Abstract
BACKGROUND
Results of previous single center observational studies suggest that daily bathing of patients with chlorhexidine may prevent hospital-acquired bloodstream infections (HABSIs) and acquisition of multidrug-resistant organisms (MDROs).
METHODS
We conducted a multicenter, cluster randomized, non-blinded crossover trial to evaluate the effect of daily bathing with chlorhexidine impregnated washcloths on the acquisition of MDROs and incidence of HABSIs. Nine intensive care and bone marrow transplant units in 6 hospitals were randomly assigned to bathe patients with either 2% no-rinse chlorhexidine-impregnated or non-antimicrobial washcloths for a six-month period, exchanged for the alternate product during the subsequent six months. The incidence rates of acquisition of MDRO and HABSI rates were compared between the two time periods by Poisson regression analysis.
RESULTS
A total of 7735 patients were enrolled during the study. The overall MDRO acquisition rate was 21% lower when chlorhexidine bathing was used (5.10 cases per 1000 patient days) than when non-antimicrobial washcloths were used (6.60 cases per 1000 patient days, p=0.028). The overall HABSI rate was 31% lower when chlorhexidine was used (4.45 cases per 1000 patient days) than when non-antimicrobial cloths were used (6.60 cases per 1000 patient days, p=0.007) No serious skin reactions were noted in either study period.
CONCLUSIONS
Daily bathing with chlorhexidine-impregnated washcloths significantly reduced the risk of acquiring MDROs and developing HABSI.
Keywords: Staphylococcus aureus, Enterococcus, methicillin resistance, vancomycin resistance, bacteremia, chlorhexidine
Introduction
Multidrug-resistant organisms (MDROs), including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE), have become endemic in many acute and long term care facilities (1–5). Infections caused by these organisms are often difficult to treat due to a dwindling armamentarium of active antimicrobials. The Centers for Disease Control and Prevention (CDC) has promulgated a variety of strategies including hand hygiene and use of isolation precautions to limit the spread of these organisms between patients, but these require consistent adherence to practices by large numbers of healthcare personnel during frequent patient encounters, and can be difficult to sustain. (6) In addition, healthcare-associated infections (HAI) associated with these and other microorganisms (7, 8) inflict considerable morbidity and mortality and incur substantial excess costs that in some cases are no longer reimbursed by third party payers, including the Centers for Medicare and Medicaid Services (CMS)(9, 10). Targeted interventions, particularly in intensive care units, can substantially reduce the risk of hospital-acquired bloodstream infections (HABSIs) associated with the use of central venous catheters. Several large studies have demonstrated that improving catheter insertion processes, including standardizing the use of insertion site antisepsis with chlorhexidine-containing products, can decrease infection risks (11–13). However, the use of antiseptic agents for patient bathing is currently considered controversial.
Chlorhexidine gluconate (CHG) is an antiseptic that has broad spectrum activity against many organisms, including Staphylococcus aureus and Enterococcus spp. Unlike many other antiseptics, CHG has residual antibacterial activity, making it ideal for decreasing microbial burden on patients’ skin and preventing secondary environmental contamination. Vernon et al. found that daily bathing with CHG-impregnated cloths decreased VRE skin contamination by 2.5 logs compared to soap and water bathing, as well as decreasing VRE contamination of healthcare workers’ hands by 40% and environmental surfaces by 30% (14). By controlling the source, these investigators reduced patient acquisition of VRE by 66%.
Because HABSIs often result from the ingress of skin organisms into the bloodstream along vascular catheters or other breaks in skin integrity, skin decontamination could theoretically also decrease HABSI risk. Bleasdale et al. showed that daily bathing with 2% CHG-impregnated cloths reduced the incidence of primary BSI by 60% (15). Our previous observational study evaluating CHG bathing in six ICUs demonstrated a 66% reduction in VRE bacteremia (16). Previous studies evaluating CHG bathing have been mainly single-center, “before and after” observational studies, limiting the general applicability of results. We therefore conducted a multicenter, randomized cluster trial to evaluate the utility of CHG bathing to reduce MDRO acquisition and HABSI risks for patients at high risk for HAI.
Materials and Methods
Study Design
We performed a multicenter, cluster randomized crossover study involving patients hospitalized in intensive care or bone marrow transplant units between August 2007 and February 2009. Units were randomized to either perform daily bathing of patients with non-antimicrobial washcloths (control) [Comfort Bath, SAGE Products, Inc.] or washcloths impregnated with 2% CHG (intervention) [2% Chlorhexidine Gluconate Cloth Patient Preoperative Skin Preparation, SAGE Products inc.] during the initial 6-month study period, followed by daily bathing with the alternate product for the second six-month period. The order in which units were assigned to the control or intervention arms was stratified by unit type and by facility. The investigators and clinical staff were not blinded to the use of control or intervention bathing.
Before the study was initiated, nurses were educated on the proper techniques for bathing patients with both washcloth products. Skin care products not compatible with CHG were eliminated prior to study initiation. Nursing personnel monitored patients for skin reactions and reported them to the investigators who graded skin reactions on a scale of 1–4 based on severity and determined whether reactions were attributable to bathing.
All units performed active surveillance testing (AST) for MRSA and VRE throughout the study period. Unit staff obtained nares (MRSA) and peri-rectal (VRE) swabs form patients ≤48 hours after unit admission and on unit discharge. The microbiology laboratories at each institution processed surveillance specimens using either standard culture-based or molecular-based (PCR) identification of MRSA and VRE. All patients found to be colonized or infected with MRSA and or VRE were placed on contact precautions once test results became available. Patients with a previous history of MRSA or VRE were placed on contact precautions upon admission.
Each participating unit submitted at least ten separate MRSA and VRE isolates obtained from unit patients to the coordinating center each month for CHG susceptibility testing. Susceptibility testing was completed by the agar dilution method with CHG concentrations ranging from 0.1 μg/ml to 1024 μg/ml (17).
The work was supported by the Centers for Disease Control Prevention and by SAGE Products, Inc. SAGE Products, Inc. supplied CHG-impregnated and non-antimicrobial washcloths to participating units for the duration of the study, provided technical and educational support and participated in weekly teleconferences with the Study Group during the conduct of the studybut was not involved in study design, analysis or preparation of this manuscript. Approval was obtained from individual center and CDC institutional review boards (IRBs). Waiver of written informed consent was obtained at each institution based on the minimal risk nature of the study. Patients who refused participation were not bathed with CHG-impregnated washcloths. The study was registered with clinicaltrials.gov (NCT00502476).
Definitions
Incident and prevalent cases of MRSA or VRE were classified as previously described (16). Bloodstream infections (BSI) were identified using National Healthcare Safety Network (NHSN] definitions (18). HABSI were defined as BSI detected >48 hours after unit admission. Primary BSI were defined as HABSI detected > 48 hours without an attributable secondary infection source. Central line-associated bloodstream infections (CLABSI) were defined as primary BSI in a patient with at least one central venous catheter in place within 48 hours of BSI detection.
Statistical Analysis
We evaluated changes in the mean MRSA and VRE acquisition and HABSI rates. We tested the null hypothesis that the rates during the control period equaled the rates during the intervention period using PROC GENMOD in SAS (version 8.2, Cary, NC) to fit a Poisson regression model that accounts for monthly MRSA and VRE prevalence in each unit as possible confounders.
We used a Cox proportional-hazards regression model to compare the time from admission until the first primary BSI between the control and intervention groups. We calculated the survival time as: 1) the interval between admission and discharge from the study unit for those patients who did not acquire primary BSI and as 2) the interval between admission and the first positive culture for patients with primary BSI.
We examined the impact of these unit characteristics on primary BSI rates: unit size, unit type, mean length of stay, utilization rate of central venous catheters, median patient age, distribution of patient gender, monthly incident MRSA ratemonthly incident VRE rate prevalence rate of MRSA at admission, and prevalence rate of VRE at admission. We compared changes in the incidence rates of primary BSI between the control period and intervention periods. Continuous variables were examined using two sample t tests and linear regression modeling and categorical variables were examined by Fisher’s exact test.
Treatment Interruption
On June 28th, 2008 SAGE Products, Inc. initiated a nationwide recall of the 2% CHG-impregnated washcloths, because of Burkholderia cepacia contamination of some product lots. Units using the CHG product at the time of the recall were switched to non-antimicrobial washcloths and local and CDC IRBs were immediately notified. Following remediation and approval of IRBs, use of the CHG product was resumed. Data from units assigned to CHG bathing during the recall period were censored from the final analysis.
Results
Twelve units from seven hospitals were recruited to participate in the planned 12-month study. One unit withdrew from the study and two units were eliminated from the analysis because of low compliance with the study protocol. The final nine study units included medical, coronary care, surgical, and cardiac surgery ICUs and one bone marrow transplant unit (Table 1). Patient participation refusal rate was low (<0.5%) and data from all patients admitted to participating units were included in an intention to treat analysis.
Table 1.
Characteristics of Participating Study Units
| Participating Units† | Mean Monthly Admissions (range) | Mean Monthly Patient Days (range) | Mean length of Stay (days) | MRSA Prevalence (% of admissions) | VRE Prevalence (% of admissions) | Baseline Primary BSI rate‡ |
|---|---|---|---|---|---|---|
| Hospital A | ||||||
| MICU | 56.70 (98–126) | 598.75 (449–641 | 5.37 | 21.81% | 20.99% | 3.11 |
| Hospital B | ||||||
| MICU | 123.75 (114–142) | 692.33 (504–773) | 5.59 | 11.04% | 21.01% | 8.09 |
| Hospital C | ||||||
| MICU/CCU | 55.75 (43–73) | 299.08 (211–345) | 5.36 | 16.14% | 9.72% | 8.54 |
| SICU | 46.25 (31–59) | 285.67 (251–314) | 6.18 | 11.35% | 4.32% | 9.55 |
| Hospital D | ||||||
| SICU 1 | 62.33 (47–76) | 316.33 (266–356) | 5.07 | 10.83% | 8.16% | 2.21 |
| SICU 2 | 51.58 (32–71) | 285.67 (227–338) | 5.54 | 4.42% | 2.83% | 0 |
| Hospital E Hospital | ||||||
| MICU | 72.67 (56–88) | 467.08 (404–525) | 6.43 | 23.28% | 27.87% | 8.70 |
| CSICU | 85.33 (80–100) | 425.92 (375–486) | 4.99 | 6.64% | 8.30% | 0.38 |
| Hospital F | ||||||
| BMT | 41.75 (32–58) | 786.25 (725–858) | 18.83 | 2.4% | 21.56% | 5.50 |
Abbreviations: MICU, medical intensive care unit; CSICU, cardiac surgery intensive care unit; MICU/CCU, combined medical intensive care unit and coronary care unit, SICU, surgical intensive care unit: BMT, bone marrow transplant unit
Number of new cases per 1000 eligible patient days during the control study period.
Acquisition of MRSA and VRE
During the periods when non-antimicrobial cloths were used, 165 new cases of MRSA or VRE acquisition were detected compared with 127 during the CHG bathing periods. The overall MRSA or VRE acquisition rate was 23% lower during the intervention period (Table 2; 5.10 vs. 6.60 cases per 1000 patient days; P=0.028). Reductions in the incidence of VRE and MRSA acquisition were unrelated to the monthly prevalence of either MRSA or VRE.
Table 2.
Effect of Daily Chlorhexidine Bathing on the Incidence of Hospital-acquired Bloodstream Infections and Acquisition of the Multi-drug Resistant Organisms, MRSA and VRE
| Intervention Period | Control Period | p value | |
|---|---|---|---|
| Admissions (n) | 3,970 | 3,842 | 0.32 |
| Gender | |||
| Male (%) | 61.3 | 60.8 | 0.63 |
| Female (%) | 38.7 | 39.2 | 0.63 |
| Bed Days of Care | 24,902 | 24,983 | 0.85 |
| Central line days | 13,425 | 13,049 | 0.14 |
| Mean Length of Stay (days) | 6.35 | 6.39 | 0.53 |
| MRSA prevalencea | 13.8% | 12.8% | 0.14 |
| VRE prevalencea | 16.3% | 15.1% | 0.24 |
| MDRO Acquisition (n) | 127 | 165 | |
| Incidence rated | 5.10 | 6.60 | 0.028 |
| VRE Acquisition (n) | 80 | 107 | |
| Incidence rateb | 3.21 | 4.28 | 0.052 |
| MRSA Acquisition (n) | 47 | 58 | |
| Incidence rateb | 1.89 | 2.32 | 0.29 |
| Total Hospital Acquired BSIs (n) | 119 | 165 | |
| Incidence Rateb | 4.78 | 6.60 | 0.007 |
| Total Primary BSIs (n) | 90 | 131 | |
| Incidence rateb | 3.61 | 5.24 | 0.006 |
| Catheter Associated BSIs (n) | 21 | 43 | |
| Incidence Ratec | 1.55 | 3.30 | 0.004 |
| Secondary BSIs (n) | 29 | 34 | |
| Incidence Rateb | 1.20 | 1.40 | 0.45 |
Total number of prevalent cases per 100 patients admitted to study unit
Total number of cases per 1000 patient days
Total number of cases per 1000 device days
Total number of acquired cases of MRSA or VRE among eligible patients per 1000 patient days
The overall VRE acquisition rate was 27% lower during the intervention compared to control periods (3.21 vs. 4.28 cases per 1000 patient days, p= 0.052). The overall MRSA acquisition rate was 19% lower during the intervention period, but this difference did not reach statistical significance (1.89 vs. 2.32 cases per 1000 patient days, p=0.29)
Bloodstream Infections
Overall, 165 HABSIs were detected among patients during the control period compared to 119 HABSIs during the intervention period. The HABSI rate was 30% lower during intervention compared to control periods (4.78 vs. 6.60 cases per 1000 patient days; p=0.007, Table 2.), reflecting the 31% lower primary BSI rate during intervention compared to control periods (3.61 vs. 5.24 cases per 1000 patient days; p=0.006) and the CLABSI rate was 51% lower during the intervention compared to control periods (1.55 vs. 3.30 cases per 1000 catheter days; p=0.004). The rate of secondary BSI did not differ significantly between the intervention and the control periods.
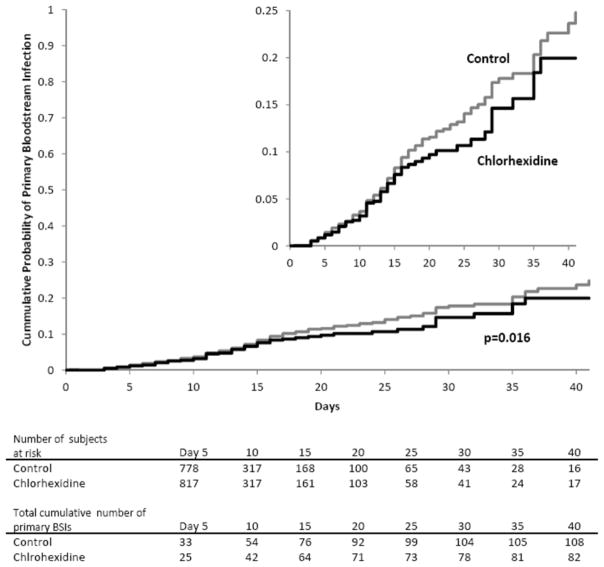
Based on Cox proportional-hazards survival regression analysis, the risk of acquiring a primary BSI was significantly lower among patients bathed with CHG than among those bathed with the non-antimicrobial cloths (p=0.016, Figure 1). This effect was greatest among patients with longer lengths of unit stay. Patients with lengths of unit stay >7 days who were bathed with chlorhexidine were 0.69 times less likely to acquire a primary BSI compared with patients bathed with CHG (RR=0.69, 95% CI 0.47–0.99). Patients with lengths of unit stay >14 days who were bathed with chlorhexidine were 0.51 times less likely to acquire a primary BSI (RR 0.51, 95% CI 030–0.87).
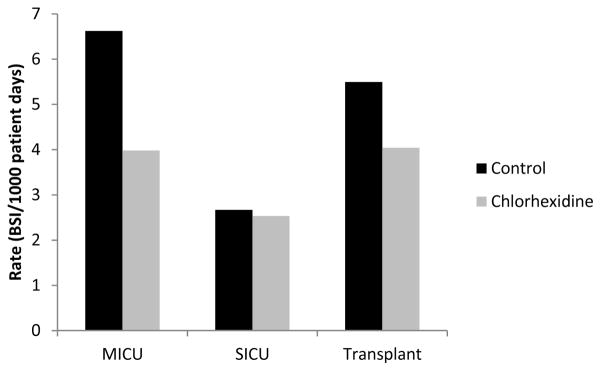
Figure 1. Reductions in primary bloodstream infections observed with the use of daily chlorhexidine bathing.
Incidence rates of hospital acquired primary bloodstream infections are shown among units utilizing daily bathing with either Chlorhexidine impregnated washcloths (black) or non-antimicrobial washcloths (gray).
Among the 221 primary BSIs, staphylococci (30%), gram-negative bacilli (23%), enterococci (20%), and yeast and fungi (12%) were the most common pathogens (Table 3). The incidence rate of primary BSI caused by coagulase-negative staphylococci was 56% lower during the intervention periods than during the control periods (0.60 vs. 1.36 cases per 1000 patient days, p=0.008). Similarly, the incidence rate of primary BSIs caused by fungi was 53% lower during the intervention compared to control periods but this did not reach statistical significance (0.36 cases per 1000 patient days vs. 0.76 cases per 1000 patient days, p=0.06).
Table 3.
Etiologic Agents of Identified Primary Bloodstream Infections
| Chlorhexidine | Control | P value | |||
|---|---|---|---|---|---|
| Number | Incidence Ratea | Number | Incidence Ratea | ||
|
|
|||||
| Staphylococci | 24 | 0.96 | 42 | 1.68 | 0.03 |
| S. aureus | 9 | 0.36 | 8 | 0.32 | 0.80 |
| Coagulase-negative staphylococci | 15 | 0.60 | 34 | 1.36 | 0.01 |
| Enterococci | 19 | 0.76 | 26 | 1.04 | 0.30 |
| E. faecalis | 13 | 0.52 | 19 | 0.76 | 0.29 |
| E. faecium | 6 | 0.24 | 6 | 0.24 | ns |
| Gram negatives | 23 | 0.92 | 27 | 1.08 | 0.58 |
| Acinetobacter | 1 | 0.04 | 2 | 0.08 | ns |
| Escherichia | 8 | 0.32 | 6 | 0.24 | ns |
| Enterobacter | 2 | 0.08 | 8 | 0.32 | 0.06 |
| Klebsiella | 5 | 0.20 | 5 | 0.20 | ns |
| Pseudomonas | 4 | 0.16 | 2 | 0.08 | 0.41 |
| Serratia | 2 | 0.08 | 1 | 0.04 | ns |
| Sternotrophomonas | 0 | 0.00 | 1 | 0.04 | ns |
| Others | 1 | 0.04 | 2 | 0.08 | ns |
| Fungi and yeast | 9 | 0.36 | 19 | 0.76 | 0.06 |
| Candida | 7 | 0.28 | 16 | 0.64 | 0.06 |
| Others | 2 | 0.08 | 3 | 0.12 | ns |
| Polymicrobial | 9 | 0.36 | 12 | 0.48 | 0.52 |
| Others | 6 | 0.24 | 5 | 0.20 | 0.76 |
|
| |||||
| Totals | 90 | 3.61 | 131 | 5.24 | 0.01 |
Number of primary bloodstream infections per 1000 patient days
The incidence of CLABSI caused by gram-positive organisms (0.89 vs. 1.76 cases per 1000 line days, p=0.05) and by fungi (0.07 vs. 0.77 cases per 1000 line days, p<0.001) were significantly lower during intervention periods. Overall, the incidence of CLABSI-associated fungemia was 90% lower during intervention periods. CHG bathing was not associated with a significant reduction in incidence of CLABSI caused by gram-negative organisms or VRE or MRSA BSI, likely related to the low number of infections caused by these organisms.
Due to concerns that the interruption of treatment may have affected the observed outcomes, we performed additional analysis of the BSI incidence rates including those months when non-antimicrobial cloths were used by units affected by the national recall. Addition of data obtained during the 4 months of treatment interruption when only non-antimicrobial bathing cloths were used did not alter the BSI analysis results. In this analysis, 58 months of use of non-antimicrobial cloths for bathing was compared to 54 months of 2% chlorhexidine cloth use. The overall results remained the same. The overall incidence of HABSIs was decreased in those months where chlorhexidine was in use (6.32 vs 4.78, p= 0.018).
Unit Characteristics
Reductions in primary BSI rates were highest among MICUs. The primary BSI rate in MICUs was 40% lower during the intervention compared to control periods (3.98 vs 6.62 cases per 1000 patient days). In contrast, the primary BSI rate in other units was 17% lower during the intervention periods than during the control periods (3.10 vs. 3.73 cases per 1000 patient days) (figure 1). However, the observed reductions in primary BSIs among MICUs was not statistically significantly associated with the unit type. Other unit characteristics--unit size, mean length of stay, baseline primary BSI rate, median age of patients, MRSA and VRE prevalence, catheter utilization, and gender distribution--were not associated with changes in the rates of primary BSI.
Chlorhexidine Susceptibility
We performed antimicrobial susceptibility on clinical isolates collected during the entire study period. We tested a total of 1106 VRE and MRSA isolates for susceptibility to chlorhexidine by the agar dilution method. Chlorhexidine was slightly more active against MRSA with an MIC90 of 4 μg/ml compared an MIC90 of 8 μg/ml for VRE isolates.
Adverse Reactions
CHG bathing was well tolerated. The overall incidence of skin reactions among patients assigned to CHG bathing was 1.9% (78/3973 patients) compared to 3.4% (130/3857 patients) among patients assigned to bathing with the control product. All 208 reported skin reactions were felt to be unrelated to the bathing intervention and overall 85% were classified as mild to moderate in nature (grade 1 or 2).
Discussion
Our study, the first multicenter cluster randomized study evaluating daily CHG bathing, confirmed the results of prior single center trials suggesting that CHG bathing reduces transmission of resistant organisms and HABSI risk among intensive care and bone marrow transplant unit patients (14–16, 19, 20). In addition, the participation of facilities from different U.S. geographic regions supports the generalizability of these results to other academic medical centers.
Our results support the findings of Bleasdale et al. and Vernon et al. and suggest that CHG bathing may be particularly effective at reducing BSI risk among ICU patients (14, 15). In contrast to these previous studies that involved a limited number of units, our multicenter design allowed a more robust examination of whether the reductions in BSIs were related to the type of unit. We found no statistically significant interaction between the type of unit and the development of BSIs suggesting that chlorhexidine bathing may be beneficial in many unit settings. In addition, CHG bathing may be particularly beneficial for patients with long ICU stays.
Our study also had some unanticipated findings. First, CHG bathing was associated with lower rates of CLABSI-associated fungemia. Previous studies have found lower risks for gram-positive CLABSI associated with CHG bathing (14–16) but have not reported reduced fungemia rates. CHG has biphasic fungicidal activity (21) but topical use of CHG has not been suggested as a possible intervention to reduce in the incidence of fungemia among patients with indwelling central lines. Previous efforts to reduce the incidence of fungemia have relied mostly on systemic antifungal prophylaxis, which can increase the incidence of antifungal resistance among fungal isolates (22). If our results are confirmed, topical use of CHG could be added to strategies to prevent fungal infections.
The use of chlorhexidine bathing was associated with significant reductions in the incidence of gram positive bacteremias, similar to previous studies. The majority of these reductions were related to the reductions seen among coagulase negative staphylococci. Despite the overall reduction in the acquisition of MRSA and VRE, significant reductions in the incidence of MRSA and VRE bacteremias were not seen. This was likely related to the overall low number of hospital acquired bacteremias due to these two organisms.
We did not identify any serious adverse effects associated with daily CHG bathing. Serious allergic reactions have been reported with the topical use of CHG but these reactions appear to be rare (23–26). We did not detect the emergence of MRSA or VRE isolates with high-level CHG resistance during the study. Concerns for increased resistance to biocides and disinfectants like CHG among nosocomial bacteria have tempered enthusiasm for wider adoption of their use in hospitals for skin antisepsis (27–34). However, the potential for emergence of CHG resistance remains a substantial concern and should be monitored over time (35).
Identifying simple, cost-effective, and safe strategies for HAI prevention is essential. Daily bathing with CHG-impregnated washcloths is a strategy that is relatively straightforward to implement and sustain because it does not require a significant change from patient bathing practices that are currently routine. We demonstrated that this intervention was associated with lower MRSA and VRE acquisition rates and decreased HABSI rates. The results of this multicenter cluster randomized study strongly suggest that daily CHG bathing can be an important weapon in the ongoing battle to prevent HAIs and improve patient safety.
Figure 2. Kaplan–Meier Estimates of Time to Primary Bloodstream Infections.
The cumulative probability of developing a primary bloodstream infection is compared for patients who received Chlorhexidine based bathing (gray) to Controls (Black). The overall protective efficacy of chlorhexidine bathing was 30% (p=0.0158). The inset graph shows a more detailed version of the larger graph with a probability up to 0.25.
Acknowledgments
Funding: This work was supported by a cooperative program award (5U01CI000395-02) from the Center for Disease Control and Prevention and a grant from SAGE Products, Inc..
References
- 1.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public health threat. Lancet. 2006;368:874–85. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 2.Bouchillon SK, Johnson BM, Hoban DJ, et al. Determining incidence of extended spectrum β-lactamase producing Enterobacteriaceae, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus in 38 centres from 17 countries: the PEARLS study 2001–2. Int J Antimicrob Agents. 2004;24:119–24. doi: 10.1016/j.ijantimicag.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Kreman T, Hu J, Pottinger J, Herwaldt LA. Survey of long-term-care facilities in Iowa for policies and practices regarding residents with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2005 Oct;26(10):811–5. doi: 10.1086/502498. [DOI] [PubMed] [Google Scholar]
- 4.Streit JM, Jones RN, Sader HS, Fritsche TR. Assessment of pathogen occurrences and resistance profiles among infected patients in the intensive care unit: report from the SENTRY Antimicrobial Surveillance Program (North America, 2001) Int J Antimicrob Agents. 2004 Aug;24(2):111–8. doi: 10.1016/j.ijantimicag.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Furuno JP, Hebden JN, Standiford HC, Perencevich EN, Miller RR, Moore AC, Strauss SM, Harris AD. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008 Sep;36(7):468–71. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel JD, Rhinehart E, Jackson M, Chiarello L Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007 Dec;35(10 Suppl 2):S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006 Aug;34(8):2084–9. doi: 10.1097/01.CCM.0000227648.15804.2D. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RR, Scott RD, 2nd, Hota B, Kampe LM, Abbasi F, Schabowski S, Ahmad I, Ciavarella GG, Cordell R, Solomon SL, Hagtvedt R, Weinstein RA. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care. 2010 Nov;48(11):1026–35. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- 9.Stone PW, Glied SA, McNair PD, Matthes N, Cohen B, Landers TF, Larson EL. CMS changes in reimbursement for HAIs: setting a research agenda. Med Care. 2010 May;48(5):433–9. doi: 10.1097/MLR.0b013e3181d5fb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sipkoff M. Hospitals asked to account for errors on their watch. CMS and states may stop paying for specific hospital-acquired conditions. Will health plans follow suit? Manag Care. 2007 Jul;16(7):30, 35–7. [PubMed] [Google Scholar]
- 11.Warren DK, Cosgrove SE, Diekema DJ, Zuccotti G, Climo MW, Bolon MK, Tokars JI, Noskin GA, Wong ES, Sepkowitz KA, Herwaldt LA, Perl TM, Solomon SL, Fraser VJ Prevention Epicenter Program. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006 Jul;27(7):662–9. doi: 10.1086/506184. Epub 2006 Jun 9. [DOI] [PubMed] [Google Scholar]
- 12.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006 Dec 28;355(26):2725–32. doi: 10.1056/NEJMoa061115. Erratum in: N Engl J Med 2007 Jun 21;356(25):2660. [DOI] [PubMed] [Google Scholar]
- 13.Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM, Thompson DA, Sinopoli DJ, Cosgrove S, Sexton JB, Marsteller JA, Hyzy RC, Welsh R, Posa P, Schumacher K, Needham D. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010 Feb 4;340:c309. doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA Chicago Antimicrobial Resistance Project (CARP) Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006 Feb 13;166(3):306–12. doi: 10.1001/archinte.166.3.306. [DOI] [PubMed] [Google Scholar]
- 15.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007 Oct 22;167(19):2073–9. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 16.Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, Speck K, Jernigan JA, Robles JR, Wong ES. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009 Jun;37(6):1858–65. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing: Fourteenth Informational Supplement M100-S14. Wayne, PA, USA: NCCLS; 2004. [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008 Jun;36(5):309–32. doi: 10.1016/j.ajic.2008.03.002. Erratum in: Am J Infect Control. 2008 Nov;36(9):655. [DOI] [PubMed] [Google Scholar]
- 19.Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009 Oct;30(10):959–63. doi: 10.1086/605925. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Price LS, Hota B, Stemer A, Weinstein RA. Prevention of bloodstream infections by use of daily chlorhexidine baths for patients at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2009 Nov;30(11):1031–5. doi: 10.1086/644751. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12(1):147–79. doi: 10.1128/cmr.12.1.147. Review. Erratum in: Clin Microbiol Rev 2001 Jan;14(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockhart SR, Wagner D, Iqbal N, Pappas PG, Andes DR, Kauffman CA, Brumble LM, Hadley S, Walker R, Ito JI, Baddley JW, Chiller T, Park BJ. Comparison of in vitro susceptibility characteristics of Candida species from cases of invasive candidiasis in solid organ and stem cell transplant recipients: Transplant-Associated Infections Surveillance Network (TRANSNET), 2001 to 2006. J Clin Microbiol. 2011 Jul;49(7):2404–10. doi: 10.1128/JCM.02474-10. Epub 2011 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sijbesma T, Röckmann H, van der Weegen W. Severe anaphylactic reaction to chlorhexidine during total hip arthroplasty surgery. A case report. Hip Int. 2011 Sep;21(5):630–2. doi: 10.5301/HIP.2011.8644. [DOI] [PubMed] [Google Scholar]
- 24.Bringué Espuny X, Soria X, Solé E, Garcia J, Marco JJ, Ortega J, Ortiz M, Pueyo A. Chlorhexidine-methanol burns in two extreme preterm newborns. Pediatr Dermatol. 2010 Nov-Dec;27(6):676–8. doi: 10.1111/j.1525-1470.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng CE, Kroshinsky D. Iatrogenic skin injury in hospitalized patients. Clin Dermatol. 2011 Nov;29(6):622–32. doi: 10.1016/j.clindermatol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Liippo J, Kousa P, Lammintausta K. The relevance of chlorhexidine contact allergy. Contact Dermatitis. 2011 Apr;64(4):229–34. doi: 10.1111/j.1600-0536.2010.01851.x. Epub 2011 Jan 13. [DOI] [PubMed] [Google Scholar]
- 27.McGann P, Kwak YI, Summers A, Cummings JF, Waterman PE, Lesho EP. Detection of qacA/B in Clinical Isolates of Methicillin-Resistant Staphylococcus aureus from a Regional Healthcare Network in the Eastern United States. Infect Control Hosp Epidemiol. 2011 Nov;32(11):1116–9. doi: 10.1086/662380. Epub 2011 Sep 20. [DOI] [PubMed] [Google Scholar]
- 28.Smith K, Gemmell CG, Hunter IS. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J Antimicrob Chemother. 2008 Jan;61(1):78–84. doi: 10.1093/jac/dkm395. Epub 2007 Nov 2. [DOI] [PubMed] [Google Scholar]
- 29.Sheng WH, Wang JT, Lauderdale TL, Weng CM, Chen D, Chang SC. Epidemiology and susceptibilities of methicillin-resistant Staphylococcus aureus in Taiwan: emphasis on chlorhexidine susceptibility. Diagn Microbiol Infect Dis. 2009 Mar;63(3):309–13. doi: 10.1016/j.diagmicrobio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Alam MM, Kobayashi N, Uehara N, Watanabe N. Analysis on distribution and genomic diversity of high-level antiseptic resistance genes qacA and qacB in human clinical isolates of Staphylococcus aureus. Microb Drug Resist. 2003 Summer;9(2):109–21. doi: 10.1089/107662903765826697. [DOI] [PubMed] [Google Scholar]
- 31.Longtin J, Seah C, Siebert K, McGeer A, Simor A, Longtin Y, Low DE, Melano RG. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus aureus isolated in Toronto, Canada, from 2005 to 2009. Antimicrob Agents Chemother. 2011 Jun;55(6):2999–3001. doi: 10.1128/AAC.01707-10. Epub 2011 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vali L, Davies SE, Lai LL, Dave J, Amyes SG. Frequency of biocide resistance genes, antibiotic resistance and the effect of chlorhexidine exposure on clinical methicillin-resistant Staphylococcus aureus isolates. J Antimicrob Chemother. 2008 Mar;61(3):524–32. doi: 10.1093/jac/dkm520. Epub 2008 Jan 28. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J Clin Microbiol. 2006 Jun;44(6):2119–25. doi: 10.1128/JCM.02690-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee AS, Macedo-Vinas M, François P, Renzi G, Schrenzel J, Vernaz N, Pittet D, Harbarth S. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: a case-control study. Clin Infect Dis. 2011 Jun 15;52(12):1422–30. doi: 10.1093/cid/cir233. [DOI] [PubMed] [Google Scholar]
- 35.Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007 Dec;51(12):4217–24. doi: 10.1128/AAC.00138-07. Epub 2007 Oct 1. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]