Abstract
The use of light-emitting electronic devices before bedtime may contribute to or exacerbate sleep problems. Exposure to blue-wavelength light in particular from these devices may affect sleep by suppressing melatonin and causing neurophysiologic arousal. We aimed to determine if wearing amber-tinted blue light-blocking lenses before bedtime improves sleep in individuals with insomnia. Fourteen individuals (n=8 females; age ± SD 46.6 ± 11.5 y) with insomnia symptoms wore blue light-blocking amber lenses or clear placebo lenses in lightweight wraparound frames for 2 h immediately preceding bedtime for 7 consecutive nights in a randomized crossover trial (4-wk washout). Ambulatory sleep measures included the Pittsburgh Insomnia Rating Scale (PIRS) completed at the end of each intervention period, and daily post-sleep questionnaire and wrist-actigraphy. PIRS total scores, and Quality of Life, Distress, and Sleep Parameter subscales, were improved in amber vs. clear lenses condition (p-values <0.05). Reported wake-time was significantly delayed, and mean subjective total sleep time (TST), overall quality, and soundness of sleep were significantly higher (p-values <0.05) in amber vs. clear lenses condition over the 7-d intervention period. Actigraphic measures of TST only were significantly higher in amber vs. clear lenses condition (p=0.035). Wearing amber vs. clear lenses for 2-h preceding bedtime for 1 week improved sleep in individuals with insomnia symptoms. These findings have health relevance given the broad use of light-emitting devices before bedtime and prevalence of insomnia. Amber lenses represent a safe, affordable, and easily implemented therapeutic intervention for insomnia symptoms.
Keywords: sleep, insomnia, blue blocker, behavioral intervention, actigraphy, randomized controlled trial
INTRODUCTION
Insomnia symptoms, including difficulty falling or staying asleep, frequently awakening, feeling that sleep is unrefreshing or not sound, or having daytime consequences like feelings of sleepiness, irritability, or trouble concentrating, described in the International Classification of Sleep Disorders-3rd Edition (Sateia, 2014), occur in as much as 33–50% of adults (Schutte-Rodin et al., 2008). While the etiology of insomnia is multifactorial and involves cognitive, behavioral, and physiological factors (Roth, 2007), clinicians and researchers are becoming increasingly aware of how nocturnal light exposure contributes to poor sleep (Czeisler, 2013). In humans, the circadian system enables a consolidated nocturnal sleep phase which coincides with ambient darkness and increased circulating levels of the pineal hormone melatonin (Turek and Gillette, 2004). Melatonin acts as the hormonal signal for the onset of the biological night and has been conceptualized as the factor which “opens the sleep gate” (Cajochen et al., 2003). Environmental light can phase delay rhythms of melatonin and alertness when presented during nighttime hours (Cajochen et al., 2014). A delay in melatonin onset, therefore, may be expected to be a factor contributing to subsequent delays in sleep initiation mechanisms. This may play a role in the development of sleep complaints.
Evening light exposure from normal ambient room lighting (Gooley et al., 2011), eBooks (Chang et al., 2015), and light-emitting diode (LED)-backlit computer screens (Cajochen et al., 2011) causes reductions and delays in melatonin secretion. Light exposure from these sources during the hours preceding habitual bedtime can also decrease subjective and objective sleepiness (Cajochen et al., 2011; Chang et al., 2015), prolong sleep onset latency (SOL) (Chang et al., 2015), and decrease rapid eye movement (REM) sleep (Chang et al., 2015) and slow wave sleep (SWS) (Munch et al., 2006). Light also has acute alerting effects, independent of the circadian system, which can interfere with sleep initiation and maintenance (Cajochen, 2007). The circadian photoreceptor system shows peak sensitivity to ~450–480 nm light within the blue portion of the spectrum (Brainard et al., 2001; Thapan et al., 2001), which accounts for the high efficacy of blue light to suppress melatonin and increase alertness (Cajochen et al., 2005). Most modern computer, TV, smartphone, and tablet screens, as well as an increasing number of domestic light bulbs, are lit by LEDs which have a peak wavelength in the blue range of ~460 nm (Cajochen et al., 2011).
The ramifications for these observations are widespread and important since 90% of responders in a representative survey of American adults reported using some type of light-emitting electronic device within the hour before bedtime (Gradisar et al., 2013). Considering the near ubiquity of personal light-emitting devices, huge portions of the population are voluntarily engaging in avoidable behaviors that may worsen their sleep and are associated with insomnia (Fossum et al., 2014). However, patients can be resistant to instructions made by clinicians to limit the use of these devices in the evening for purposes of improving sleep (Phelps, J., 2008). The development of methods to reduce the adverse effects of evening ambient light exposure, while still allowing for the maintained use of light-emitting devices, could have high impact in shaping clinical practice paradigms for improving sleep in individuals with insomnia.
By selectively filtering out blue-wavelength light in the hours preceding bedtime, the impact of light of the circadian system may be ameliorated. This can be accomplished by wearing amber-tinted, blue-blocking (BB) lenses. Indeed, prior work has demonstrated that BB lenses can prevent light-induced melatonin suppression (Kayumov et al., 2005; Sasseville et al., 2006), and there is some evidence of a therapeutic benefit of these lenses for sleep in a variety of pathological states (Burkhart and Phelps, 2009; Fargason et al., 2013; Henriksen et al., 2014; Phelps, James, 2008). To our knowledge, no study to date has utilized a randomized crossover design to assess the impact of BB lenses on subjective and objective sleep quality and duration in individuals with an insomnia diagnosis. We aimed to test the hypothesis that amber lenses worn for 2 hours before bedtime will improve sleep quality and duration, compared to clear lenses, in individuals with insomnia symptoms.
MATERIAL AND METHODS
Participants
A total of 15 men and women, age 18–65 y were recruited and enrolled. Participants were recruited from the New York City area. None of the participants were patients of study investigators. The primary inclusion criterion was reporting chronic insomnia symptoms for >3 mo. Insomnia identification in study participants was achieved via a validated symptom questionnaire, the Insomnia Symptoms Questionnaire (ISQ) (Okun et al., 2009), which is guided by established diagnostic criteria. The ISQ is a 13-item self-report measure that provides a dichotomous outcome (“present” or “absent”) of the definition of insomnia based on diagnostic criteria from the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM), Fourth Edition, and is also consistent with the American Academy of Sleep Medicine Research Diagnostic Criteria (Okun et al., 2009). All participants met the diagnostic criteria for insomnia based on the ISQ. Exclusion criteria included prior diagnosis of obstructive sleep apnea (apnea-hypopnea index ≥15 events/h, via polysomnography, obtained from medical history in individuals who had previously undergone a diagnostic study), a score >5 on the STOP-Bang Questionnaire (Chung et al., 2016) which is indicative of a high risk of sleep apnea (completed by all potential participants), or other sleep disorders such as periodic limb movement disorder, restless leg syndrome or narcolepsy (assessed via medical history); current night shift workers; and travel across time zones within 2 wk preceding study. Further exclusion criteria included current cigarette smoking, taking beta-blockers, diagnosis of a psychiatric disorder (based on self-report, medical history, or current use of any anti-depressive or anti-anxiety medications), child at home <1 y old, pregnancy, breastfeeding, or excessive caffeine intake (>400 mg/d). None of the women enrolled were taking hormonal contraceptives. Participants completed a medical history questionnaire, and also underwent a physical examination and an interview by the study physician during both experimental phases. None of the participants indicted any eye diseases. Participants received monetary compensation for completion of study procedures. All participants provided written informed consent. Procedures were approved by the Columbia University Medical Center (CUMC) Institutional Review Board, and all procedures were in accordance with the Declaration of Helsinki. Data were collected while participants were free-living and at the Clinical Research Resource of the Irving Institute for Clinical and Translational Research at CUMC.
Intervention
This was a randomized, placebo-controlled crossover trial. The intervention consisted of wearing amber lenses or clear placebo lenses for the 2 h preceding bedtime each night for 1 wk. The BB lenses are amber lenses worn in wraparound frames (Bandit style frames, Uvex, Honeywell Safety, Smithfield, RI, USA). The amber lenses filter out blue-wavelength light, while allowing the other visible spectrum light (green, red) to pass, resulting in a blue-light absorption (BLA) of 65% and a visible light transmission (VLT) of 90%. The placebo lenses are clear lenses worn in wraparound frames identical to those in the BB condition. The clear lenses have a VLT of 92%, while allowing for the almost complete transmission of blue-wavelength light (~90%) based on manufacturer specifications (Table 1). The frames are lightweight and resemble sunglasses. For the intervention period, participants were instructed to wear their frames containing the lenses each night, from 2 h before bedtime until bedtime (removing frames at lights-out) while they were living at home on their habitual sleep-wake schedule. Participants were told to wear the frames containing the lenses during any nocturnal awakenings in which a light is turned on, an electronic device is used in bed, or if they get out of bed (bathroom, drink, etc.). Participants also completed a log which included the times they wore the frames. The frames could be worn over contact lenses or eyeglasses. After enrollment, participants were randomized via simple randomization with a computer-generated random numbers generator into an intervention sequence (clear condition followed by amber condition for even numbers; amber condition followed by clear condition for odd numbers). Intervention phases were separated by a 4-wk washout period, followed by crossover to the alternate intervention phase. The 4-wk washout period was used to reduce carry-over effects, minimize the influence of the initial treatment on phase 2 subjective outcomes (demand characteristic), and to study menstruating women at the roughly the same menstrual cycle phase. Participants were instructed to remain on the habitual sleep-wake schedule, and were free to self-select their bed- and wake-times throughout the intervention.
Table 1.
Summary of light transmittance for clear and amber lenses used in the intervention.
| Lenses | Visible Light Transmittance | Blue Light Transmittance |
|---|---|---|
|
|
|
|
| CLEAR | 92% | ~90% |
| AMBER | 90% | 35% |
Clear and amber lenses were worn in Bandit wraparound frames (Uvex, Honeywell Safety). Transmittance levels of visible and blue wavelength light were provided by manufacturer.
Outcomes/measures
The primary outcome was Pittsburgh Insomnia Rating Scale (PIRS) score (Moul et al., 2002; Moul et al., 2004). The PIRS is a 65-item 4-point scale which considers the domains of nighttime/daytime symptom distress, sleep parameters, and quality of life (Moul et al., 2004). A total score (range: 0 [good] to 195 [poor]), and 3 subscales are computed. The distress score subscale (indicating how much distress the sleep disturbance is causing) ranges from 0 (not bothered) to 138 (severely bothered). The sleep parameters subscale (indicating sleep initiation, maintenance, duration) ranges from 0 (good sleep) to 30 (disturbed sleep). The quality of life subscale (indicating how insomnia symptoms impact quality of life, satisfaction, inter-personal interaction) ranges from 0 (excellent) to 27 (poor). In addition to being designed as an instrument to determine broad-based clinical efficacy of sleep interventions for insomnia, the PIRS was used here since it assesses sleep over the preceding 7-d period (asking participants to consider their sleep “over the past week”), coinciding with the current intervention period. The PIRS was completed at ~18:00–19:00 h following the last day of the 7-d intervention.
Secondary outcomes included subjective and objective sleep parameters and vital signs. Each day throughout the 7-d intervention periods, participants completed a sleep diary documenting bedtime (lights out), wake time, and the time at which lenses were worn, and a post-sleep questionnaire (PSQ). The PSQ, completed in the morning and reflecting the preceding sleep episode, includes estimates of sleep onset latency (SOL), total sleep time (TST), wakefulness after sleep onset (WASO), and ratings on a 7-point scale of overall evaluation of sleep (1: extremely bad, 7: extremely good), and soundness of sleep (1: extremely light, 7: extremely sound) (O’Donnell et al., 2009). A subjective measure of sleep efficiency (SE) was calculated (estimated TST divided by time in bed). During the 7-d intervention periods, participants continuously wore an accelerometer on their non-dominant wrist (wGT3X-BT Actigraph monitor; ActiLife LLC, Pensacola, FL, USA). ActiLife 6 data analysis software was used to obtain measures of SOL, TST, SE, and WASO based on Cole-Kripke criteria (Cole et al., 1992). All individual actigraph recordings were visually inspected for missing data (i.e. nights where actigraph was not worn), and these were excluded from analyses. Missing data occurred in 12% of the total nights recorded.
Analyses
Sample size estimates were for paired-samples, two-tailed level of significance at 0.05 and 80% power. We expected to be able to detect an effect size (Cohen’s d) of 0.95 for significantly improved subjective sleep quality in amber vs. placebo lenses with n=11, and to be able to detect an effect size of 0.92 for significantly decreased subjective SOL in BB vs. placebo lenses with n=12 (Fargason et al., 2013). Assuming a 20% drop-out rate, we aimed to recruit n=15 to have power to detect statistically significant improvements in sleep with BB lenses. Mean values throughout the 7-d intervention periods were calculated for sleep parameters. Data were checked for normality with the Shapiro-Wilks test. To determine potential effects of treatment order on outcomes, univariate ANCOVA, with treatment condition (fixed factor), condition-at-first-visit (covariate), age (covariate) and condition x order, condition x age, and order x age interactions, was conducted on all outcomes. If no order effects or interactions of order with the covariates age and condition were seen, comparisons of outcomes between conditions were made via paired-samples t-tests, or Wilcoxon signed-rank test for parameters that were not normally distributed (Shapiro-Wilks p-values <0.05). Cohen’s d was calculated to determine effects sizes for changes in sleep parameters. Analyses were conducted using SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA).
RESULTS
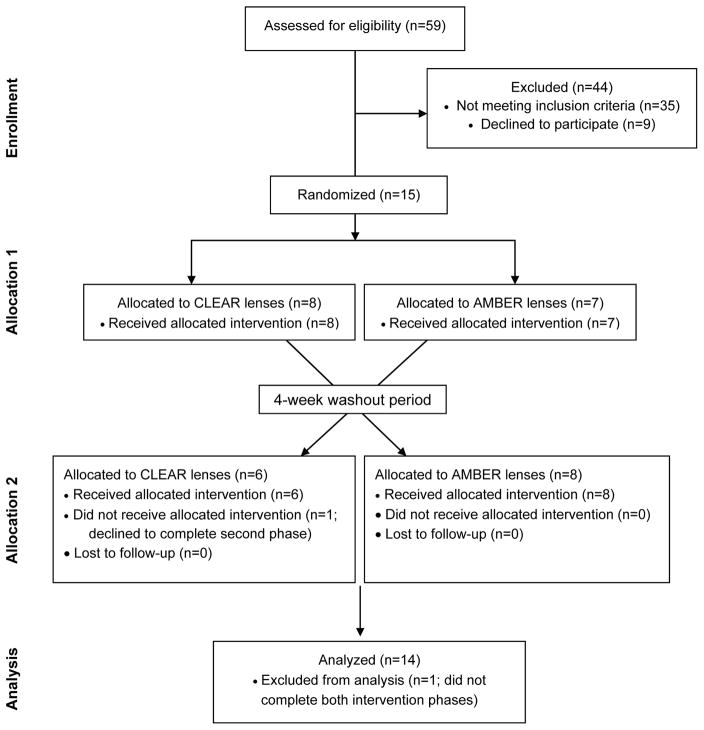
A total of 15 participants were enrolled and randomized to intervention phases (Figure 1). Eight participants were randomized to clear lenses followed by amber lenses, and 7 participants were randomized to amber lenses followed by clear lenses (Figure 1). One participant in the amber lenses-first condition declined to complete the second intervention phase, leaving 14 participants who completed both phases and were analyzed for primary and secondary outcomes. Of the completers, 57% were female, and mean ± SD age and body mass index were 46.6 ± 11.5 y and 26.8 ± 4.2 kg/m2, respectively (Table 2). No harm or unintended side-effects were reported for either intervention phase.
Figure 1.
CONSORT diagram showing the flow of participants through each stage of the randomized trial.
Table 2.
Participant characteristics at baseline.
| Variable | All complete participants | CLEAR first | AMBER first |
|---|---|---|---|
|
|
|
||
| Sample size | 14 | 8 | 6 |
| Sex, females, n (%) | 8 (57%) | 5 (63%) | 3 (50%) |
| Age, y | 46.6 ± 11.5 (27–61) | 47.0 ± 10.9 (27–60) | 46.0 ± 13.3 (27–61) |
| Body mass index, kg/m2 | 26.8 ± 4.2 (19.2–33.3) | 27.3 ± 3.0 (23.3–31.3) | 26.1 ± 5.6 (19.2–33.3) |
Values are expressed as mean ± SD (range).
A summary of the specific sleep complaints at baseline is presented in Table 3. The most prevalent sleep complaints were the feeling that sleep is not refreshing or that sleep is not sound, with 78.6% and 71.4% of participants reporting these as occurring 5–7 times per week. Difficulty staying asleep was the next most prevalent complaint, with 64% of participants reporting this as occurring 5–7 times per week. In terms of difficulty falling asleep, 50% of participants reported this as always occurring (5–7 times/week), with 21.4% reporting this frequently (3–4 times/week), and 28.6% reporting this to occur sometimes (1–2 rimes/week) (Table 3).
Table 3.
Summary of specific sleep complaints in participants at baseline.
| Variable | Never | Don’t know | Rarely (< 1 x/week) | Sometimes (1–2 x/week) | Frequently (3–4 x/week) | Always (5–7 x/week) |
|---|---|---|---|---|---|---|
|
|
|
|||||
| Difficulty falling asleep | 0 (0%) | 0 (0%) | 0 (0%) | 4 (28.6%) | 3 (21.4%) | 7 (50%) |
| Difficulty staying asleep | 0 (0%) | 1 (7.1%) | 1 (7.1%) | 0 (0%) | 3 (21.4%) | 9 (64.3%) |
| Frequent awakenings from sleep | 0 (0%) | 1 (7.1%) | 1 (7.1%) | 2 (14.3%) | 3 (21.4%) | 7 (50%) |
| Feeling that sleep is not sound | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (28.6%) | 10 (71.4%) |
| Feeling that sleep is unrefreshing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (21.4%) | 11 (78.6%) |
Responses are based on the Insomnia Symptoms Questionnaire, and reflect frequency of occurrence over the past month. Data are expressed as n (%).
PIRS scores after 1 wk of clear and amber lenses worn 2-h before bedtime are presented in Table 4. We examined a potential interaction between treatment condition, order of treatment, and age using univariate ANCOVA with condition (fixed factor), condition-at-first-visit (covariate), age (covariate). There were no significant main effects of order or condition x order, condition x age, or order x age interactions (p-values ≥0.05) on any PIRS outcomes. This suggests that the effects of treatment did not vary across age, and that order did not influence treatment outcomes. PIRS total score was significantly reduced after the amber condition compared to the clear lenses condition (mean ± SD: 72.64 ± 28.14 vs. 88.93 ± 33.19; p=0.023). In addition, significant reductions in the Distress Score (46.57 ± 23.23 vs. 56.93 ± 27.36; p=0.046), Sleep Parameter Score (11.21 ± 6.17 vs. 13.36 ± 6.11; p=0.047), and Quality of Life Score (14.86 ± 4.33 vs. 18.57 ± 4.20; p=0.003) subscales were observed after amber compared to clear lenses condition.
Table 4.
Pittsburgh Insomnia Rating Scale scores after 1 week of clear and amber lenses (in frames) worn 2-h before bedtime.
| CLEAR | AMBER | P-value | |
|---|---|---|---|
|
|
|
||
| PIRS Total Score | 88.93 ± 33.19 (45–152) | 72.64 ± 28.14 (31–133) | 0.023 |
| Distress Score | 56.93 ± 27.36 (20–116) | 46.57 ± 23.23 (17–96) | 0.046 |
| Sleep Parameter Score | 13.36 ± 6.11 (3–27) | 11.21 ± 6.17 (4–24) | 0.047 |
| Quality of Life Score | 18.57 ± 4.20 (10–24) | 14.86 ± 4.33 (7–22) | 0.003 |
PIRS: Pittsburgh Insomnia Rating Scale. Data were analyzed using paired-samples t-tests and are expressed as means ± SD (range). Statistically significant p-values (<0.05) are denoted in bold.
Mean values of subjective and actigraphic sleep parameters obtained throughout each intervention phase are presented in Table 5. Based on univariate ANCOVA with condition (fixed factor), condition-at-first-visit (covariate), age (covariate), there were no main effects of order or significant condition x order, condition x age, or order x age interactions (p-values ≥0.05) for any subjective or actigraphic sleep outcomes. Mean bedtime was unchanged between experimental conditions (23:27 ± 01:46 vs. 23:25 ± 01:14; p=0.833). However, mean wake-time was significantly later in amber vs. clear (07:15 ± 01:33 vs. 06:47 ± 01:27; p=0.033; Cohen’s d=0.64). In amber vs. clear lenses condition, subjective measures of TST (399.33 ± 80.31 vs. 347.11 ± 70.50 min; p<0.01; d=1.22) were significantly increased. Subjective SE was slightly increased (87.35 ± 12.50 vs. 82.56 ± 15.87; p=0.055; d=0.64), and subjective WASO was slightly decreased (35.66 ± 39.64 vs. 52.38 ± 60.95; p=0.060; d=0.90), in amber vs. clear lenses condition, but differences were not statistically significant. Subjective SOL was unchanged in amber vs. clear lenses condition (31.23 ± 20.72 vs. 43.26 ± 44.77 min; p=0.209; d=0.76). Ratings on the overall quality and the soundness of the sleep episodes were both significantly improved in the amber vs. clear lenses condition (p≤0.03; Table 5). Based on actigraphy, TST was significantly increased in the amber vs. clear lenses condition (358.80 ± 66.29 vs. 330.33 ± 66.01 min; p=0.035; d=0.65). Actigraphic measures of SOL, SE, and WASO were unchanged between conditions (Table 5).
Table 5.
Subjective and actigraphic sleep parameters throughout 1 week of clear and amber lenses (in frames) worn 2-h before bedtime.
|
|
|
||
|---|---|---|---|
| SUBJECTIVE | |||
|
|
|
||
| Measure | CLEAR | AMBER | P-value |
|
|
|
||
| Bedtime, h:m | 23:35 ± 01:14 (20:21–02:51) | 23:37 ± 01:46 (20:00–01:41) | 0.833 |
| Waketime, h:m | 06:47 ± 01:27 (04:13–08:40) | 07:15 ± 01:33 (04:17–10:04) | 0.033 |
| SOL, min | 43.26 ± 44.77 (3.5–126) | 31.23 ± 20.72 (5–81) | 0.209a |
| TST, min | 347.11 ± 70.50 (231–459) | 399.33 ± 80.31 (225–488) | <0.01 |
| SE, % | 82.56 ± 15.87 (58–99) | 87.35 ± 12.50 (57–100) | 0.055a |
| WASO, min | 52.38 ± 60.95 (1–227) | 35.66 ± 39.64 (0–142) | 0.060a |
| Sleep quality | 3.31 ± 0.91 (2.1–4.7) | 4.00 ± 1.39 (1.9–7) | 0.032 |
| Sleep soundness | 3.32 ± 1.15 (1.9–5.1) | 4.34 ± 1.27 (2.3–7) | 0.004 |
|
|
|
||
| ACTIGRAPHIC | |||
|
|
|
||
| Measure | CLEAR | AMBER | P-value |
|
|
|
||
| SOL, min | 16.21 ± 23.42 (1–92) | 11.27 ± 12.23 (1–42) | 0.221a |
| TST, min | 330.33 ± 66.01 (244–514) | 358.80 ± 66.29 (268–525) | 0.035a |
| SE, % | 77.01 ± 8.67 (66–93) | 78.35 ± 9.00 (65–92) | 0.285 |
| WASO, min | 83.95 ± 35.53 (25–140) | 88.43 ± 38.45 (38–173) | 0.546 |
Subjective (post-sleep questionnaire; top) and actigraphic (wrist-mounted; bottom) measures are 7 day mean throughout each intervention phase. SOL; sleep onset latency; TST: total sleep time; SE: sleep efficiency; WASO: wakefulness after sleep onset. Data were analyzed using paired-samples t-tests, or Wilcoxon signed-rank test for non-normally distributed data (indicated by a), and are expressed as means ± SD (range). Statistically significant p-values (<0.05) are shown in bold.
DISCUSSION
We report here that wearing BB amber lenses for the 2 hours preceding bedtime for one week resulted in significant improvements in sleep, compared to clear lenses, in individuals with insomnia symptoms. Specifically, scores on the PIRS (total and subscales) were reduced in the amber vs. clear lenses condition, indicating a reduction in insomnia severity. Subjective measures of sleep duration and quality, as well as actigraphy-derived sleep duration, were also significantly improved by wearing amber lenses before sleep.
There is a growing awareness of the adverse effects of light-emitting personal electronic devices (e.g. computers, tablets, smart phones) used before bedtime on circadian physiology, vigilance, and sleep quality. A 5-hour evening exposure to an LED-backlit computer, compared to a non-LED backlit computer, reduced melatonin secretion and also caused decreased subjective and neurophysiologic sleepiness (Cajochen et al., 2011). Another important study compared the effects of a 4-hour pre-bedtime reading session with a blue-wavelength enriched light-emitting eBook vs. a printed book and noted significant suppression and phase delays of melatonin secretion (Chang et al., 2015). This delay in melatonin secretion was associated with reduced subjective and neurophysiologic measures of sleepiness in the evening, a prolonged SOL, and decreased nocturnal REM sleep (Chang et al., 2015). Exposure to light from a tablet (iPad set to full brightness) as short as 2-hours produces similar effects on melatonin suppression compared to a dark control (Wood et al., 2013), an effect that is reproducible in an ecologically valid model of 1 and 2 hours of exposure (Figueiro and Overington, 2016).
Prior work has demonstrated that use of BB lenses prevented bright-light induced melatonin suppression (Kayumov et al., 2005; Sasseville et al., 2006). Importantly, BB lenses were also found to prevent melatonin suppression after exposure to self-luminous personal devices in the evening before bedtime (Figueiro and Overington, 2016). Our underlying theory is that the reduction in blue-light exposure is the likely mechanism whereby amber lenses, compared to clear lenses, improve sleep in individuals with insomnia symptoms. Although we assume a lesser degree of melatonin suppression and/or higher nocturnal melatonin secretion in the amber vs. clear lenses condition, this was not currently assessed. It should also be noted that much of the work on the effects of blue light on the sleep-wake and circadian system (e.g. Cajochen et al., 2011; Chang et al., 2015) was done under laboratory conditions wherein the light exposure occurred following or on a backdrop of dim ambient light. This is important to consider, since prior light history is known to influence the effects of light on the circadian system (Hébert et al., 2002). Specifically, the effects of light exposure on melatonin suppression may be enhanced after both short- (Chang et al., 2011) and long-term (Hébert et al., 2002) dim light exposure. Therefore, since ambient light and melatonin levels were not collected during the intervention, currently observed effects should be interpreted with caution, particularly in terms of imputing a biological mechanism of action. Until the current findings on sleep quality are replicated while simultaneously assessing circadian phase, it remains difficult to separate physiologic and psychologically-driven effects of the intervention. As a follow-up to current findings, and to more confidently suggest a biological mechanism of action, it will be important to measure ambulatory melatonin secretion in response to this intervention, possibly via analysis of 6-sulfatoxymelatonin in morning urine samples. Habitual evening light levels in the home environment should be also recorded via photometer. In the current study, participants were free to self-select their bedtimes, and in addition, we did not characterize circadian timing or preference either by physiological tracking or questionnaire. Therefore, some inter-individual differences in the circadian phase of participants at the time of the intervention may have influenced the findings.
To our knowledge, this is the first time amber lenses have been tested as a means of improving sleep in adults with insomnia symptoms, in a randomized crossover trial. A prior study demonstrated a beneficial effect of a similar intervention in individuals with self-reported difficulty falling or staying asleep (Burkhart and Phelps, 2009). In that randomized parallel-arm study, the amber lenses group reported higher ratings of sleep on a 10-point Likert scale compared to a yellow-lenses control group after wearing the lenses 3 hours before bedtime for 2 weeks (Burkhart and Phelps, 2009). However, in that study, baseline group differences in sleep quality were also reported, and no quantitative assessment of insomnia symptoms was used to identify eligible participants. Our current investigation, which utilized a validated measure for assessing insomnia symptoms before enrollment (Okun et al., 2009), a crossover design, and both subjective and objective assessments of several sleep parameters, is consistent with their initial report. Prior investigations have also demonstrated that this therapeutic approach has several other beneficial applications. These include improving sleep in individuals with comorbid attention deficit hyperactivity disorder and circadian rhythm sleep disorder, delayed sleep phase type (Fargason et al., 2013), as an adjunctive to antipsychotic treatment to reduce bipolar disorder-related mania (Henriksen et al., 2014), and to improve sleep in night shift workers (Sasseville et al., 2009).
Insomnia is a heterogeneous disorder, with various subtypes (e.g. difficulty initiating sleep; difficulty maintaining sleep; early morning awakening), causes, and associated contributing characteristics such as personality traits and life history (Benjamins et al., 2016). Here, we did not enroll participants based on a specific subtype, but rather included participants based on scores indicative of the presence of symptoms based on the DSM and American Academy of Sleep Medicine-recommended definition of insomnia (Okun et al., 2009). In addition, the current sample size is too small for a subgroup analysis. It is possible that this particular intervention approach is more beneficial for specific subpopulations, for instance, those with sleep initiation insomnia as opposed to sleep maintenance insomnia. This should be tested in subsequent interventions. Insomnia also commonly shows comorbidity with psychiatric disorders, and we excluded for the presence of psychiatric disorders. However, this was based on self-report, and a stronger approach would have been to conduct a diagnostic assessment (e.g. the Structured Clinical Interview for DSM). In addition to the heterogeneity of the sample in terms of specific sleep complaint at baseline, the relatively large range of ages may limit the generalizability of some results. This is an important consideration, since a reduction in the transparency of the eyeball lens as occurs with age (Bloemendal et al., 2004) could influence the transmission of light through the retino-hypothalamic tract.
Although we did observe a significant increase in objective sleep duration, the current discrepancy between an improvement in self-reported sleep quality parameters after amber vs. clear lenses that is not paralleled by robust improvements in objective measures is also relevant to discuss. The current DSM-5 criteria for insomnia are based on subjective experience. Furthermore, individuals with insomnia often report poor sleep quality in the absence of objectively-assessed sleep impairments (Harvey and Tang, 2012). In one study, individuals with self-reported chronic insomnia symptoms also did not show poor sleep, either reflective of their subjective complaint or relative to a no-complaint control group, when assessed with electroencephalography leading authors to suggest a de-emphasis of objective measures in favor of subjective assessment for insomnia (Rosa and Bonnet, 2000). This indicates that an improvement in self-reported sleep duration, maintenance, quality and soundness, as observed here, even in the absence of pronounced objective improvements, is clinically relevant. Given the subjective phenomenology and multi-faceted etiology of insomnia, an effect of improving subjective sleep is beneficial.
In conclusion, we observed that in individuals with insomnia symptoms, wearing amber lenses compared to clear lenses before bedtime improved sleep. Current findings have public health relevance given the high rates of insomnia and prevalent use of light-emitting devices before bedtime. Amber lenses represent a safe, affordable, and easily implemented non-pharmacologic behavioral therapeutic intervention for insomnia symptoms.
Acknowledgments
Funding: This research was funded by a Focused-Project Award (144-FP-16; AS) from the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine. This publication was also supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873.
Footnotes
Clinical Trials Registration: ClinicalTrials.gov Identifier: NCT02698800
Conflicts of Interest: None.
Author Contributions: AS designed the study, analyzed and interpreted data, and wrote the manuscript. EWK conducted the study, analyzed data, and contributed to writing the manuscript. MPSO was involved in data interpretation and manuscript preparation. AJW provided medical supervision for the study, assisted with data interpretation, and manuscript preparation.
References
- Benjamins JS, Migliorati F, Dekker K, Wassing R, Moens S, Blanken TF, te Lindert BH, Mook JS, Van Someren EJ. Insomnia heterogeneity: characteristics to consider for data-driven multivariate subtyping. Sleep Medicine Reviews. 2016 doi: 10.1016/j.smrv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in biophysics and molecular biology. 2004;86(3):407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart K, Phelps JR. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiology international. 2009;26(8):1602–1612. doi: 10.3109/07420520903523719. [DOI] [PubMed] [Google Scholar]
- Cajochen C. Alerting effects of light. Sleep medicine reviews. 2007;11(6):453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Chellappa SL, Schmidt C. Circadian and Light Effects on Human Sleepiness–Alertness. In: Garbarino S, Nobili L, Costa G, editors. Sleepiness and Human Impact Assessment. Springer; Milan: 2014. pp. 9–22. [Google Scholar]
- Cajochen C, Frey S, Anders D, Spati J, Bues M, Pross A, Mager R, Wirz-Justice A, Stefani O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. Journal of applied physiology. 2011;110(5):1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Krauchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. Journal of neuroendocrinology. 2003;15(4):432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. The Journal of clinical endocrinology and metabolism. 2005;90(3):1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(4):1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. The Journal of physiology. 2011;589(5):1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. CHEST Journal. 2016;149(3):631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. Perspective: casting light on sleep deficiency. Nature. 2013;497(7450):S13. doi: 10.1038/497S13a. [DOI] [PubMed] [Google Scholar]
- Fargason RE, Preston T, Hammond E, May R, Gamble KL. Treatment of attention deficit hyperactivity disorder insomnia with blue wavelength light-blocking glasses. bipolar disorder. 2013;24:25. [Google Scholar]
- Figueiro M, Overington D. Self-luminous devices and melatonin suppression in adolescents. Lighting Research & Technology. 2016;48(8):966–975. [Google Scholar]
- Fossum IN, Nordnes LT, Storemark SS, Bjorvatn B, Pallesen S. The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behavioral sleep medicine. 2014;12(5):343–357. doi: 10.1080/15402002.2013.819468. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. The Journal of clinical endocrinology and metabolism. 2011;96(3):E463–472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. doi: 10.5664/jcsm.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Tang NK. (Mis) perception of sleep in insomnia: A puzzle and a resolution. Psychological Bulletin. 2012;138(1):77. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. Journal of pineal research. 2002;33(4):198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen TE, Skrede S, Fasmer OB, Hamre B, Grønli J, Lund A. Blocking blue light during mania–markedly increased regularity of sleep and rapid improvement of symptoms: a case report. Bipolar disorders. 2014;16(8):894–898. doi: 10.1111/bdi.12265. [DOI] [PubMed] [Google Scholar]
- Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. The Journal of clinical endocrinology and metabolism. 2005;90(5):2755–2761. doi: 10.1210/jc.2004-2062. [DOI] [PubMed] [Google Scholar]
- Moul D, Pilkonis P, Miewald J, Carey T, Buysse D. Sleep. AMER ACAD SLEEP MEDICINE; 6301 BANDEL RD, STE 101, ROCHESTER, MN 55901 USA: 2002. Preliminary study of the test-retest reliability and concurrent validities of the Pittsburgh Insomnia Rating Scale (PIRS) pp. A246–A247. [Google Scholar]
- Moul DE, Hall M, Pilkonis PA, Buysse DJ. Self-report measures of insomnia in adults: rationales, choices, and needs. Sleep medicine reviews. 2004;8(3):177–198. doi: 10.1016/S1087-0792(03)00060-1. [DOI] [PubMed] [Google Scholar]
- Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. American journal of physiology Regulatory, integrative and comparative physiology. 2006;290(5):R1421–1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Silva EJ, Munch M, Ronda JM, Wang W, Duffy JF. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. Journal of sleep research. 2009;18(2):254–263. doi: 10.1111/j.1365-2869.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ, Hall M. Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2009;5(1):41–51. [PMC free article] [PubMed] [Google Scholar]
- Phelps J. Dark therapy for bipolar disorder using amber lenses for blue light blockade. Medical hypotheses. 2008;70(2):224–229. doi: 10.1016/j.mehy.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosomatic Medicine. 2000;62(4):474–482. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Roth T. Insomnia: definition, prevalence, etiology, and consequences. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- Sasseville A, Benhaberou-Brun D, Fontaine C, Charon MC, Hebert M. Wearing blue-blockers in the morning could improve sleep of workers on a permanent night schedule: a pilot study. Chronobiology international. 2009;26(5):913–925. doi: 10.1080/07420520903044398. [DOI] [PubMed] [Google Scholar]
- Sasseville A, Paquet N, Sevigny J, Hebert M. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. Journal of pineal research. 2006;41(1):73–78. doi: 10.1111/j.1600-079X.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- Sateia MJ. International classification of sleep disorders-: highlights and modifications. Chest Journal. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. The Journal of physiology. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Gillette MU. Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep medicine. 2004;5(6):523–532. doi: 10.1016/j.sleep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Applied ergonomics. 2013;44(2):237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]