Abstract
TCRβ chain repertoire of peripheral αβ T cells is generated through the step-wise assembly and subsequent selection of TCRβ variable region exons during thymocyte development. To evaluate the influence of a two-step recombination process on Vβ rearrangement and selection, we generated mice with a pre-assembled Dβ1Jβ1.1 complex on the Jβ1ω allele, an endogenous TCRβ allele that lacks the Dβ2-Jβ2 cluster, creating the Jβ1DJβ allele. As compared to Jβ1ω/ω mice, both Jβ1DJβ/ω and Jβ1DJβ/DJβ mice exhibited grossly normal thymocyte development and TCRβ allelic exclusion. In addition, Vβ rearrangements on Jβ1DJβ alleles occurred at the same frequency as Vβ rearrangements on Jβ1ω alleles, and were similarly regulated by TCRβ mediated feedback regulation. However, thymocytes with VβDJβ rearrangements assembled on Jβ1DJβ alleles overall were preferentially selected and the Vβ repertoire of αβ T cells was significantly altered during αβ TCR selection in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice. Our data indicate that the diversity of DJβ complexes assembled during thymocyte development influences TCRβ chain selection and peripheral Vβ repertoire.
Keywords: T cells, T cell receptor, gene rearrangements, molecular biology, transgenic/knockout mice
Introduction
Generation and expression of a diverse repertoire of antigen receptors on the cell surface of lymphocytes is essential for adaptive immunity. TCR and Ig chains consist of variable regions that bind antigen and constant (C) regions. During lymphocyte development, TCR and Ig variable region exons ("genes") are assembled through the rearrangement of germline variable (V), diversity (D), and joining (J) gene segments (1). V(D)J recombination is initiated by the lymphocyte-specific RAG1/RAG2 (RAG) endonuclease, which induces DNA double strand breaks between participating gene segments and their flanking recombination signal sequences (RSSs), generating blunt signal ends and hairpin-sealed coding ends (2). Signal ends are repaired by generally expressed core non-homologous end-joining (NHEJ) proteins to form signal joins, while coding ends are opened by additional NHEJ proteins and then repaired by the core NHEJ factors to form V(D)J coding joins (3, 4). This process generates antigen receptor chain diversity through the combination of joining events, the inherent imprecision of NHEJ, and the random addition of non-template (N) nucleotides by the lymphocyte-specific terminal TdT protein (1).
In humans and mice, αβ T lymphocytes develop through a differentiation program that involves the assembly, expression, and selection of TCR genes (5). TCRβ genes are assembled through an ordered process in which Dβ-to-Jβ rearrangements are detectable in CD117(c-kit)+CD44+CD25− early T lineage progenitors (ETPs) and CD117+CD44+CD25+ (stage II) CD4−/CD8− (double negative or DN) thymocytes, and Vβ rearrangements initiate in CD117−CD44−CD25+ (stage III) DN cells (6, 7). Although Dβ-to-Jβ recombination is not required for Vβ rearrangement, the deletion of sequences between Dβ and Jβ segments, such as the 3'Dβ RSSs, may facilitate Vβ-to-DJβ recombination (Sleckman, c-fos, Khor). The assembly and expression of a productive (in-frame) VβDJβ rearrangement on the first allele generates TCRβ chains that pair with pTα molecules to form pre-TCRs that select DNIII cells for further development (5). This β-selection process involves signals that rescue DNIII thymocytes from apoptosis, promote rapid cellular proliferation, direct differentiation into c-kit−CD44−CD25− DN IV cells and then CD4+/CD8+ (double positive, DP) thymocytes, and prevent Vβ-to-DJβ rearrangements on the second allele to ensure TCRβ chains are expressed from only one allele (5). In DP thymocytes, TCRα genes are assembled on both alleles from Vα and Jα segments (8). Productive VαJα rearrangements generate TCRα chains that can associate with TCRβ chains to form αβ TCR receptors, which are then subject to selection (9). Negative selection of αβ TCRs leads to cell death, while positive selection rescues DP cells from apoptosis and promotes their further development to CD4+ or CD8+ (single positive, SP) thymocytes, which exit the thymus as αβ T cells (9). Due to the imprecision of V(D)J joining, only one-third of VβDJβ rearrangements are assembled in-frame. Thymocytes that assemble a non-productive (out-of-frame) VβDJβ rearrangement on the first allele can undergo Vβ rearrangement on the second allele, which drives differentiation if assembled in-frame (10). Thus, in addition to their selected VβDJβ rearrangements, ~60% of normal αβ T cells contain DJβ rearrangements and ~40% contain out-of-frame VβDJβ rearrangements on their non-selected alleles, a phenomenon referred to as the 60/40 ratio (10, 11). This pattern of TCRβ rearrangements indicates that Vβ-to-DJβ rearrangements occur on one allele at a time in DNIII cells (Mostoslavasky), however there is no evidence that distinguishes whether Dβ-to-Jβ rearrangements occur asynchronously between alleles or on both alleles simultaneously.
The TCRβ chain repertoire of peripheral αβ T lymphocytes is generated through the assembly and selection of VβDJβ rearrangements during thymocyte development. Generation of primary TCRβ repertoires is not random since rearrangements between both Dβ and Jβ segments and Vβ segments and DJβ complexes occur at varying relative levels in DN thymocytes as determined, at least in part, by genetic variations of their flanking RSSs (12–16). The relative frequency at which Vβs are expressed in DN thymocytes prior to β-selection and in DP cells prior to αβ TCR selection are similar, suggesting that Vβ repertoire is not substantially altered during β-selection (17). In contrast, the relative frequency at which Vβs are expressed in DP thymocytes versus SP thymocytes and peripheral αβ T cells can be dramatically different, indicating that the Vβ repertoire of TCRβ chains is shaped during αβ TCR selection (18–24). The length of VβDJβ joins also is modulated during thymocyte development with shorter sequences selected for during DP to SP differentiation (25, 26).
There are many gaps in our understanding of the mechanisms that evolved to control the regulated assembly and selection of TCRβ chains. The tri partite joining of Vβ, Dβ, and Jβ segments is required for generation of TCRβ chains with normal-sized third complementarity determining regions, which are involved in antigen binding (27). Accordingly, selective pressure enforced by antigens may have caused TCRβ loci to evolve Vβ, Dβ, and Jβ segments such that TCRβ chains include amino acids encoded by Dβ nucleotides and gain the increased junctional diversity of two joining events. The assembly of Ig heavy (H) chain genes from VH, DH, and JH segments occurs through DJH intermediates and is subject to allelic exclusion (11), while the assembly of TCRδ genes occurs through both DJδ and VDδ intermediates and exhibits allelic inclusion (28). In this context, an ordered two-step recombination process also could have evolved to provide an additional level of regulatory control important for preventing Vβ to DJβ rearrangements on both alleles and enforcing TCRβ allelic exclusion (15).
The mouse TCRβ locus consists of 20 functional Vβ segments and two Dβ-Jβ clusters (Dβ1-Jβ1 and Dβ2-Jβ2) that each contains a single Dβ segment (Dβ1 or Dβ2) and six functional Jβ segments (Jβ1.1-Jβ1.6 or Jβ2.1-Jβ2.7) (Accession Numbers). In a population of DN thymocytes, Dβ2 rearranges to all six functional Jβ2 segments and Dβ1 rearranges to all 12 functional Jβ segments, creating DβJβ complexes of 18 Dβ-Jβ joining combinations (reference). In the DNIII population, all Vβ segments rearrange to these Dβ1Jβ1, Dβ1Jβ2, and Dβ2Jβ2 complexes (reference), with deletion of Dβ1Jβ1 complexes and germline Jβ1 segments upon joining to either Dβ1Jβ2 or Dβ2Jβ2 complexes. Moreover, in DNIII cells with primary Vβ rearrangements to Dβ1Jβ1 complexes, secondary Vβ rearrangements theoretically can occur to Dβ2Jβ2 complexes, resulting in deletion of the VβDβ1Jβ1 complexes. Since the number and complexity of TCRβ rearrangements present obstacles for the investigation of mechanisms that regulate the assembly of TCRβ variable region exons, we previously used gene targeting to delete the endogenous Dβ2-Jβ2 cluster and create the Jβ1ω allele on which Vβ rearrangements only can be targeted to DβJβ1 complexes of six Dβ-Jβ joining combinations (Bassing). Heterozygous and homozygous Jβ1ω mice exhibit αβ T cell development, TCRβ rearrangement, Vβ repertoire, and TCRβ allelic exclusion indistinguishable from wild-type mice with un-modified TCRβ loci (15). Thus, to evaluate the influence of Dβ-to-Jβ rearrangement on Vβ rearrangement and TCRβ selection, we generated and analyzed mice with a pre-assembled Dβ1Jβ1.1 complex on the Jβ1ω allele. Due to practical considerations, our analysis was unfortunately limited to one particular Jβ1 segment and one DβJβ coding join of a defined sequence and length, which could introduce biases in Vβ rearrangement and TCRβ selection. Yet, such potential biases also would indicate unequivocally that DJβ complexes assembled in DN thymocytes influences these downstream processes.
Materials and Methods
Targeting Construct and Probes
The DJβ targeting vector was constructed in pLNTK (29). The 5’ homology arm is a 2.8 kb KpnI/BamHI fragment containing a pre-assembled Dβ1Jβ1.1 complex PCR-cloned from splenocytes, which was then blunted and ligated into the SalI site of pLNTK. The 3’ homology arm is a 1.8 kb BamHI/SacI genomic fragment containing Jβ1.2 through Jβ1.6, which was blunted and ligated into the XhoI site of pLNTK. This fragment also contained a HindIII site that was inserted just inside the BamHI site prior to subcloning. The completed targeting vector was sequenced to confirm the integrity of the pre-assembled Dβ1Jβ1.1 complex. The 5’KO probe is a 300 bp PCR product amplified with primers 5’-GGATCCTGAGAACTGGACATAAGGG-3’ and 5’-TTTAATCACTGTGTACTTCC-3’. The 3’KO probe is a 546 bp EcoRI/KpnI fragment.
Gene Targeting and Generation of ES Cells
The DJβ targeting vector was electroporated into Jβ1ω/ω ES cells (15) as previously described (30) to generate Jβ1DJβNeo/ω ES cells. Targeted clones were identified by Southern blot analysis with the 5’Dβ1 probe on EcoRI digested genomic DNA (5.5 kb Jβ1DJβNeo, 9.3 kb Jβ1ω) and confirmed with the 3’Jβ1 probe on HindIII digested DNA (5.7 kb Jβ1DJβNeo, 8.9 kb Jβ1ω). Targeted ES cells were infected with recombinant AdenoCre and subcloned. Cre-deleted subclones were identified by Southern blot analysis with the 5’Dβ1 probe on EcoRI digested genomic DNA (5.5 kb Jβ1DJβNeo, 8.7 kb Jβ1DJβ, 9.3 kb Jβ1ω) and confirmed with the 3’Jβ1 probe on BamHI digested DNA (8.7 kb Jβ1DJβNeo, 13.5 kb Jβ1DJβ, 8.3 kb Jβ1ω).
Mice
Generation of Jβ1ω/ω mice was previously described (15). DO11.10 TCRβ transgenic mice (31) were bred with Jβ1DJβ/DJβ mice to generate Vβ8TgJβ1DJβ/DJβ mice. Germline Vβ14NT/+ mice were generated from LN2 ES cells (32) as described (33).
FACS
Cells from single cell suspensions of the thymuses and spleens of 4–6 week-old mice were counted and then stained with indicated combinations of FITC-conjugated anti-CD8, anti-Vβ5, anti-Vβ8, anti-Vβ10b, and anti-Vβ14 antibodies; PE-conjugated anti-CD4, anti-CD8, anti-Cβ, and anti-Vβ10b antibodies; APC- conjugated anti-Cβ and anti-CD4; biotin-conjugated anti-Vβ14 and anti-Vβ12; and SA-PE-Cy7, SA-FITC, and SA-APC reagents (BD Pharmingen). For analysis of DN subsets, thymocytes were stained with a cocktail of PE-conjugated antibodies for TCRβ, TCRδ, CD8, CD45R, CD19, CD11c, CD11b, Ter119, and NK.1, as well as PE-Cy7-conjugated anti-CD25 and APC-conjugated anti-CD117 antibodies (BD Pharmingen). Data acquisition was conducted on a BD FACSCalibur equipped with BD CellQuest Pro and data analysis performed with FlowJo software (Tree Star). Each FACS experiment was done at least three separate times on independent mice of each genotype.
Hybridoma Analysis
Hybridomas were generated by fusion of the BW-1100.129.237 thymic lymphoma cell line (34) with concanavalin A and IL-2 stimulated αβ T cells as previously described (30). Genomic DNA was isolated and subjected to Southern blot analysis. The Southern blot analysis of TCRβ rearrangements was conducted with the 5’Dβ1 and 3’Jβ1 probes on either EcoRI or HindIII digested genomic DNA isolated from the hybridomas. The 5’Dβ1 probe is a 400 bp NheI fragment. The 3’Jβ1 probe is a 777 bp DrdI fragment.
Results
Generation of Mice with a Pre-Assembled DβJβ1.1 Complex
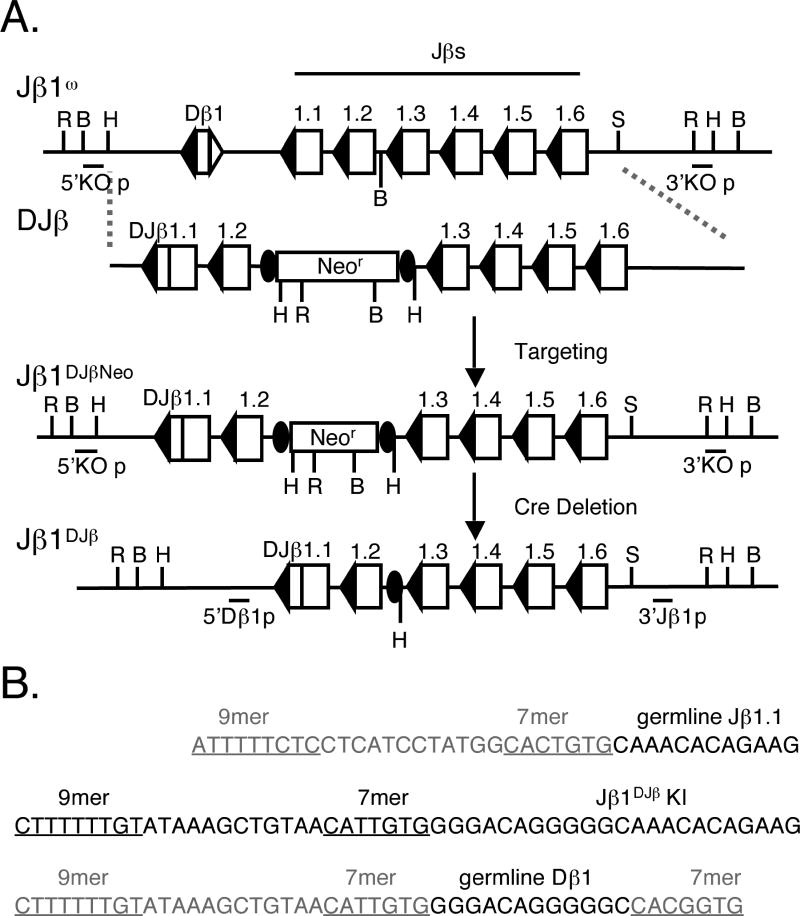
Our previous gene-targeted modification of the endogenous Dβ1Jβ1 cluster demonstrated that a loxP site and a novel BamHI site inserted just 3’ of Jβ1.2 on one allele could be used to distinguish between VβDJβ1.1 and VβDJβ1.2 rearrangements and had no discernable effects on thymocyte development, Vβ rearrangement, or VβDJβ1.1 and VβDJβ1.2 expression (15). Thus, we used gene-targeted mutation to replace the germline sequences spanning the Dβ1 and Jβ1.1 segments with a pre-assembled Dβ1Jβ1.1 complex (Figure 1) on a single allele of Jβ1ω/ω embryonic stem (ES) cells. This Dβ1Jβ1.1 complex was isolated by PCR amplification and subcloning of Dβ1Jβ1.1 joins on DJβ rearranged alleles of wild-type splenic αβ T cells. The isolated Dβ1Jβ1.1 complex does not contain N or P nucleotides and compared to the full sequences of Dβ1 and Jβ1.1 is missing one C nucleotide. The initial targeting event resulted in the replacement of the endogenous sequences with the Dβ1Jβ1.1 complex and insertion of a neomycin resistant gene (Neor) flanked by loxP sites just 3’ of the endogenous Jβ1.2 segment, creating the Jβ1DJβNeo allele (Figure 1). Next, we deleted the Neor gene through transient expression of the Cre recombinase in Jβ1DJβNeo/ω ES cells to leave a single loxP site inserted just 3’ of the endogenous Jβ1.2 segment, creating the Jβ1DJβ allele (Figure 1). We also inserted a novel HindIII site next to the loxP site to distinguish between TCRβ rearrangements on the Jβ1DJβ and Jβ1ω alleles (Figure 1). Finally, we used Jβ1DJβ/ω ES cells to generate germline Jβ1DJβ/ω mice and then bred these with Jβ1ω/ω mice and also with each other to generate Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, respectively. The comparative analysis of Jβ1ω/ω and Jβ1DJβ/DJβ mice will allow us to evaluate whether complete subversion of the tri partite TCRβ recombination process influences αβ T cell development, TCRβ rearrangement, Vβ repertoire, and/or TCRβ allelic exclusion. In addition, the analysis of TCRβ rearrangements in Jβ1DJβ/ω will reveal whether the assembly of a DJβ complex could affect Vβ rearrangement or influence TCRβ selection.
Figure 1. Gene-targeted generation of Jβ1DJβ/ω ES cells.
(A) This schematic diagram illustrates the Jβ1ω allele that lacks the Dβ2-Jβ2.7 segments, positioning of the DJβ targeting construct, and the resulting Jβ1DJβNeo and Jβ1DJβ alleles. Open boxes depict the Dβ1 and Jβ1 segments; their adjacent RSSs are triangles. Upon gene-targeting, the pre-assembled Dβ1Jβ1.1 complex replaced the germline Dβ1 and Jβ1.1 segments and a neomycin resistance gene (neor, rectangle) flanked by loxP sites (filled ovals) was inserted between the Jβ1.2 and Jβ1.3 segments. Following Cre-mediated deletion of the neor gene, the remaining segments align approximately to the position of an endogenous Dβ1Jβ1.1 complex. The solid horizontal bars indicate the relative locations of the 5’KO, 3’KO, 5’Dβ1, and 3’Jβ1 probes. Restriction site designations: B, BamHI; H, HindIII; R, EcoRI; S, SacI. (B) Shown are the sequences of the germline Dβ1 segment with the 5'Dβ1 RSS and the heptamer of the 3'Dβ1 RSS, (top sequence), the pre-assembled Dβ1Jβ1.1 complex (middle sequence), and the germline Jβ1.1 segment with the Jβ1.1 RSS (bottom sequence).
Normal αβ T Cell Development in Jβ1DJβ/ω and Jβ1DJβ/DJβ Mice
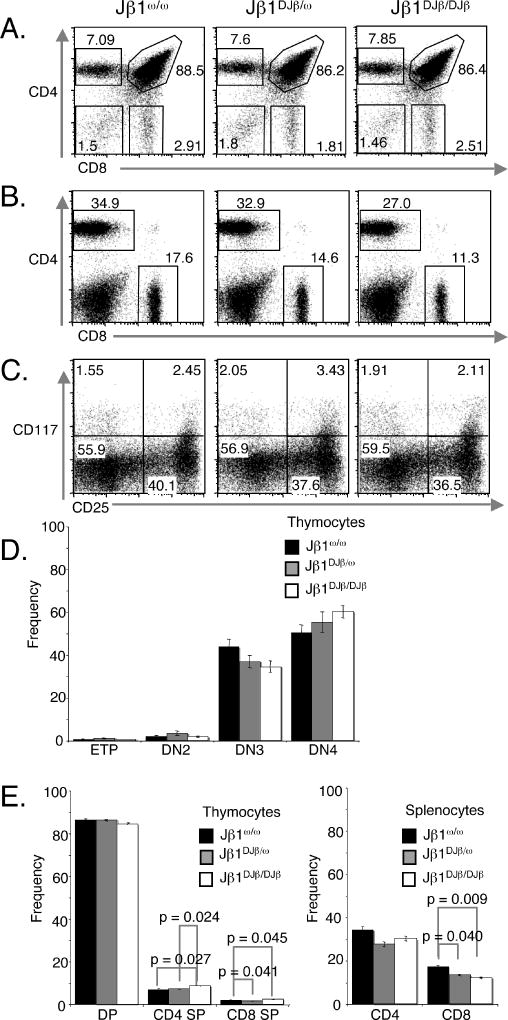
To evaluate the potential influence of Dβ-to-Jβ rearrangement on αβ T cell development, we analyzed thymocytes and splenocytes isolated from Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ mice. Both Jβ1DJβ/ω and Jβ1DJβ/DJβ mice exhibited similar numbers of thymocytes and splenocytes as Jβ1ω/ω mice (data not shown). FACS analysis of Jβ1DJβ/ω and Jβ1DJβ/DJβ thymocytes with anti-CD4 and anti-CD8 antibodies revealed a distribution of DN, DP, and SP populations similar to those in Jβ1ω/ω thymocytes (Figure 2A,D,E). In addition, FACS analysis of Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ splenocytes with anti-CD4 and anti-CD8 antibodies revealed similar percentages of CD4+ and CD8+ αβ T cells in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice (Figure 2B,E). FACS analysis of Jβ1DJβ/ω and Jβ1DJβ/DJβ thymocytes with anti-CD117 and anti-CD25 antibodies showed a similar distribution of ETPs, stage II, stage III, and stage IV DN cells as Jβ1ω/ω thymocytes (Figure 2C,D). Despite these similarities, there were detectable differences in the DN III and DN IV populations and statistically significant differences in SP thymocytes and CD4+ and CD8+ splenic αβ T cell populations among Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice (Figure 2D,E). These data demonstrate that, although neither a single fixed DJβ rearrangement nor the sequence of the particular DJβ complex used has any substantial effect on thymocyte development, the inability to assemble a diverse DJβ repertoire has subtle or significant influences on different stages of αβ T cell differentiation. The decreased ratios of DNIII to DNIV cell populations that correlate with increased copy number of the Jβ1DJβ allele may reflect that Vβ rearrangements occur at a higher frequency on the Jβ1DJβ allele in DNIII thymocytes and/or DNIII cells expressing VβDJβ chains from Jβ1DJβ alleles are preferentially selected. The statistically significant differences in SP thymocytes and CD4+ and CD8+ splenic αβ T cell populations among Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice suggest that selection of αβ TCR containing VβDJβ chains with the pre-assembled Dβ1Jβ1.1 complex may be altered.
Figure 2. Normal αβ T cell development in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice.
(A–B) Shown are representative anti-CD4 and anti-CD8 FACS analysis of cells isolated from the (A) thymuses and (B) spleens of Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice. The percentage of (A) DN, DP, CD4+ SP, and CD8+ SP thymocytes and (B) CD4+ and CD8+ αβ T cells is indicated. (C) Shown are representative anti-CD117 and anti-CD25 FACS analysis of thymocytes negative for mature cells markers (TCRβ, TCRδ, CD4, CD8α, CD19, CD11c, CD11b, B220 and NK1.1). (D,E) Bar graphs showing the average frequency of cells within each thymocyte developmental stage and peripheral T cell population from at least five mice of each genotype. The error bars are standard error of the mean. Significant differences have been calculated using a two-tailed student t-test.
Jβ1DJβ/ω and Jβ1DJβ/DJβ αβ T Cells Exhibit TCRβ Allelic Exclusion
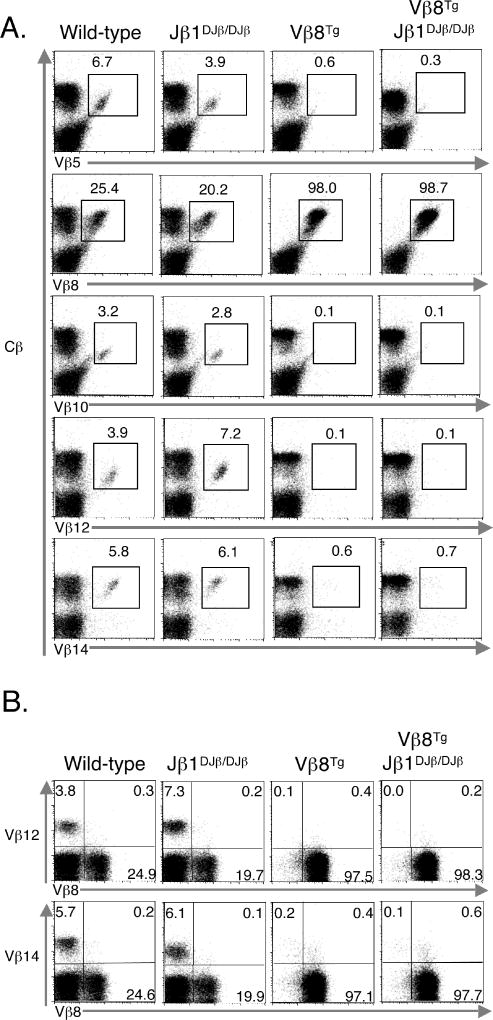
To evaluate the contribution of a two-step recombination process on enforcement of TCRβ allelic exclusion, we assayed for the expression of two distinct TCRβ chains on the cell surface of Jβ1DJβ/DJβ αβ T cells. Since an allotypic marker has not been reported for TCRβ chains, we conducted FACS analysis with antibodies specific for two different Vβ segments. FACS analysis of Jβ1DJβ/DJβ splenocytes with anti-Vβ5/anti-Vβ10 or anti-Vβ8/anti-Vβ14 antibodies failed to reveal any obvious αβ T lymphocyte populations that expressed two Vβs on the cell surface (Figure 3A). Many studies of TCRβ allelic exclusion have been conducted using transgenic mice that express a pre-rearranged, in-frame VβDJβ rearrangement randomly integrated into the genome (10, 37). Thus, we also generated and analyzed TCRβ allelic exclusion in Jβ1DJβ/DJβ mice expressing a transgenic in-frame Vβ8DJβ rearrangement (Vβ8Tg). FACS analysis of Vβ8Tg and Vβ8TgJβ1DJβ/DJβ splenocytes with an anti-Cβ antibody and an anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 antibody revealed the absence of αβ T cells expressing any Vβ on the cell surface without expression of Vβ8 in either mouse (Figure 3B). In addition, FACS analysis of Vβ8Tg and Vβ8TgJβ1DJβ/DJβ splenocytes with an anti-Vβ8 antibody and either an anti-Vβ12 or anti-Vβ14 antibody did not detect any difference in αβ T cells expressing both Vβ8 and another Vβ on the cell surface (Figure 3C). Collectively, these data indicate that a two-step recombination process is not required for enforcement of TCRβ allelic exclusion, at least at the level of detection of FACS using anti-Vβ antibodies specific for TCRβ chains with two different Vβs.
Figure 3. Jβ1DJβ/DJβ αβ T cells exhibit TCRβ allelic exclusion.
(A) Shown are representative anti-Vβ5 and anti-Vβ10 or anti-Vβ8 and anti-Vβ14 FACS analysis of Cβ+ cells isolated from the spleens of Jβ1ω/ω and Jβ1DJβ/DJβ mice. The percentage of Vβ5+, Vβ10+, and Vβ5+Vβ10+ or Vβ8+, Vβ14+, and Vβ8+Vβ14+ cells is indicated. (B) Shown are representative anti-Cβ and anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 FACS analysis of cells isolated from the spleens of wild-type, Jβ1DJβ/DJβ, Vβ8Tg, and Vβ8TgJβ1DJβ/DJβ mice. The percentage of Cβ+ cells that express Vβ5, Vβ8, Vβ10, Vβ12, or Vβ14 is indicated. (C) Shown are representative anti-Vβ12 and anti-Vβ8 or anti-Vβ14 and anti-Vβ8 FACS analysis of Cβ+ cells isolated from the spleens of wild-type, Jβ1DJβ/DJβ, Vβ8Tg, and Vβ8TgJβ1DJβ/DJβ mice. The percentage of Vβ12+, Vβ8+, and Vβ12+Vβ8+ or Vβ14+, Vβ8+, and Vβ14+Vβ8+ cells is indicated.
Normal TCRβ Feedback Regulation in Jβ1DJβ/ω and Jβ1DJβ/DJβ and αβ T Cells
The observed enforcement of TCRβ allelic exclusion in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice suggests that Vβ-to-DJβ rearrangements on Jβ1DJβ alleles are subject to TCRβ-mediated feedback inhibition. However, despite impaired TCRβ-mediated feedback inhibition in pTα deficient thymocytes, dual Vβ expressing αβ T cells are not observed in either pTα deficient mice or pTα deficient mice containing a TCRβ transgene, indicating that TCRβ allelic exclusion also can be enforced by post Vβ-to-DJβ recombination mechanisms (Fred, von Boehmer). The 60/40 ratio of αβ T cells with VβDJβ/DJβ and VβDJβ/VβDJβ rearrangements reflects the regulation of Vβ rearrangement by TCRβ-mediated feedback inhibition (10). Therefore, to evaluate the influence of a two-step recombination process on TCRβ-mediated feedback regulation, we generated in a panel of 88 Jβ1DJβ/DJβ αβ T cell hybridomas and analyzed TCRβ rearrangements by Southern blot analysis. We first assayed TCRβ rearrangements on EcoRI-digested genomic DNA using the 5'Dβ1 and 3'Jβ1 probes, each of which hybridizes to the same 5.5 kb EcoRI germline fragment from the Jβ1DJβ allele (Figure 1). We found that 55 of 88 (62%) Jβ1DJβ/DJβ αβ T cell hybridomas retained 5.5 kb 5'Dβ1 and 3'Jβ1 bands, indicating they contained only one VβDJβ rearrangement (Table IA). The remaining 33 of 88 (38%) lacked the 5.5 kb 5'Dβ1 and 3'Jβ1 bands, but contained two novel-sized 3'Jβ1 bands, revealing they carried VβDJβ rearrangements on both Jβ1DJβ alleles (Table IA). These data demonstrate that Jβ1DJβ/DJβ αβ T cells exhibit the normal 60/40 ratio of VβDJβ/DJβ and VβDJβ/VβDJβ rearrangements. Thus, Vβ rearrangements on Jβ1DJβ alleles are subject to normal TCRβ feedback regulation, consistent with the observed enforcement of TCRβ allelic exclusion in Jβ1DJβ/DJβ and Vβ8TgJβ1DJβ/DJβ αβ T cells.
Table I.
| A. TCRβ rearrangement phenotypes in Jβ1DJβ/DJβ and Jβ1DJβ/ω αβ T cell hybridomas1 | |||
|---|---|---|---|
| Genotype | Total | VβDJβ/DJβ | VβDJβ/VβDJβ |
| Jβ1DJβ/DJβ | 88 | 55 (62.5%) | 33 (37.5%) |
| Jβ1DJβ/ω | 247 | 144 (58.0%) | 103 (42.0%) |
| B. Frequency of complete VβDJβ exon rearrangements on the DJβ allele versus the ω allele in heterozygous VβDJβ/DJβ Jβ1DJβ/ω αβ T cell hybridomas (144 total)2 | ||
|---|---|---|
| Genotype | Allele | Vβ-to-DJβ |
| Jβ1DJβ/ω | DJβ | 99 (69.0%) |
| ω | 45 (31.0%) | |
Results for sections A and B were collected from hybridomas grown from at least three independent fusions. See Materials and Methods for details.
VβDJβ rearrangements in Jβ1DJβ/ω mice could be linked to the different alleles using a unique restriction digest pattern introduced in the DJβ targeting. See Materials and Methods and FIGURE 1 for details.
VβDJβ Rearrangements are Preferentially Selected on Jβ1DJβ Alleles
To evaluate whether the presence of the pre-assembled Dβ1Jβ1.1 complex might either enhance or impair Vβ-to-DJβ rearrangement, we first sought to determine whether overall Vβ rearrangements occur at a similar level on the Jβ1DJβ and Jβ1ω alleles. Unfortunately, due to the inherent biases of amplifying VβDβJβ1 rearrangements of one fixed size on Jβ1DJβ alleles versus of six different sizes on Jβ1ω alleles, solid conclusions are not possible from PCR-based analysis of Vβ-to-DJβ rearrangements in non-selected DNIII thymocytes. The novel HindIII site inserted just downstream of the pre-assembled DJβ1.1 complex enables us to distinguish between TCRβ rearrangements on the Jβ1DJβ and Jβ1ω alleles in Jβ1DJβ/ω αβ T cells. Thus, we generated a panel of 247 Jβ1DJβ/ω αβ T cell hybridomas and analyzed VβDJβ rearrangements in these cells by Southern blot analysis and by PCR amplification and sequencing. We first assayed TCRβ rearrangements on EcoRI-digested genomic DNA using the 5'Dβ1 and 3'Jβ1 probes, each of which hybridizes to the same 9.4 kb germline EcoRI fragment on the Jβ1ω allele (Figure 1). We found that all 247 hybridomas lacked the 9.4 kb 3'Jβ1 band, but contained a novel-sized 3'Jβ1 band, revealing they had DJβ or VβDJβ rearrangements on the Jβ1ω allele. In addition, we found that 144 (58%) contained a single 5'Dβ1 band and 103 (42%) lacked any 5'Dβ1 bands. These data indicate that Jβ1DJβ/ω αβ T cells also exhibit the normal ratio of VβDJβ/DJβ and VβDJβ/VβDJβ rearrangements.
If Vβ-to-DJβ rearrangements occur at equal frequency on the Jβ1DJβ and Jβ1ω alleles and VβDJβ rearrangements involving the single pre-assembled Dβ1Jβ1.1 complex and the normal repertoire of rearranged Dβ1Jβ1 complexes are similarly selected, Jβ1DJβ/ω αβ T cells of the VβDJβ/DJβ configuration should contain an equal frequency of VβDJβ rearrangements on the Jβ1DJβ and Jβ1ω alleles. Thus, we next assayed TCRβ rearrangements using the 5'Dβ1 probe on HindIII-digested genomic DNA of the 144 Jβ1DJβ/ω αβ T cell hybridomas with VβDJβ rearrangements on a single allele. The 5'Dβ1 probe hybridizes to an 8.9 kb germline HindIII fragment on the Jβ1ω allele and to a 2.5 kb germline fragment on the Jβ1DJβ allele (Figure 1). We found that 45 of 144 (31%) retained and 99 of 144 (69%) lost the 2.5 kb 5'Dβ1 band (Table IB), revealing that the majority of Jβ1DJβ/ω αβ T cells contain VβDJβ rearrangements on the Jβ1DJβ allele. This observation indicates that either Vβ rearrangements occur at a higher frequency on the Jβ1DJβ allele in DN cells or thymocytes expressing VβDJβ chains from Jβ1DJβ alleles are preferentially selected during development.
In addition to their in-frame and selected VβDJβ rearrangements, ~60% of normal αβ T cells contain DJβ rearrangements and ~40% contain out-of-frame VβDJβ rearrangements on their non-selected alleles (10, 11). Since Jβ1DJβ/ω αβ T cell hybridomas exhibit this normal 60/40 ratio, the frequency at which out-of-frame VβDJβ rearrangements occur on the Jβ1DJ allele of Jβ1DJβ/ω αβ T cells with VβDJβ/VβDJβ rearrangements can be used to distinguish between increased recombination versus preferential selection. If Vβ-to-DJβ rearrangements occurred at an increased frequency on Jβ1DJβ alleles and were selected equally as those on Jβ1ω alleles, two-thirds (66%) of VβDJβ joins on Jβ1DJβ alleles would be out-of-frame; whereas, if VβDJβ rearrangements were preferentially selected on the Jβ1DJβ allele, greater than one-third of these VβDJβ joins would be in-frame. Thus, we cloned and sequenced VβDJβ1.1 coding joins on the Jβ1DJβ allele of representative Jβ1DJβ/ω αβ T cell hybridomas with VβDJβ rearrangements on both alleles. We conducted PCR reactions on the genomic DNA isolated from 25 of these hybridomas using primers that hybridize to each specific Vβ and a primer that hybridizes 3’ of the HindIII site on the Jβ1DJβ allele (Figure 1). PCR products were digested with either HindIII or BamHI to distinguish between amplified Vβ rearrangements to the pre-assembled Dβ1Jβ1.1 complex on the Jβ1DJβ allele versus to Dβ1Jβ1.1 or Dβ1Jβ1.2 complexes on the Jβ1ω allele. Sequence analysis of 25 PCR products representing Vβ rearrangements to the pre-assembled DJβ1.1 complex revealed that 13 (52%) were out-of-frame and 12 (48%) were in-frame (Table II). This data suggests that cells expressing VβDJβ chains from Jβ1DJβ alleles overall are preferentially selected during thymocyte development, which is consistent with the decreased ratios of DNIII to DNIV cell populations that correlate with increased Jβ1DJβ copy number (Figure 1C, D). However, these experiments do not address whether the rearrangement frequencies of particular Vβ segments to the pre-assembled DJβ1.1 complex on the Jβ1DJβ allele are increased or decreased.
Table II.
Sequence analysis of VβDJβ coding joins on the Jβ1DJβ alleles of Jβ1DJβ/ω αβ T cell hybridomas with bi-allelic VβDJβ rearrrangements
| Vβ | Sequence (Codons) | P | N | P | DJβ Sequence (Codons) | Frame |
|---|---|---|---|---|---|---|
| Vβ16 | AGC TTA GCC | AG GGG GCA | Out | |||
| Vβ11 | AGC AGC CTC | AG GGG GCA | Out | |||
| Vβ8.2 | AGC GGT GA | ATA | A CAG GGG GCA | In | ||
| Vβ10 | GCC AGC AGC | TAT G | G GGG GCA | Out | ||
| Vβ13 | AGC AGT TTC | CCG GGA CCA | Out | |||
| Vβ12 | GCC AGC AGG | CAG GGG GCA | In | |||
| Vβ9 | AGC AGT AGA | G GGG GCA | Out | |||
| Vβ15 | TGT GGT GCT | CC | TC | GA CAG GGG GCA | In | |
| Vβ8.2 | GCC AGA GGT | GAA A | GA CAG GGG GCA | In | ||
| Vβ7 | AGC AGT TTA | TAC | CAG GGG GCA | In | ||
| Vβ6 | GCC AGC AGT A | AC | CAG GGG GCA | In | ||
| Vβ3 | AGC AGT CTC | CTA | A CAG GGG GCA | Out | ||
| Vβ8.2 | AGC GGT GAT | G | C | G GGG GCA | In | |
| Vβ1 | AGC AGC CAA | GA CAG GGG GCA | Out | |||
| Vβ1 | AGC AGC | CA | A CAG GGG GCA | In | ||
| Vβ11 | AGC AGC CT | A CAG GGG GCA | In | |||
| Vβ11 | AGC AGC | TTA GG | T | GGG GCA | Out | |
| Vβ12 | AGC AGT C | A | CCC | G GGA CAG GGG GCA | In | |
| Vβ10 | AGC AGC | TA | GA CAG GGG GCA | Out | ||
| Vβ11 | AGC AGC CT | T CAA CCC T | GA CAG GGG GCA | In | ||
| Vβ9 | AGC AGC C | CA | GA CAG GGG GCA | Out | ||
| Vβ16 | AGC TTA G | TG | C | G GGG GCA | Out | |
| Vβ1 | AGC AGC CA | GA CAG GGG GCA | Out | |||
| Vβ11 | AGC AGC | TT | G GGA CAG GGG GCA | In | ||
| Vβ11 | AGC AGC C | CA | GA CAG GGG GCA | Out |
Altered Vβ Repertoire in Jβ1DJβ/ω and Jβ1DJβ/DJβ Mice
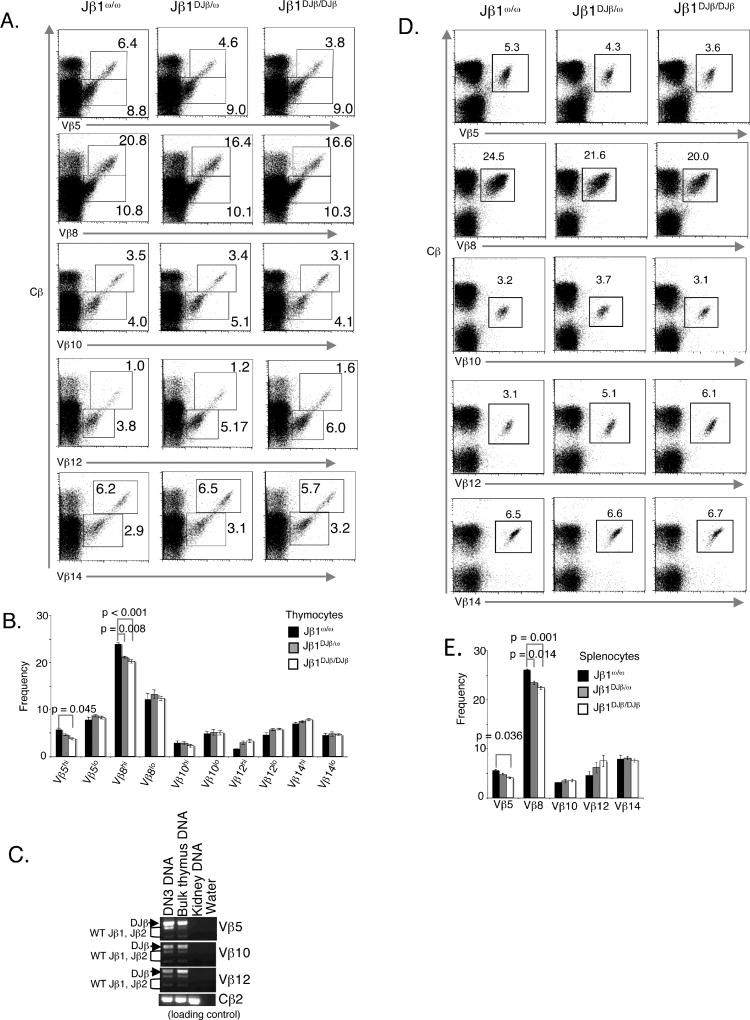
Although the Vβ repertoire is not substantially altered during β-selection (17), the relative frequency at which Vβs are expressed in DP versus SP thymocytes can be dramatically different, indicating that the Vβ repertoire of TCRβ chains is shaped during αβ TCR selection (18–24). The statistically significant differences in SP cells among Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice suggest that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements may influence αβ TCR selection of DP thymocytes. To investigate this issue, we sought to evaluate whether the repertoire of TCRβ chains is altered upon DP to SP differentiation in Jβ1DJβ/ω or Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice. TCRβ chains are expressed at "low/intermediate" levels on DP thymocytes undergoing αβ TCR selection and at "high" levels on positively selected SP cells (9). Thus, we conducted FACS analysis of Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ thymocytes with an anti-Cβ antibody and an anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 antibody. As we have done previously to evaluate αβ TCR selection (Wu), we quantified the percentages of cells expressing particular Vβ segments in TCRβ "low/intermediate" (DP) and TCRβ "high" (SP) thymocytes. We found similar percentages of Vβ10+ and Vβ14+ TCRβ "intermediate/low" and "high" thymocytes in Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ mice (Figure 4A,B). In contrast, we detected similar percentages of Vβ8+ and Vβ5+ TCRβ "low/intermediate" thymocytes in Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ mice, but significant decreases in the percentages of Vβ8+ and Vβ5+ TCRβ "high" thymocytes in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice (Figure 4A,B). We also observed a similar percentage of Vβ12+ TCRβ "low/intermediate" thymocytes in Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ mice, and a subtle decrease in the percentage of Vβ12+ TCRβ "high" thymocytes in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice (Figure 4A,B). These data demonstrate that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements influences αβ TCR selection of DP thymocytes expressing particular Vβs.
Figure 4. Altered αβ TCR selection and Vβ repertoire in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice.
(A) Shown are representative anti-Cβ and anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 FACS analysis of cells isolated from the thymuses of Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice. The percentage of Cβ+ "low/intermediate" and "high" cells that express Vβ5, Vβ8, Vβ10, Vβ12, or Vβ14 is indicated. (B) Bar graphs showing the average percentage Cβ+ "low/intermediate" and "high" thymoctyes that express Vβ5, Vβ8, Vβ10, Vβ12, or Vβ14 is indicated. These values were obtained from three mice of each genotype. The error bars are standard error of the mean. Significant differences have been calculated using a two-tailed student t-test. (C) Shown are representative anti-Cβ and anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 FACS analysis of cells isolated from the spleens of Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice. The percentage of Cβ+ cells that express Vβ5, Vβ8, Vβ10, Vβ12, or Vβ14 is indicated. (D) Bar graphs showing the average percentage Cβ+ splenic αβ T cells that express Vβ5, Vβ8, Vβ10, Vβ12, or Vβ14 is indicated. These values were obtained from three mice of each genotype. The error bars are standard error. Significant differences have been calculated using a two-tailed student t-test.
The relative frequency at which Vβs are expressed in peripheral αβ T cells also can be dramatically different due to αβ TCR selection in DP thymocytes (references). The statistically significant differences in CD4+ and CD8+ splenic αβ T cells among Jβ1ω/ω, Jβ1DJβ/ω and Jβ1DJβ/DJβ mice suggest that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements also may influence peripheral Vβ repertoire. To investigate this issue, we also evaluated whether the repertoire of TCRβ chains is altered in Jβ1DJβ/ω or Jβ1DJβ/DJβ splenic αβ T lymphocytes, as compared to in Jβ1ω/ω cells. To this aim, we conducted FACS analysis of Jβ1ω/ω, Jβ1DJβ/ω, and Jβ1DJβ/DJβ splenocytes with an anti-Cβ antibody and an anti-Vβ5, anti-Vβ8, anti-Vβ10, anti-Vβ12, or anti-Vβ14 antibody. We found a similar percentage of Vβ14+ and Vβ10+ splenic αβ T cells as in Jβ1ω/ω mice (Figure 4C,D). In contrast, we detected significant decreases in the percentage of Vβ8+ and Vβ5+ splenic αβ T cells in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice (Figure 4C,D). We also observed an increase in the percentage of Vβ12+ splenic αβ T cells in Jβ1DJβ/ω and Jβ1DJβ/DJβ mice, as compared to in Jβ1ω/ω mice (Figure 4C,D), however, these differences were not statistically significant due to variation in the percentage of Vβ12+ cells in mice among experiments. These data demonstrate that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements also influences the Vβ repertoire of peripheral αβ T cells.
Discussion
To evaluate the influence of a two-step recombination process on Vβ rearrangement and selection, we generated mice with a pre-assembled Dβ1Jβ1.1 complex on an endogenous TCRβ allele that also lack Dβ2-Jβ2 segments, creating the Jβ1DJβ allele on which Vβ rearrangements only can be targeted to this one particular Dβ1Jβ1 complex of a defined sequence and length. Our comparative analysis of Jβ1DJβ/DJβ mice and Jβ1ω/ω mice in which Vβ rearrangements can be targeted to Dβ1Jβ1 complexes of six possible Dβ-Jβ joining combinations demonstrated that complete subversion of the tri partite TCRβ recombination process did not detectably alter Vβ rearrangement or TCRβ allelic exclusion. However, our analysis of TCRβ rearrangements in Jβ1DJβ/ω revealed that cells expressing VβDJβ chains from Jβ1DJβ alleles overall are preferentially selected during thymocyte development. In addition, we found that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements influences αβ TCR selection of DP thymocytes expressing particular Vβs and also the Vβ repertoire of peripheral αβ T cells. Collectively, our findings indicate that a two-step recombination process is not essential for normal regulation of Vβ rearrangement, but the sequence of DJβ complexes assembled during thymocyte development can influence TCRβ chain selection and peripheral Vβ repertoire.
TCRβ genes are assembled in an ordered fashion such that Dβ-to-Jβ and Vβ-to-DJβ, but not Vβ-to-Dβ, rearrangements occur during thymocyte development. This ordered assembly of TCRβ genes is controlled, at least in part, through the developmental stage-specific initiation of Dβ/Jβ in ETP and DNII cells and Vβ recombinational accessibility in DNIII thymocytes (12, 38, 39), most likely directed by activation of germline Dβ-Jβ and Vβ promoters independent of TCRβ gene recombination events (Sikes, Sikes, Vb reference). Recent data demonstrating that c-fos deposits RAG onto 3'Dβ RSSs and that Vβ-to-Dβ rearrangements occur in c-fos−/− thymocytes (40) supports the model that RAG occupancy of 3'Dβ RSSs prevents synaptic complex formation between the RAG proteins and Vβ RSSs and 5'Dβ RSSs until Dβ-to-Jβ rearrangement and deletion of 3'Dβ RSSs (40, 41). The lack of a detectable increase in the level of overall Vβ-to-DJβ rearrangements on Jβ1DJβ alleles as compared to on Jβ1ω alleles in Jβ1DJβ/ω αβ T cell hybridomas argues against a predominant role of such a steric hindrance mechanism for enforcement of ordered TCRβ gene rearrangements. However, the genomic deletion associated with the pre-assembled Dβ1Jβ1.1 complex could alter germline Dβ1-Jβ1 transcription (Khor) and/or nucleosome positioning over the 5'Dβ1 RSS (Baumann, Golding, Kwon, and Nightingale) in a manner that reduces RAG accessibility and Vβ-to-DJβ recombination on the Jβ1DJβ allele. In addition, chromatin changes associated with RAG-mediated cleavage during Dβ-to-Jβ rearrangement (Chen), which would not occur on the Jβ1DJβ allele, also may facilitate RAG accessibility and Vβ-to-DJβ rearrangement. Finally, it is conceivable that Dβ-to-Jβ rearrangements may occur asynchronously between alleles where RAG-cleavage activates DNA damage signals that prevent recombination events on the other allele. If so, Dβ-to-Jβ rearrangements that occur first on Jβ1ω alleles could inhibit Vβ-to-DJβ rearrangements on Jβ1DJβ alleles.
TCRβ genes are also regulated such that in-frame VβDJβ rearrangements form only on a single allele in the majority of developing thymocytes. Despite intense efforts, the manner by which Vβ rearrangements are restricted to one allele at a time is completely unknown; though evidence for both stochastic and directed control mechanisms has been provided (10, 42, 43). Our current observations that Vβ rearrangements occur at a similar level on Jβ1DJβ and Jβ1ω alleles in Jβ1DJβ/ω αβ T cell hybridomas and TCRβ allelic exclusion is maintained in Jβ1DJβ/DJβ mice have implications for the potential mechanisms that restrict Vβ rearrangement to one allele at a time. First, our data formally shows that Dβ-to-Jβ rearrangement per se is not required for mono-allelic assembly and expression of TCRβ genes, and TCRβ allelic exclusion is achieved exclusively through regulation of the Vβ-to-DJβ rearrangement step in developing αβ T cells. Second, within the context of stochastic models of TCRβ allelic exclusion that invoke low recombination efficiency of the Vβ rearrangement step as the underlying mechanism (10), our data demonstrate that deletion of 3'Dβ RSSs upon Dβ-to-Jβ rearrangement to relieve potential steric hindrance of 5'Dβ RSSs is not the sole rate-limiting mechanism that restricts Vβ rearrangements to only one allele at a time.
The assembly and expression of TCRβ genes and the selection of TCRβ chains associated with pTα molecules is required for the differentiation of DNIII thymocytes into DNIV and then DP cells (5). Expression of transgenic in-frame VβDJβ rearrangements leads to a reduction in the percentage of DNIII cells and a concomitant increase in the percentage of DNIV thymocytes, demonstrating that the assembly and expression of TCRβ chains is the rate-limiting step in early thymocyte development (44, 45). In this study, we demonstrate that Jβ1DJβ/ω and Jβ1DJβ/DJβ mice exhibit slight reductions in the frequency of DNIII thymocytes and concomitant increases in the frequency of DNIV cells. The magnitude of these changes corresponds to the number of Jβ1DJβ alleles, suggesting that the DNIII to DNIV transition and β-selection are slightly enhanced by the presence of the pre-assembled DJβ complex. The lack of a detectable increase in the level of overall Vβ-to-DJβ rearrangements on Jβ1DJβ alleles as compared to on Jβ1ω alleles in Jβ1DJβ/ω αβ T cell hybridomas suggests that VβDJβ rearrangements involving the particular DJβ join used in this study are better able to pair and/or signal with pTα chains than VβDJβ rearrangements involving the population of DJβ joins normally assembled on Jβ1ω alleles. However, we cannot rule out the possibility that increased rearrangement frequencies of particular Vβ segments to the pre-assembled DJβ1.1 complex on Jβ1DJβ alleles as compared to the Dβ1Jβ1 complexes of six Dβ-Jβ joining possibilities on Jβ1ω alleles in DNIII thymocytes contributes to this slightly "accelerated" early thymocyte development. In this regard, recombination efficiencies can be influenced by the nucleotide composition of coding sequences flanking participating RSSs (references). Unfortunately, firm conclusions require the accurate quantification of VβDJβ rearrangements in non-selected DNIII thymocytes, which is not possible due to the inherent biases of amplifying VβDβJβ1 rearrangements of one fixed size on Jβ1DJβ alleles versus of six different sizes on Jβ1ω alleles.
The generation and expression of a broad repertoire of antigen receptors on the surface of lymphocytes is critical for development and function of an effective adaptive immune system. For example, under representation of a particular Vκ segment (VκA2) in the peripheral Igκ repertoire of humans due to allelic polymorphisms is associated with an increased susceptibility to Haemophilus influenzae type b (Hib) since VκA2 segments are often used in anti-Hib antibodies (46). It has been known for quite some time that the Vβ repertoire assembled in DN thymocytes can be substantially shaped during αβ TCR selection in the DP thymocytes of mice expressing super-antigens (18–24). Our current observations that incorporation of the pre-assembled Dβ1Jβ1.1 complex in productive VβDJβ rearrangements affects αβ TCR selection of DP thymocytes expressing particular Vβs and the Vβ repertoire of peripheral αβ T cells demonstrates that the sequence of DJβ complexes assembled during thymocyte development can influence TCRβ chain selection and peripheral Vβ repertoire, even in the absence of super-antigens. In mice, restriction of endogenous Vα rearrangements to a single functional Jα segment substantially impairs positive selection of αβ TCR in DP thymocytes and leads to a marked reduction in peripheral αβ T cell numbers (Huang). Moreover, mice containing a single endogenous DH segment exhibit reduced numbers of bone marrow B cells and defective immune responses to a particular T-independent antigen (Schelonka). Furthermore, we previously demonstrated that the frequency of Vβ2 and Vβ14 rearrangements is reduced by the presence of other Vβ segments that compete with Vβ2 and Vβ14 for the productive coupling with DJβ1 complexes (Bassing). Thus, we hypothesize that the two Dβ-Jβ clusters and the six Jβ segments within each cluster may have evolved under selective pressure to ensure the most beneficial representation of Vβ segments expressed in the peripheral TCRβ repertoire of αβ T cells.
Acknowledgments
We thank Heikyung Suh and Megan Gleason for technical help and Brenna Brady for critical evaluation of the manuscript.
This work was supported by the National Institutes of Health Grant AI20047 (to F.W.A.) and the Department of Pathology and Laboratory Medicine and Center for Childhood Cancer Research of the Children's Hospital of Philadelphia (to C.H.B.). A.C.C. is supported by the Training Program in Immune System Development and Regulation at the University of Pennsylvania. C.H.B. is a Pew Scholar in the Biomedical Sciences. F.W.A. is an Investigator of the Howard Hughes Medical Institute
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. doi: 10.1016/s0092-8674(04)00039-x. [DOI] [PubMed] [Google Scholar]
- 4.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, Fischer A, Durandy A, de Villartay JP, Revy P. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 5.von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 6.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8-thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 8.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 9.Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–874. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- 10.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Alt FW, Yancopoulos GD, Blackwell TK, Wood C, Thomas E, Boss M, Coffman R, Rosenberg N, Tonegawa S, Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Ranganath S, Gleason M, Woodman BB, Borjeson TM, Alt FW, Bassing CH. Restriction of endogenous TCRbeta rearrangements to Vbeta14 through selective recombination signal sequence modifications. Proc Natl Acad Sci U S A. 2007;104:4002–4007. doi: 10.1073/pnas.0700081104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Bassing CH, Jung D, Woodman BB, Foy D, Alt FW. Dramatically increased rearrangement and peripheral representation of Vbeta14 driven by the 3'Dbeta1 recombination signal sequence. Immunity. 2003;18:75–85. doi: 10.1016/s1074-7613(02)00515-0. [DOI] [PubMed] [Google Scholar]
- 14.Posnett DN, Vissinga CS, Pambuccian C, Wei S, Robinson MA, Kostyu D, Concannon P. Level of human TCRBV3S1 (V beta 3) expression correlates with allelic polymorphism in the spacer region of the recombination signal sequence. J Exp Med. 1994;179:1707–1711. doi: 10.1084/jem.179.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassing CH, Alt FW, Hughes MM, D'Auteuil M, Wehrly TD, Woodman BB, Gartner F, White JM, Davidson L, Sleckman BP. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature. 2000;405:583–586. doi: 10.1038/35014635. [DOI] [PubMed] [Google Scholar]
- 16.Livak F, Burtrum DB, Rowen L, Schatz DG, Petrie HT. Genetic modulation of T cell receptor gene segment usage during somatic recombination. J Exp Med. 2000;192:1191–1196. doi: 10.1084/jem.192.8.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson A, Marechal C, MacDonald HR. Biased V beta usage in immature thymocytes is independent of DJ beta proximity and pT alpha pairing. J Immunol. 2001;166:51–57. doi: 10.4049/jimmunol.166.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Speiser DE, Kolb E, Schneider R, Pircher H, Hengartner H, MacDonald HR, Zinkernagel RM. Tolerance to Mlsa by clonal deletion of V beta 6+ T cells in bone marrow and thymus chimeras. Thymus. 1989;13:27–33. [PubMed] [Google Scholar]
- 19.MacDonald HR, Lees RK, Schneider R, Zinkernagel RM, Hengartner H. Positive selection of CD4+ thymocytes controlled by MHC class II gene products. Nature. 1988;336:471–473. doi: 10.1038/336471a0. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald HR, Schneider R, Lees RK, Howe RC, Acha-Orbea H, Festenstein H, Zinkernagel RM, Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332:40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 21.Wade T, Bill J, Marrack PC, Palmer E, Kappler JW. Molecular basis for the nonexpression of V beta 17 in some strains of mice. J Immunol. 1988;141:2165–2167. [PubMed] [Google Scholar]
- 22.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332:35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 23.Blackman MA, Marrack P, Kappler J. Influence of the major histocompatibility complex on positive thymic selection of V beta 17a+ T cells. Science. 1989;244:214–217. doi: 10.1126/science.2784868. [DOI] [PubMed] [Google Scholar]
- 24.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 25.Yassai M, Ammon K, Goverman J, Marrack P, Naumov Y, Gorski J. A molecular marker for thymocyte-positive selection: selection of CD4 single-positive thymocytes with shorter TCRB CDR3 during T cell development. J Immunol. 2002;168:3801–3807. doi: 10.4049/jimmunol.168.8.3801. [DOI] [PubMed] [Google Scholar]
- 26.Yassai M, Gorski J. Thymocyte maturation: selection for in-frame TCR alpha-chain rearrangement is followed by selection for shorter TCR beta-chain complementarity-determining region 3. J Immunol. 2000;165:3706–3712. doi: 10.4049/jimmunol.165.7.3706. [DOI] [PubMed] [Google Scholar]
- 27.Hughes MM, Yassai M, Sedy JR, Wehrly TD, Huang CY, Kanagawa O, Gorski J, Sleckman BP. T cell receptor CDR3 loop length repertoire is determined primarily by features of the V(D)J recombination reaction. Eur J Immunol. 2003;33:1568–1575. doi: 10.1002/eji.200323961. [DOI] [PubMed] [Google Scholar]
- 28.Sleckman BP, Khor B, Monroe R, Alt FW. Assembly of productive T cell receptor delta variable region genes exhibits allelic inclusion [In Process Citation] J Exp Med. 1998;188:1465–1471. doi: 10.1084/jem.188.8.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorman JR, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt FW. The Ig(kappa) enhancer influences the ratio of Ig(kappa) versus Ig(lambda) B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 30.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR alpha enhancer in alphabeta and gammadelta T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 32.Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter AC, Yang-Iott K, Hochedlinger K, Jaenisch R, Bassing CH. TCRb mediated feedback regulation prevents the coupling of accessible Vb14 segments and DJb complexes. Manuscript in Preperation 2008 [Google Scholar]
- 34.White J, Blackman M, Bill J, Kappler J, Marrack P, Gold DP, Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989;143:1822–1825. [PubMed] [Google Scholar]
- 35.Davodeau F, Peyrat MA, Romagne F, Necker A, Hallet MM, Vie H, Bonneville M. Dual T cell receptor beta chain expression on human T lymphocytes. J Exp Med. 1995;181:1391–1398. doi: 10.1084/jem.181.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 37.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 38.Ranganath S, Carpenter AC, Gleason M, Shaw AC, Bassing CH, Alt FW. Productive coupling of accessible Vbeta14 segments and DJbeta complexes determines the frequency of Vbeta14 rearrangement. J Immunol. 2008;180:2339–2346. doi: 10.4049/jimmunol.180.4.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourigny MR, Mazel S, Burtrum DB, Petrie HT. T cell receptor (TCR)-beta gene recombination: dissociation from cell cycle regulation and developmental progression during T cell ontogeny. J Exp Med. 1997;185:1549–1556. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Xiao G, Zhang Y, Wen X, Gao X, Okada S, Liu X. Regulation of Tcrb recombination ordering by c-Fos-dependent RAG deposition. Nat Immunol. 2008;9:794–801. doi: 10.1038/ni.1614. [DOI] [PubMed] [Google Scholar]
- 41.Sleckman BP, Bassing CH, Hughes MM, Okada A, D'Auteuil M, Wehrly TD, Woodman BB, Davidson L, Chen J, Alt FW. Mechanisms that direct ordered assembly of T cell receptor beta locus V, D, and J gene segments. Proc Natl Acad Sci U S A. 2000;97:7975–7980. doi: 10.1073/pnas.130190597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skok JA, Gisler R, Novatchkova M, Farmer D, de Laat W, Busslinger M. Reversible contraction by looping of the Tcra and Tcrb loci in rearranging thymocytes. Nat Immunol. 2007;8:378–387. doi: 10.1038/ni1448. [DOI] [PubMed] [Google Scholar]
- 43.Schlimgen RJ, Reddy KL, Singh H, Krangel MS. Initiation of allelic exclusion by stochastic interaction of Tcrb alleles with repressive nuclear compartments. Nat Immunol. 2008;9:802–809. doi: 10.1038/ni.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serwold T, Hochedlinger K, Inlay MA, Jaenisch R, Weissman IL. Early TCR expression and aberrant T cell development in mice with endogenous prerearranged T cell receptor genes. J Immunol. 2007;179:928–938. doi: 10.4049/jimmunol.179.2.928. [DOI] [PubMed] [Google Scholar]
- 45.Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR alpha expression critically influences T cell development and selection. J Exp Med. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feeney AJ, Atkinson MJ, Cowan MJ, Escuro G, Lugo G. A defective Vkappa A2 allele in Navajos which may play a role in increased susceptibility to haemophilus influenzae type b disease. J Clin Invest. 1996;97:2277–2282. doi: 10.1172/JCI118669. [DOI] [PMC free article] [PubMed] [Google Scholar]