/
1 and 4
exp Guillain-Barre Syndrome/
exp Immunization/
6 and 7
exp Vaccination/
6 and 9
exp Campylobacter/
6 and 11 and incidence.ti,ab.
exp Respiratory Tract Infections/
6 and 13
6 and rate.ti,ab.
6 and influenza.ti,ab.
3 or 5 or 8 or 10 or 12 or 14 or 16
Cinahl
S2 (guillain-barre syndrome and (immunization or vaccination or (campylobacter and incidence) or respiratory tract infections or rate or influenza))
S1 (polyradiculoneuropathy and (incidence or epidemiology))
S3 (s1 or s2)
CABI
(((guillain-barre syndrome) AND (influenza))) OR (((guillain-barre syndrome) AND (rate))) OR (((guillain-barre syndrome) AND (respiratory tract infections))) OR (((guillain-barre syndrome) AND (campylobacter AND incidence))) OR (((guillain-barre syndrome) AND (immunization OR vaccination))) OR (((polyradiculoneuropathy) AND (incidence OR epidemiology)))
(guillain-barre syndrome) AND (influenza)
(guillain-barre syndrome) AND (rate)
(guillain-barre syndrome) AND (respiratory tract infections)
(guillain-barre syndrome) AND (campylobacter AND incidence)
(guillain-barre syndrome) AND (immunization OR vaccination)
(polyradiculoneuropathy) AND (incidence OR epidemiology)
Footnotes
Disclosure Statement
All authors are employed by the Centers for Disease Control and Prevention and no additional funding was used for this study. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 2.Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barré syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 3.Levin MC, Krichavsky M, Berk J, Foley S, Rosenfeld M, Dalmau J, Chang G, Posner JB, Jacobson S. Neuronal molecular mimicry in immune-mediated neurologic disease. Ann Neurol. 1998;44:87–98. doi: 10.1002/ana.410440115. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T. Infectious agents as the triggers for the pathogenesis of the neuroimmunological disorders. Nippon Rinsho. 2008;66:1056–1064. [PubMed] [Google Scholar]
- 5.Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 6.Hemachudha T, Griffin DE, Chen WW, Johnson RT. Immunologic studies of rabies vaccination-induced Guillain-Barré syndrome. Neurology. 1988;38:375–378. doi: 10.1212/wnl.38.3.375. [DOI] [PubMed] [Google Scholar]
- 7.Fenichel GM. Neurological complications of immunization. Ann Neurol. 1982;12:119–128. doi: 10.1002/ana.410120202. [DOI] [PubMed] [Google Scholar]
- 8.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, Eddins DL, Bryan JA. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110:105–123. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 9.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, Vannice K, Izurieta HS, Akhtar A, Gold M, Oselka G, Zuber P, Pfeifer D, Vellozzi C. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374:2115–2122. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 11.Griffin JW, Li CY, Ho TW, Xue P, Macko C, Gao CY, Yang C, Tian M, Mishu B, Cornblath DR. Guillain-Barré syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases. Brain. 1995;118:577–595. doi: 10.1093/brain/118.3.577. [DOI] [PubMed] [Google Scholar]
- 12.Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978;3:565–566. doi: 10.1002/ana.410030628. [DOI] [PubMed] [Google Scholar]
- 13.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(suppl):S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 14.Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, Burwen DR, Cornblath DR, Cleerbout J, Edwards KM, Heininger U, Hughes R, Khuri-Bulos N, Korinthenberg R, Law BJ, Munro U, Maltezou HC, Nell P, Oleske J, Sparks R, Velentgas P, Vermeer P, Wiznitzer M Brighton Collaboration GBS Working Group. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Bagos P, Nikolopoulos G. Mixed-effects poisson regression models for meta-analysis of follow-up studies with constant or varying durations. Int J Biostat. http://works.bepress.com/pbagos/19.
- 16.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guillain-Barré syndrome variants in Emilia-Romagna, Italy, 1992–3: incidence, clinical features, and prognosis. Emilia-Romagna Study Group on Clinical and Epidemiological Problems in Neurology. J Neurol Neurosurg Psychiatry. 1998;65:218–224. doi: 10.1136/jnnp.65.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aladro-Benito Y, Conde-Sendin MA, Muñoz-Fernández C, Pérez-Correa S, Alemany-Rodríguez MJ, Fiuza-Pérez MD, Alamo-Santana F. Guillain-Barré syndrome in the northern area of Gran Canaria and the island of Lanzarote (in Spanish) Rev Neurol. 2002;35:705–710. [PubMed] [Google Scholar]
- 19.Beghi E, Bogliun G. The Guillain-Barré syndrome (GBS). Implementation of a register of the disease on a nationwide basis. Italian GBS Study Group. Ital J Neurol Sci. 1996;17:355–361. doi: 10.1007/BF01999898. [DOI] [PubMed] [Google Scholar]
- 20.Beghi E, Kurland LT, Mulder DW, Wiederholt WC. Guillain-Barré syndrome. Clinicoepidemiologic features and effect of influenza vaccine. Arch Neurol. 1985;42:1053–1057. doi: 10.1001/archneur.1985.04060100035016. [DOI] [PubMed] [Google Scholar]
- 21.Bogliun G, Beghi E. Incidence and clinical features of acute inflammatory polyradiculoneuropathy in Lombardy, Italy, 1996. Acta Neurol Scand. 2004;110:100–106. doi: 10.1111/j.1600-0404.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Q, Jiang GX, Fredrikson S, Link H, De Pedro-Cuesta J. Incidence of Guillain-Barré syndrome in Sweden 1996. Eur J Neurol. 2000;7:11–16. [PubMed] [Google Scholar]
- 23.Chio A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R Piemonte, and Valle d’Aosta Register for Guillain-Barré Syndrome. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003;60:1146–1150. doi: 10.1212/01.wnl.0000055091.96905.d0. [DOI] [PubMed] [Google Scholar]
- 24.Cuadrado JI, de Pedro-Cuesta J, Ara JR, Cemillán CA, Díaz M, Duarte J, Fernández MD, Fernández O, García-López F, García-Merino A, García-Montero R, Martínez-Matos JA, Palomo F, Pardo J, Tobías A Spanish GBS Epidemiological Study Group. Guillain-Barré syndrome in Spain, 1985–1997: epidemiological and public health views. Eur Neurol. 2001;46:83–91. doi: 10.1159/000050769. [DOI] [PubMed] [Google Scholar]
- 25.Cuadrado JI, de Pedro-Cuesta J, Ara JR, Cemillán CA, Díaz M, Duarte J, Fernández MD, Fernandez O, García-López F, García- Merino A, Velasquez JM, Martínez-Matos JA, Palomo F, Pardo J, Tobías A Spanish GBS Epidemiological Study Group. Public health surveillance and incidence of adulthood Guillain-Barré syndrome in Spain, 1998– 1999: the view from a sentinel network of neurologists. Neurol Sci. 2004;25:57–65. doi: 10.1007/s10072-004-0231-6. [DOI] [PubMed] [Google Scholar]
- 26.Deceuninck G, Boucher RM, De Wals P, Ouakki M. Epidemiology of Guillain-Barré syndrome in the province of Quebec. Can J Neurol Sci. 2008;35:472–475. doi: 10.1017/s0317167100009136. [DOI] [PubMed] [Google Scholar]
- 27.Govoni V, Granieri E, Manconi M, Capone J, Casetta I. Is there a decrease in Guillain-Barré syndrome incidence after bovine ganglioside withdrawal in Italy? A population-based study in the Local Health District of Ferrara, Italy. J Neurol Sci. 2003;216:99–103. doi: 10.1016/s0022-510x(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 28.Rees JH, Thompson RD, Smeeton NC, Hughes RA. Epidemiological study of Guillain-Barré syndrome in south east England. J Neurol Neurosurg Psychiatry. 1998;64:74–77. doi: 10.1136/jnnp.64.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedano MJ, Calleja J, Canga E, Berciano J. Guillain-Barré syndrome in Cantabria, Spain. An epidemiological and clinical study. Acta Neurol Scand. 1994;89:287–292. doi: 10.1111/j.1600-0404.1994.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Koningsveld R, Van Doorn PA, Schmitz PI, Ang CW, Van der Meché FG. Mild forms of Guillain-Barré syndrome in an epidemiologic survey in The Netherlands. Neurology. 2000;54:620–625. doi: 10.1212/wnl.54.3.620. [DOI] [PubMed] [Google Scholar]
- 31.Winner SJ, Evans JG. Age-specific incidence of Guillain-Barré syndrome in Oxfordshire. Q J Med. 1990;77:1297–1304. doi: 10.1093/qjmed/77.3.1297. [DOI] [PubMed] [Google Scholar]
- 32.Langmuir AD, Bregman DJ, Kurland LT, Nathanson N, Victor M. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am J Epidemiol. 1984;119:841–879. doi: 10.1093/oxfordjournals.aje.a113809. [DOI] [PubMed] [Google Scholar]
- 33.Bogliun G, Beghi E. Validity of hospital discharge diagnoses for public health surveillance of the Guillain-Barré syndrome. Neurol Sci. 2002;23:113–117. doi: 10.1007/s100720200036. [DOI] [PubMed] [Google Scholar]
- 34.Koobatian TJ, Birkhead GS, Schramm MM, Vogt RL. The use of hospital discharge data for public health surveillance of Guillain-Barré syndrome. Ann Neurol. 1991;30:618–621. doi: 10.1002/ana.410300418. [DOI] [PubMed] [Google Scholar]
- 35.Hicks CW, Kay B, Worley SE, Moodley M. A clinical picture of Guillain-Barré syndrome in children in the United States. J Child Neurol. 2010;25:1504–1510. doi: 10.1177/0883073810370481. [DOI] [PubMed] [Google Scholar]
- 36.Pier DB, Hallbergson A, Peters JM. Guillain-Barré syndrome in a child with pain: lessons learned from a late diagnosis. Acta Paediatr. 2010;99:1589–1591. doi: 10.1111/j.1651-2227.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- 37.Voskuhl RR. Sex differences in autoimmune disease. Biol Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao ZF, Yang LX, Mo XA, Qin C, Lai YR, He NY, Li T, Hackett ML. Frequency of autoimmune diseases in myasthenia gravis: a systematic review. Int J Neurosci. 2010;121:121–129. doi: 10.3109/00207454.2010.539307. [DOI] [PubMed] [Google Scholar]