Abstract
Depot medroxyprogesterone acetate (Depo-Provera) has been associated with an increased risk of HIV acquisition. In a longitudinal study, we investigated the impact of Depo-Provera use by healthy women on expression of immune markers for HIV preference and on HIV infection ex vivo at baseline (visit 1), one month (visit 2) and three months (visit 3) after Depo-Provera treatment. We found a significant increase in the frequency and expression of integrin α4β7 on CD4+ T cells at visit 2. Interestingly, Hispanic but not black women exhibited a significant increase in integrin α4β7 cell numbers and expression levels at visit 2, whereas, black but not Hispanic women exhibited a significant change in CCR5 and CD38 expression levels between visit 2 and visit 3. The frequency of terminal effector memory CD4+ T cells decreased significantly in black women from visit 1 to visit 3. Virus production following ex vivo HIV infection of PBMCs was increased at visit 3 compared to visit 1. In black women, the frequency of HIV p24+CD4+ T cells was higher at visit 3 than at visit 1. Expression of integrin α4β7 on HIV p24+CD4+ T cells following ex vivo infection at visit 2 was significantly less than at visit 1. These results demonstrate that Depo-Provera alters the immune profile of peripheral CD4+ T cells and increases susceptibility to HIV infection ex vivo. The observation that these effects differed between women of different ethnicities has implications for developing effective and targeted strategies for HIV prevention.
Introduction
More than 210 million women worldwide used hormonal contraception in 2015 (1). For decades, a putative, adverse impact of hormonal contraception on HIV acquisition and transmission has been a concern, but results on this subject have remained controversial (2–12). Several studies indicate that use of Depo-Provera (depot medroxyprogesterone acetate, or MPA), an injectable form of hormonal contraceptive used by 35 million women globally, is associated with heightened risk of HIV acquisition and transmission (4, 6, 7, 9, 11, 13, 14). Two recent meta-analyses of multiple studies with stringent inclusion criteria indicate an increased risk of HIV acquisition in Depo-Provera users with an adjusted hazard ratio of 1.4–1.5 compared to subjects not using hormonal contraception (15, 16). Furthermore, an updated meta-analysis by Polis et al. analyzing high power studies published between 2014 and 2016 found a hazard ratio of 1.4 in women using Depo-Provera, compared to non-hormonal contraceptive controls (17). Despite these findings, Wall and colleagues report Depo-Provera use resulted in no increased risk of HIV acquisition among HIV-negative women with their HIV-positive partners or among HIV-positive women with HIV-negative men in Zambian discordant couples (18, 19). The possibility that hormonal contraceptive use increases risk of HIV acquisition has profound implications for family planning polices, particularly in countries with high rates of HIV transmission. Thus, understanding how Depo-Provera modulates immune responses and affects HIV infection is critical for developing strategies for HIV prevention as well as for shaping policies for preventing unintended pregnancy.
Depo-Provera is known to modulate immune responses that can impact HIV susceptibility or infection (reviewed in (9)). The synthetic progestin MPA has a high affinity for the progesterone receptor but has low affinity interactions with other steroid receptors including glucocorticoid, androgen, and mineralocorticoid receptors (20–23). MPA is thought to exhibit an immunosuppressive effect through its interaction with the glucocorticoid receptor (24). MPA but not progesterone (P4) suppresses cytokine production in T cell receptor (TCR)-stimulated PBMCs and in toll-like receptor (TLR)-stimulated plasmacytoid dendritic cells (pDCs) (25, 26). MPA blocks down-regulation of CXCR4 and CCR5 in PBMCs in response to TCR activation (25, 26). HIV replication is increased by MPA in activated CD8-depleted PBMCs or CD4+ T cells in vitro (25, 26). In contrast to MPA, progesterone (P4) has been reported to down-regulate CXCR4 and CCR5 in PBMCs in vitro, which is associated with reduced susceptibility to HIV (27). These results indicate that MPA and P4 mediate distinct immune functions despite the fact that both bind the progesterone receptor, and illustrate the complexity of the role of hormonal contraceptives in immune regulation.
The immunomodulatory effects of Depo-Provera on peripheral blood cells in women has been studied to a limited extent. One cross-sectional study showed no significant difference in the percentage of CCR5+CD8+ or CCR5+CD4+ T cells, but found a significant increase in expression (mean fluorescent intensity, MFI) of CCR5 on CD4+ T cells of Depo-Provera users compared to women not using hormonal contraception (28). TLR-9 stimulation of pDCs resulted in reduced production of TNFα and IFNα in Depo-Provera users compared to women not using hormonal contraception (29). Depo-Provera users also had decreased plasma levels of IFNα and IL-8 but no change in T cell subsets compared to non-hormonal contraception users (29). In HIV-infected women on cART (median age of 32) no significant changes in cell-mediated immunity or plasma cytokine levels were observed except for a small, albeit significant, decrease in TGFβ (30). A significant decrease in the frequency of CD4+CD25+ cells and a nearly significant decrease in CD8+CD38+HLADR+ cells were found in this population 3 months after Depo-Provera injection, whereas an increased frequency of CD8+ Foxp3 (Treg) cells was observed 4 weeks after Depo-Provera administration (30).
Here, we have examined changes in immunological markers associated with preferential HIV infection of CD4+ T cells, including integrin α4β7, CCR5 (HIV co-receptor), and CD38 (activation), and have characterized CD4+ T cell subsets in PBMCs from women before (visit 1), one month following (visit 2), and three months following (visit 3) Depo-Provera injection. The three month schedule was chosen because serum or plasma MPA concentrations are known to decrease over time and return to near baseline three months after Depo-Provera injection (31, 32). At the time of each visit, we also assessed the susceptibility of freshly isolated PBMCs to HIV infection ex vivo, and measured HIV production and immunological markers on HIV p24+ cells. Our findings indicated that Depo-Provera treatment modified immune profiles of CD4+ T cells and increased HIV infection ex vivo. Of possible importance for clinical treatment of patients, we also observed that changes in the immune response and in susceptibility to HIV infection following Depo-Provera injection were influenced by ethnicity and duration of treatment.
Materials and Methods
Study design
These studies were approved by Rutgers, New Jersey Medical School Institutional Review Board. Participants were women receiving healthcare at Rutgers, New Jersey Medical School clinics located in Newark, NJ. Women who desired to use Depo-Provera, who met the primary screening criteria as described below, and were willing to sign the informed consent form were recruited and compensated. The enrollment eligibility criteria were: 1) healthy, non-pregnant women aged 18–35; 2) no known or suspected HIV infection; 3) no use of hormonal contraception in the previous 2 months; 4) negative for chlamydia, gonorrhea, syphilis, genital herpes, bacterial vaginosis, trichomonas, presence of condyloma accuminata, carcinoma in situ of the cervix or invasive cervical cancer; 5) no known or suspected immunosuppressive illness or condition; 6) no sexual intercourse for 3 days prior to the enrollment visit. Eligibility was ascertained at the enrollment visit. Potential participants not fulfilling all inclusion criteria were excluded.
Blood was collected before Depo-Provera injection (visit 1), one month following (visit 2), and three months following the initial Depo-Provera injection (visit 3). PBMCs were isolated immediately after blood collection by Histopaque®-1077 gradient centrifugation. Immune phenotypes of freshly isolated PBMCs were determined after isolation. PBMCs were exposed to HIV and maintained in RPMI-1640 with 10% heat-inactivated FBS and IL-2 (50 IU/mL). IL-2 was supplemented to cell cultures every 2 days.
Reagents
Histopaque®-1077, fetal bovine serum (FBS), human AB serum, RPMI-1640 medium, and PBS were from Sigma-Aldrich. Human IL-2 was from R&D systems. V500-conjugated mouse IgG1κ anti-human CD45 (clone HI30), Alexa Fluor® 700-conjugated mouse IgG2a anti-human CD197 (CCR7) (clone 150503), APC-Cy™7-conjugated mouse IgG2aκ anti-human CD195 (CCR5) (clone 2D7/CCR5), APC-conjugated mouse IgG2bκ anti-human CD45RA (clone HI100), Alexa Fluor® 700-conjugated mouse IgG2aκ isotype control (clone G155-178), APC-Cy™7-conjugated mouse IgG2aκ isotype control (clone G155-178), and anti-mouse Ig BD™ CompBead set were from BD Biosciences. PerCP-conjugated mouse IgG1κ anti-human CD4 (clone RPA-T4), PE/Cy7-conjugated mouse IgG1κ anti-human CD38 (clone HIT2), Brilliant Violet 605™-conjugated mouse IgG2aκ anti-human CD3 (clone OKT3), PE-conjugated mouse IgG1κ isotype control (clone MOPC-21), PE/Cy7-conjugated mouse IgG1κ isotype control (clone MOPC-21), and APC-conjugated mouse IgG2bκ isotype control (clone MPC-11) were from BioLegend. PE-conjugated mouse IgG1 anti-HIV-1 p24 core antigen (clone KC57) was from Beckman Coulter. FITC-conjugated chicken anti-mouse IgG secondary antibody was from Santa Cruz Biotechnology. Integrin α4β7 monoclonal antibody (Act-1, cat# 11718) was obtained from Dr. A. A. Ansari via the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
HIV-1 infection
Freshly isolated PBMCs (5 × 106 cells) were exposed to HIV-1BaL (Advanced Biotechnologies Inc.) at a multiplicity of infection (MOI) of 0.01 overnight at 37°C in the presence of IL-2. Unbound virus was washed off, and cells were plated at a density of 1 × 106 cells/ml. Cell culture supernatant was collected 7 days after viral exposure, and the level of HIV-1 p24 was measured using the AlphaLISA HIV p24 kit (PerkinElmer). AlphaLISA plates were read using a 2300 EnSpire Multilabel Plate Reader (PerkinElmer).
Flow cytometry
The frequency (the number of cells in a total population) and the mean fluorescent intensity (MFI) for the level of protein expression on cell surface were determined by flow cytometry. Live cells were selected using the Zombie UV fixable viability kit from BioLegend. Following live/dead staining, cells were blocked in wash buffer (PBS, 2% FBS, 1% human AB serum) for 20 minutes at 4°C. Cells were stained with fluorochrome-conjugated antibodies against various cell surface markers or their appropriate isotype controls in wash buffer for 20 minutes at 4°C. Cells were washed and fixed with 2% paraformaldehyde in PBS. For analysis of HIV-1 p24 positive cells, after cell surface marker staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm™ (BD Biosciences) for 20 minutes at 4°C. Cells were washed in BD Perm/Wash™ buffer (BD Biosciences) and stained with HIV-1 p24 antibody or isotype control antibody for 30 minutes at 4°C in BD Perm/Wash™ buffer. Cells were washed and fixed with 2% paraformaldehyde in PBS. Samples were analyzed using a BD LSR II (BD Biosciences). Results were analyzed with FlowJo (Tree Star Inc.).
During analysis lymphocytes were selected based on forward scatter versus side scatter. Dead cells were excluded from analysis. CD4+ T cells were identified as CD3+ and CD4+. Within the CD4+ T cell population, expression of surface markers integrin α4β7, CCR5, CD38, CCR7, CD45RA, and intracellular p24 were analyzed as we described previously (33).
Statistical analysis
Wilcoxon matched-pairs signed rank test was used to compare values between study visits. Specifically, this method analyzed the difference in immune parameters or HIV infection within the same individual at different visits. Mann-Whitney test was used to compare differences between uninfected and cells exposed to HIV-1BaL. Spearman correlation test was used to compare HIV infection (p24 levels or the p24+ cells) and surface markers expression at each visit. Statistical analysis of the immune phenotypes and correlation analysis was performed using GraphPad Prism version 7.0b (GraphPad Software, Inc.). Regression analysis of log-transformed data of HIV p24 production was conducted using generalized estimating equations (GEE) to account for the longitudinal nature of data collected from samples at baseline, one, and three months after treatment. The GEE model of log 10-transformed p24 values was implemented with an AR correlation structure that assumes that correlations between observations on the same subject decline with time (34). Re-analysis of the data using other correlation structures (e.g., exchangeable) produced similar results.
Results
Subject Characteristics
A total of 39 healthy subjects meeting the eligibility criteria were enrolled in the study. Among these, 29 participants returned for all visits, 6 participants returned only for visit 2, and 4 participants returned only for visit 3. As expected based on the demographic characteristics of the recruitment clinical site in Newark, all subjects were black or Hispanic. Median age was 26 (interquartile range (IQR), 21.5–29.5); 15 women were Hispanic (median age of 25, IQR 22–31), and 24 women were black (median age of 26.5, IQR 21.75–28.25). All subjects did not use any form of hormonal contraception including Depo-Provera for up to 10 months at the time of recruitment.
Expression of integrin α4β7, CCR5, and CD38 on peripheral CD4+ T cells from women pre- and post-Depo-Provera injection
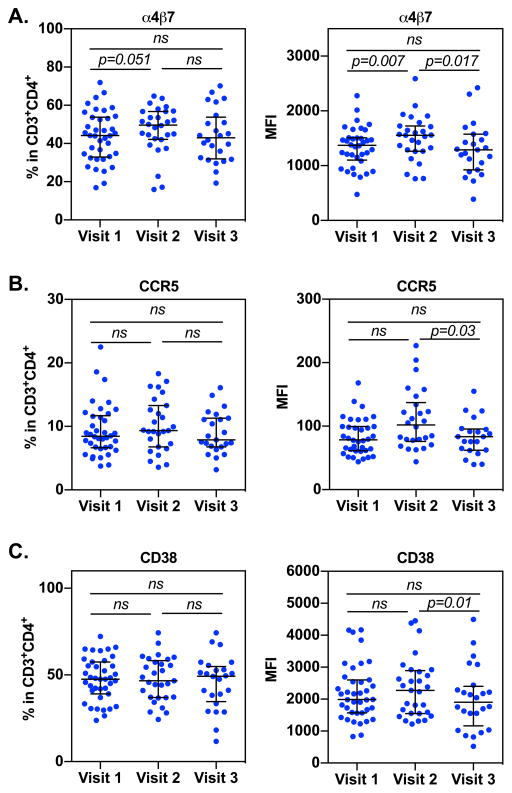
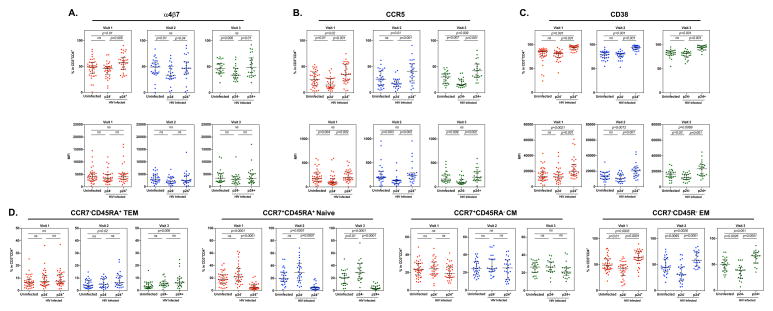
We first determined whether Depo-Provera injection resulted in changes in the cell surface expression of integrin α4β7, CCR5, and CD38, markers that are associated with preference for HIV infection in the CD4+ T cell population. The gating strategy was shown in Supplementary Fig 1. Participant peripheral blood CD4+ T cells were analyzed before (visit 1), one month following (visit 2), and three months following (visit 3) Depo-Provera injection. There was an increase in the frequency of integrin α4β7+CD4+ T cells at visit 2 (median 49.7%, IQR 42.37%–56.8%) compared to baseline (visit 1, median 44.15%, IQR 33.47%–54.55%) (Fig 1A left). There was no significant difference in the frequency of integrin α4β7+CD4+ T cells between visit 2 and visit 3 (visit 3, median 42.95%, IQR 32.37%–51.95%) or between visit 1 and visit 3. Results of analysis of MFI indicated a significant increase in expression of integrin α4β7+ on CD4+ T cells at visit 2 (median 1554, IQR 1278–1718) compared to baseline (visit 1, median 1363, IQR 1136.5–1504.5; Fig 1A right). At visit 3, there was a significant decrease in the expression of integrin α4β7 on CD4+ T cells (median 1290, IQR 988–1514) compared to visit 2, but there was no significant difference in the expression of integrin α4β7 at visit 3 compared to visit 1.
Figure 1. Depo-Provera altered the expression of cell surface markers associated with HIV susceptibility.
PBMCs from subjects before and after Depo-Provera injection were isolated and the percentage and MFI of integrin α4β7 (A), HIV co-receptor CCR5 (B), and activation marker CD38 (C) were analyzed within the CD3+CD4+ T cell population by flow cytometry. Wilcoxon matched-pairs signed rank test was used to compare values from before Depo-Provera (visit 1), one month after Depo-Provera (visit 2), and 3 months after Depo-Provera (visit 3). Each dot represents one donor. Bars represent median and interquartile range. p≤0.05 was considered significant, p>0.05 was not significant (ns).
The frequency of CCR5+ CD4+ T cells at visit 2 (median 9.33%, IQR 7.03%–12.95%) was slightly, albeit not significantly, increased compared to baseline (visit 1, median 8.24%, IQR 6.69%–11.55%) (Fig 1B, left). A similar increase in CCR5 expression from visit 1 to visit 2 was observed (visit 1, median 79.5, IQR 61.67–99.32; visit 2, median 101.8, IQR 78.2–131.24) (Fig 1B right). There was a measurable but nonsignificant decrease in the frequency of CCR5+CD4+ T cells at visit 3 (median 7.88%, IQR 6.84%–11.2%) compared to visit 2. We found a significant decrease in CCR5 MFI at visit 3 (median 83.3, IQR 62.5–94.7) compared to visit 2 (median 101.8, IQR 78.2–131.24) but not compared to visit 1.
There was no difference in the frequency of CD38+CD4+ T cells between visit 1 (median 47.6%, IQR 40.05%–54.4%) and visit 2 (median 46.7%, IQR 37.1%–57.4%). An observable but nonsignificant increase in intensity of CD38 expression on CD4+ T cells was found at visit 2 (median 2272, IQR 1553–2857) compared to visit 1 (median 1987, IQR 1577.5–2574.5) (Fig 1C). There was a slight but nonsignificant increase in the frequency of CD38+CD4+ T cells at visit 3 (median 49.3%, IQR 37.1%–53.63%) compared to visit 2. There was a significant decrease in the intensity of CD38 expression at visit 3 (median 1906, IQR 1423–2323.25) compared to visit 2. There was no significant difference in either frequency or intensity of CD38 at visit 3 compared to visit 1.
Differential immune phenotypes in blacks and Hispanics in response to Depo-Provera
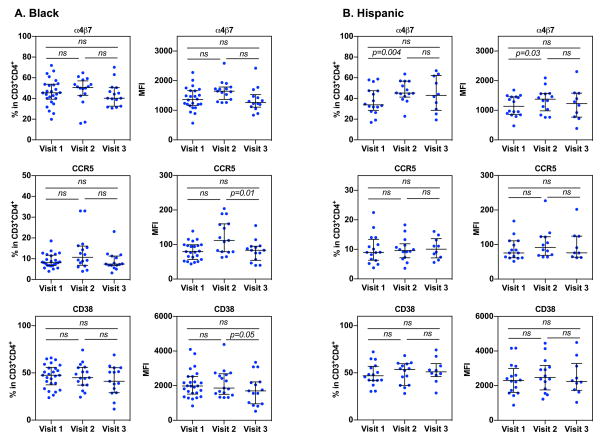
Interestingly, we observed subtle differences in immune phenotypes between black and Hispanic women following Depo-Provera injection. In black women, there was a non-significant increase in the frequency and intensity of integrin α4β7 at visit 2 (median 51.3%, IQR 43.55%–55.8%; median MFI 1636, IQR 1408.6–1771.25) compared to visit 1 (median 45.6%, IQR 40.2%–53.7%; median MFI 1382, IQR 1202–1659.5) (Fig 2A). There was a decline in the frequency and intensity of integrin α4β7 at visit 3 (median 42.24%, IQR 34.15%–49.55%; median MFI 1303, IQR 1123–1472) compared to visit 2. We observed similar patterns of CCR5 expression on CD4+ T cells from black women following Depo-Provera injection compared to all donors combined, with an increase in the frequency and intensity of these markers at visit 2 (median 9.31%, IQR 6.87%–13.88%; median MFI 110, IQR 79.15–155.25) followed by a decline to baseline levels (median 8.24%, IQR 6.79%–11.55%; median MFI 79.5, IQR 60.15–99.35) at visit 3 (median 7.5%, IQR 6.81%–11.07%; median MFI 83.5, IQR 56.9–94.7). In black women, only the change in intensity of CCR5 between visit 2 and visit 3 was significant (p<0.05). There was a decrease in CD38 expression in black women after Depo-Provera injection (visit 1, median 47.6%, IQR 38.8%–55.1%; median MFI 1978, IQR 1565–2214) with a significant difference in CD38 intensity between visit 2 (median 44.65%, IQR 37.18%–55.68%; median MFI 1833, IQR 1525–2574.75) and visit 3 (median 40.05%, IQR 30.13%–51.25%; median MFI 1627.5, IQR 969.25–2142.75).
Figure 2. Immune phenotypes of CD4+ T cells by Depo-Provera differs in black and Hispanic women.
The percentage and MFI of cell surface markers integrin α4β7, CCR5, and CD38 on CD3+CD4+ T cells from freshly isolated PBMCS was analyzed within patients who identified as either black (A) or Hispanic (B) by flow cytometry. Each dot represents one donor. Bars represent median and interquartile range. Wilcoxon matched-pairs signed rank test was used for analysis. p≤0.05 was considered significant, p>0.05 was not significant (ns).
In contrast to black women, Hispanic women had significantly higher frequency and expression levels of integrin α4β7 at visit 2 (median 45.4%, IQR 42%–56.8%; median MFI 1427, IQR 1030–1560) compared to visit 1 (median 34.6%, IQR 29.6%–47.4%; median MFI 1295, IQR 872.5–1455) (Fig 2B). There was a non-significant decrease in the frequency of integrin α4β7+CD4+ T cells at visit 3 (median 42.95%, IQR 31.2%–59.98%; median MFI 1228.5, IQR 818.5–1530.75) compared to visit 2, although the percentage at visit 3 was higher than baseline. The increase in the frequency of CCR5+CD4+ T cells in response to Depo-Provera was less pronounced in Hispanic women (visit 1, median 8.99%, IQR 6.45%–11.25; visit 2, median 9.33%, IQR 7.29%–11.4%, and visit 3, median 10.08%, IQR 7.63%–12.67%). Unlike the significant change in CCR5 expression observed in black women, there was a slight but not significant increase in CCR5 expression at visit 2 (median 91.8, IQR 70.05–122.25) compared to visit 1 (median 79.85, IQR 62.5–107.87) and visit 3 (median 75.8, IQR 64.30–99.18). Interestingly, there was a non-significant increase in the frequency of CD38+CD4+ T cells after Depo-Provera injection (visit 1, median 48.1%, IQR 42.15%–56.4%; visit 2, median 52.5%, IQR 36.8%–59.5%; visit 3, median 51.05%, IQR 47.97%–56.22%). There was no significant difference in the intensity of CD38 expression in Hispanic women before or after Depo-Provera injection (visit 1, median 2322, IQR 1815.5–2863; visit 2, median 2563, IQR 1821–3073; visit 3, median 2244, IQR 1859–2953.25).
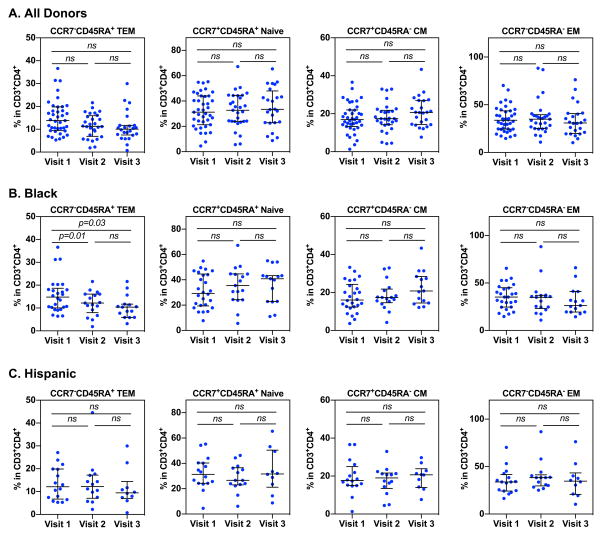
The impact of Depo-Provera on CD4+ T cell subsets including naïve (CCR7+CD45RA+), central memory (CM) (CCR7+CD45RA−), effector memory (EM) (CCR7−CD45R−), and terminal effector memory (TEM) (CCR7−CD45RA+) cells was also analyzed at each visit (Fig 3A). There was no significant change in the percentage of naïve, CM, EM or TEM subsets between visits, but there was a noticeable albeit non-significant decrease in the TEM subset from visit 1 to visit 3 (visit 1, median 13.8% IQR 9.86%–18.95%; visit 2, median 11.4% IQR 7.51%–16.1%; visit 3 median 10.06%, IQR 7.64%–11.7%) and an increase in the CM subset between visit 2 and visit 3 (visit 1, median 16.9%, IQR 13.65%–21.95%; visit 2, median 17.4%, IQR 14.4%–21.3%; visit 3, median 20.7%, IQR 14.45%-26.93%).
Figure 3. Profiles of CD4+ T cell subsets in women before and after Depo-Provera injection.
PBMCs from study participants were isolated and T cell subsets within the CD3+CD4+ T cell population were analyzed by flow cytometry. T cell subsets were analyzed based on the following phenotypes: terminal effector memory (TEM, CD45RA+CCR7−), naïve (CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), and effector memory (EM, CD45RA−CCR7−). Wilcoxon matched-pairs signed rank test was used to compare differences between study visits of all donors (A), donors who identified as black (B), and donors who identified as Hispanic (C). Each dot represents one donor. Bars represent median and interquartile range. p≤0.05 was considered significant, p>0.05 was not significant (ns).
The percentage of TEM cells in black participants significantly decreased from visit 1 (13.65% median, IQR 10.32%–13.5%) to visit 2 (12.2% median, IQR 7.98%–11.65%) and from visit 1 to visit 3 (10.5% median, IQR 7.94%–10.4%) (Fig 3B). There was a noticeable nonsignificant increase in the naïve and CM subsets in black women (naïve visit 1, median 30%, IQR 20.3%–44%; visit 2, median 37.3%, IQR 24.35%–44.63; visit 3, median 40.35%, IQR 22.93%–43.13%) (CM visit 1, median 16.3%, IQR 12.6%–23.6%; visit 2, median 17.05%, IQR 14.98%–21.4%; visit 3, median 20.6%, IQR 14.8%–27.28%). Analysis of the Hispanic patient group showed no significant change in CD4+ T cell subsets between visits despite a non-significant decrease in the frequency of TEM cells after Depo-Provera injection (TEM visit 1, median 13%, IQR 7.72%–19.85%; visit 2, median 10.85%, IQR 7.07%–15.08%; visit 3, median 9.47%, IQR 7.54%–11.48%) (Fig 3C).
Impact of Depo-Provera on susceptibility of PBMCs to HIV-1 ex vivo
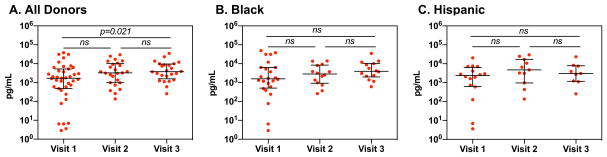
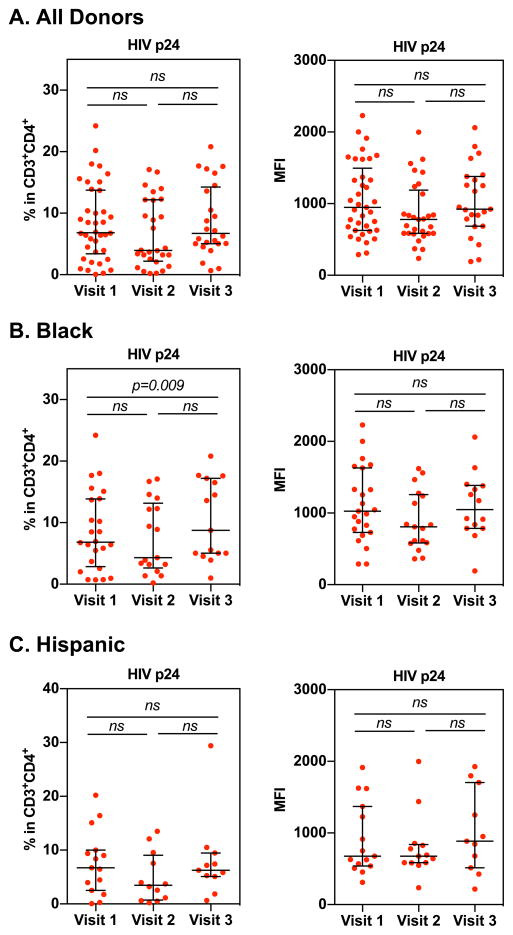
To determine whether Depo-Provera increased HIV infection of PBMCs ex vivo, freshly isolated PBMCs were exposed to HIV-1BaL at MOI 0.01. HIV production was determined by measuring HIV p24 levels in cell culture medium, as well as by flow cytometric analysis of the frequency and intensity of CD4+ T cells expressing HIV p24. There was a significant increase in HIV production in PBMCs at visit 3 compared to visit 1 (visit 1, median 1,736.97 pg/mL, IQR 593.79–4,950.55 pg/mL; visit 2, median 3,190.95 pg/mL, IQR 980.97–8,382.31, and visit 3, median 3,683.52 pg/mL, IQR 1,780.62–8,459.79 pg/mL) (Fig 4A). There was no significant difference in HIV p24 levels at day 7 between different visits among either black or Hispanic participants (Fig 4B and Fig 4C).
Figure 4. Depo-Provera significantly increases HIV-1BaL virus production in PBMCs ex vivo.
PBMCs from study participants were exposed to HIV-1BaL (MOI 0.01) overnight. Cells were washed the following day to remove unbound virus. Culture supernatant from infected cells was collected 7 days after viral exposure and the level of HIV-1 p24 release in the supernatant was measured by HIV p24 AlphaLISA. PBMCs were cultured with IL-2 (50 IU/mL) and IL-2 was supplemented to cell culture media every 2 days. Wilcoxon matched-pairs signed rank test was used to compare differences between study visits of all donors (A), donors who identified as black (B), and donors who identified as Hispanic (C). Each dot represents one donor. Bars represent median and interquartile range. p≤0.05 was considered significant, p>0.05 was not significant (ns).
The number of infected CD4+ T cells and the intensity of HIV p24+ staining measured on day 10 after HIV infection was lower, albeit non-significantly, at visit 2 than at visit 1 or visit 3 (visit 1, median 6.92%, IQR 3.82%–13.8%, MFI median 967.5, IQR 640.75–1557-5; visit 2, median 3.75%, IQR 2.54%–12.2%, MFI median 779, IQR 586–1136; visit 3, median 7.16%, IQR 5.06%–14.5%, MFI median 915, IQR 785–1401) (Fig 5A). The slight decrease in HIV p24+ cells at visit 2 was not associated with an increase in cell death (Supplementary Fig 2).
Figure 5. Effect of Depo-Provera on the susceptibility to HIV ex vivo.
PBMCs from study participants were exposed to HIV-1BaL (MOI 0.01) overnight. Cells were washed the following day to remove virus. The percentage of HIV p24+CD4+ T cells was determined by flow cytometry at day 10 after infection as described in methods. Wilcoxon matched-pairs signed rank test was used to compare differences between study visits of all donors (A), donors who identified as black (B), or Hispanic (C). Each dot represents one donor. Bars represent median and interquartile range. p≤0.05 was considered significant, p>0.05 was not significant (ns).
We found a significant increase in the frequency of HIV p24+ cells in black subjects at visit 3 (median 11.17% median, IQR 5.03%–17.02%) compared to visit 1 (median 6.84%, IQR 3.68%–10.4%). The intensity of HIV p24 expression at visit 3 in black patients was observably but not significantly higher (median 1173, IQR 840–1380) than at visit 1 (median 1035.5, IQR 764–1411) (Fig 5B). The pattern of the HIV p24+ cell frequency among different visits in Hispanic women was comparable to black women, but unlike the results in black women, the differences were not significant (Fig 5C). There was no difference in the intensity of HIV p24 expression among 3 visits in Hispanic women. These findings suggest that ethnicity plays a role in immune modulation in response to Depo-Provera that subsequently impacts HIV susceptibility.
Effect of Depo-Provera on immunological markers for HIV preference
We next determined whether Depo-Provera altered markers for HIV preference and CD4+ T cell subsets in HIV-infected cells. Prior to Depo-Provera injection (visit 1), the HIV p24+ population had a significantly higher percentage of integrin α4β7 (57.7% median, IQR 45.2%–66.6%) compared to the p24− population in HIV-infected cells (46.7% median, IQR 38.1%–50.3%) or uninfected cells (49% median, IQR 38.1%–57.8%) (Fig 6A). HIV p24+ cell population also had a higher percentage of CCR5+ cells at visit 1 (35% median, IQR 22.8%–53.8%) compared to the p24− population (14.8% median, IQR 9.17%–27.1%) or uninfected cells (25.9% median, IQR 14.7%–38.2%) (Fig 6B). The frequency of CD38+ cells was also higher in the p24+ population (95% median, IQR 90.75%–97.2%) than in the p24− cells (81.5% median, IQR 73.65%–84.45%) or uninfected cells (85.2% median, IQR 76.5%–87.4%) (Fig 6C). HIV p24+ population was mainly found in the EM subset (63.9% median, IQR 57.9%–74.8%) followed by CM subset (18.9% median, IQR 14.8%–26.1%). TEM cells comprised 7.56% of p24+ cells (median, IQR 5.45%–12.3%) and naïve cells were the least abundant (median 4.68%, IQR 2.52%–8.71%) (Fig 6D).
Figure 6. Immunological phenotypes of CD4+ T cells preferentially infected by HIV.
PBMCs exposed to HIV-1BaL (MOI 0.01) were cultured for 10 days. In HIV infected PBMCs, cell surface marker expression of integrin α4β7, CCR5, and CD38 cell surface marker expression (A–C) and T cell subsets (D) were measured on HIV p24−CD4+ T cells and HIV p24+CD4+ T cells. Uninfected cells were included as a comparison. Each dot represents one donor. Bars represent median and interquartile range. The Mann-Whitney test was used to compare cell surface marker expression between uninfected, HIV p24− or HIV p24+CD4+ T cells. p≤0.05 was considered significant, p>0.05 was not significant (ns).
Similar profiles of HIV preference were observed in PBMCs after Depo-Provera treatment at visit 2 and at visit 3. The HIV p24+ cell population had a higher frequency of cells expressing integrin α4β7, CCR5, and CD38 (Fig 6) than the HIV p24− population in infected or uninfected PBMCs.
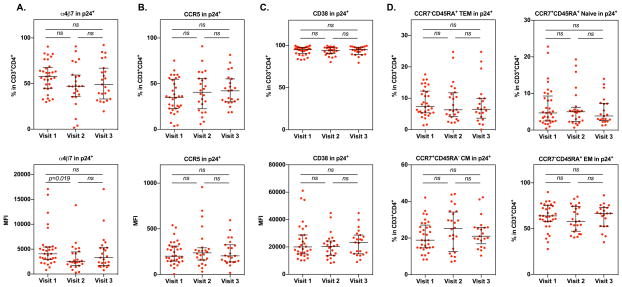
Analysis of markers for HIV preference and CD4+ T cell subsets within HIV p24+ cells from PBMCs at different visits revealed a slight, but not significant, decrease in the frequency of integrin α4β7+ cells at visit 2 (median 47.06%, IQR 37.35%–58.5%) and visit 3 (median 49%, IQR 36%–64.95%) compared to visit 1 (median 55.7%, IQR 45.2%–66.6%) (Fig 7A). The intensity of integrin α4β7+ in HIV p24+CD4+ T cells was significantly reduced from visit 1 (median 4062, IQR 2992.5–5421.5) to visit 2 (median 2470, IQR 1876.75–4248.25). There was a slight increase in CCR5+ cells in HIV p24+ cells after Depo-Provera treatment (visit 1, median 35%, IQR 22.8%–53.8%; visit 2, median 40.65%; IQR 25.92%–55.6%; visit 3, median 42.1%, 30.35%–52.95%) although the difference was not significant (Fig 7B). There was no significant change in CD38+ cells within the HIV p24+ population among different visits (visit 1, median 95%, IQR 90.75%–97.2%; visit 2, median 94.15%, IQR 90.45%–97.13%; visit 3, median 95%, IQR 90.9%–97.85%) (Fig 7C). Analysis of CD4+ T cell subsets within the HIV p24+ population indicated a notable increase in the percentage of CM cells at visit 2 (median 25.25%, IQR 13.67%–33.47%) compared to visit 1 (median 18.9%, IQR 14.8%–26.1%). The frequency of EM cells was noticeably reduced in the p24+ population at visit 2 (median 58% median, IQR 48.27%–73.32%) compared to visit 1 (median 63.9% median, IQR 57.8%–74.8%) although the changes in HIV preferred CD4+ T cell subsets in response to Depo-Provera did not reach significance (Fig 7D). Taken together, our results indicated that, while CD4+ T cells expressing integrin α4β7, CCR5 or CD38 were preferentially infected by HIV before and after Depo-Provera treatment, the level of integrin α4β7 in HIV-infected cells was significantly decreased at visit 2.
Figure 7. Impact of Depo-Provera on CD4+ T cell surface marker expression on HIV positive cells.
Changes in integrin α4β7, CCR5, and CD38 cell surface marker expression (A–C) and CD4+ T cell subsets (D) between study visits were analyzed on HIV p24+CD4+ T cells by FACS analysis. Each dot represents one donor. Bars represent median and interquartile range. Wilcoxon matched-pairs signed rank test was used to compare differences between study visits. p≤0.05 was considered significant, p>0.05 was not significant (ns).
Discussion
Our longitudinal study indicated that Depo-Provera administration increased the numbers of peripheral CD4+ T cells with markers for HIV preference and promoted HIV infection of PBMCs ex vivo. We found a significant increase in the percentage of integrin α4β7+CD4+ T cells and in the expression level of integrin α4β7 one month (visit 2) after injection of Depo-Provera, when serum levels of MPA are highest. (31, 32). There was also an increasing trend of CCR5 and CD38 expression at visit 2 compared to visit 1. The percentage and intensity of α4β7, CCR5, and CD38 decreased from visit 2 to visit 3 to levels comparable visit 1, suggesting the immunomodulatory effects of Depo-Provera may depend on the serum MPA concentration (visit 2> visit 3>,~= visit 1). There were differential changes in these markers depending on the race/ethnicity. There was a decreasing trend of TEM frequency from visit 1 to visit 3, and the reduction of TEM cells in black women was highly significant. Depo-Provera did not appear to change HIV preference significantly as HIV p24+ cells had higher levels of integrin α4β7, CCR5, and CD38 than HIV p24− cells regardless Depo-Provera treatment. Interestingly, the expression level of integrin α4β7+ on HIV p24+ cells was significantly lower at visit 2 compared to visit 1, and we also observed a non-significant decrease in the frequency of HIV p24+ cells expressing integrin α4β7 from visit 1 to visit 3 (Fig 7). We found that HIV production from PBMCs at visit 3 was significantly higher than visit 1 (Fig 4) and the frequency of HIV p24+ cells at visit 3 was significantly higher than visit 1 in black, but not Hispanic women (Fig 5). Taken together, our results indicate Depo-Provera altered the immune markers associated with preferential HIV infection and increased HIV infection ex vivo. These changes varied depending on race/ethnicity and the sample collection time after treatment.
Unlike studies in Depo-Provera-treated rhesus macaques which show a correlation between CD38+ T cell levels and HIV-RNA levels, suggesting CD38 may be an indicator of viral replication (35–37), we did not find a positive correlation between the frequency of integrin α4β7, CCR5, or CD38 expressing cells and HIV susceptibility (e.g. HIV p24+ cell numbers or HIV production). We did find a significant, but weak, negative correlation between HIV production at day 7 and cell surface expression of integrin α4β7 (r=−0.22, p=0.038) and CD38 (r=−0.21, p=0.04) on freshly isolated PBMCs (day 0) (Supplementary Fig 3), but no significant correlation between markers on Day 0 and the frequency of p24+ cells measured at day 10 post-infection. There was a weak negative correlation between HIV production and CD38 on freshly isolated PBMCs from black women (r=−0.3168, p=0.0196). There was no significant correlation between HIV infection (HIV p24 release or p24+ cells) and the frequency of immune markers on PBMCs at day 10 post-infection except a very weak negative association between the frequency of integrin α4β7 and HIV production in Hispanic women (r=−0.38, p=0.02) (Supplementary Fig 4). The weak negative correlation between HIV infection and integrin α4β7 or CD38 may be attributed to increased cell death in the HIV preferable cells population that are highly infected by HIV. Detailed analyses of the dynamics between HIV infection and cell death with a sensitive method in these highly HIV susceptible cells would offer insights into HIV pathogenesis.
We found a significant increase in HIV preference markers at visit 2 but the increase in HIV infection was found at visit 3 compared to visit 1. The increase in HIV infection and preference makers at different visits may be due to a complex interaction of Depo-Provera with various immune parameters that modulate HIV infection. For example, other regulatory cells or soluble factors in PBMCs could be involved in Depo-Provera-mediated enhancement of HIV susceptibility at the visit 3. Depo-Provera use has been shown to alter pDC function with a decreased production of IFNα and TNFα in response to TLR-9 stimulation (29). HIV host restriction factors such as interferon-inducible cellular proteins can block multiple stages of the HIV lifecycle (38, 39). Changes in cytokine levels induced by Depo-Provera may also modify levels of intrinsic host restriction factors in CD4+ T cells, resulting in a decreased ability of CD4+ T cells to control HIV infection. Some immuno-modulatory impacts on HIV infection may not be observed until the decreased concentration of Depo-Provera at the visit 3.
In the HIV infection ex vivo assay, we found a significant increase in HIV infection from visit 1 to visit 3 when analyzing HIV p24 production at day 7 post-infection (Fig 4A) but not the frequency of HIV p24+ cells at day 10 post-infection (Fig 5A). Similarly, the difference in the frequency of HIV p24+ cells at day 10 post-infection in black women in response to Depo-Provera (Fig 5B) was not found in the HIV p24 production at day 7 post-infection (Fig 4B). Multiple elements may contribute to the discrepancy between results of two assays (HIV p24 ELISA verses HIV p24+ flow cytometry) in a multi-cellular system for HIV infection of PBMCs ex vivo. These factors include 1) the impact of Depo-Provera on other immune cells (e.g. monocytes or pDCs) or metabolites that could affect CD4+ T cells, 2) the dynamics between HIV and PBMCs (e.g. compositions of immune cell types, cell activation/death state and cytokine production), and 3) the biological variability in women and subjects’ metabolism in response to Depo-Provera. Future studies using purified CD4+ T cells for HIV infection assay and determining the functions of other immune cells or immune milieu in clinical samples may offer insights into development a better HIV ex vivo assay to predict clinical outcomes.
Our results revealed ethnicity dependent changes to immune profiles and HIV susceptibility in response to Depo-Provera. For example, we observed an increase in integrin α4β7 expression from visit 1 to visit 2 in Hispanic women, with no significant change in expression from visit 2 to visit 3. In black women, there was an increasing trend of integrin α4β7 expression from visit 1 to visit 2 but the level decreased from visit 2 to visit 3. Significant decreases in TEM cells from visit 1 to visit 2 and visit 1 to visit 3 was observed in only within the black patient group. Additionally, black women had a significant increase in the percentage of p24+ cells from visit 1 to visit 3, while the Hispanic patient group showed no significant difference in the number of infected cells between visits. While there was a difference in the smoking status or BMI between these two groups (Smoking, Hispanic: 1 out of 15, black: 8 out of 24; BMI (median), Hispanic: 25.27, black: 30.3), our conclusion remains the same after stratifying for smoking and BMI. Race and ethnicity are known to contribute to disease susceptibility and vaccine responses (40, 41). Blacks comprise 12.3% of the US population but accounted for 44.3% of new HIV diagnoses while Hispanics, who comprise 17% of the total US population accounted for 16.4% of diagnoses in 2015 (42). Although racial/ethnic disparities in HIV transmission are likely multifactorial (43–45), the role of race/ethnicity-dependent immune responses in modulation of HIV transmission requires further investigation.
A recent longitudinal study by Achilles indicates no change in CCR5 or CD69 (activation marker) on peripheral or cervical CD4+ T cells in women 90 days after initiation of Depo-Provera (46). However, a cross sectional study performed by Sciaranghella detected significantly higher CCR5 MFI on CD4+ T cells from Depo-Provera users compared to a control group without hormonal contraception (28). We found a significant increase in integrin α4β7+CD4+ T cells, and an increasing trend in CCR5+CD4+ T cells 1 month after Depo-Provera injection compared to baseline or 3 months after Depo-Provera injection. There was no significant change in these markers on CD4+ T cells between the baseline first visit and 3 months (90 days) after Depo-Provera. These results suggest that study design, particularly the timeframe when samples are collected, may impact the outcome. Weinberg and colleagues found a significant decrease in activated CD4+ T cell subsets (CD4+CD25+ T cells) 3 months after Depo-Provera injection in HIV-infected women on cART (30), whereas we observed a trend of increased CD38+CD4+ T cells at visit 2 but not visit 3. It is not clear whether the different results are due to the status of HIV, cART, age or race/ethnicity in these two studies.
In addition to PBMCs, immune cells within the female genital tract are impacted in response to Depo-Provera. Depo-Provera users have higher levels of activated endometrial T cells along with a higher percentage of endometrial CD4+ naïve and CM subsets compared to women not using hormonal contraceptives, although there is no difference in their TEM subsets (47). We observed a similar increasing tread of naïve and CM CD4+ T cells subsets in response to Depo-Provera, especially in black patients, indicating changes in CD4+ T cell subsets by Depo-Provera may not be compartmentalized. However, our longitudinal study revealed a decreasing trend of TEM cells in response to Depo-Provera, with a significant reduction observed within the black patient group. It remains to be determined whether Depo-Provera-mediated alteration of TEM cell population is location dependent (blood vs. mucosal tissues).
While Depo-Provera appeared to have a sustained effect on CD4+ T cell subsets, we found a transient increase in HIV preference markers at visit 2 (one month) followed by a decrease to baseline levels at visit 3 (apart from expression of integrin α4β7 in Hispanic women). This finding differs from a study comparing immune cells in vaginal tissues before and 12 weeks (the same timing as visit 3) after receiving Depo-Provera, which showed Depo-Provera treatment resulted in a significant increase in the median numbers of CD45, CD3, CD8, CD68, CCR5, and HLA-DR positive cells regardless of whether samples were collected at follicular or luteal phases (48). It is possible that cells at the mucosal sites are regulated differentially from peripheral cells, and are less dependent on serum concentrations of Depo-Provera.
In summary, our longitudinal study demonstrated that Depo-Provera changed markers for HIV preference and CD4+ T cell subsets in PBMCs and increased HIV infection ex vivo despite a small sample size. Our results warrant future investigation on the mechanism of Depo-Provera-mediated increased HIV preference markers such as integrin α4β7. We showed differential immune profiles and HIV infection could vary depending on sampling time and the race/ethnicity of study subjects, which may explain inconsistent findings of the association of Depo-Provera and increased risk of HIV acquisition. The impact of Depo-Provera on immune homing markers and T cell subsets suggests that Depo-Provera may impact the establishment of HIV reservoirs and immune regulation during HIV pathogenesis (49, 50). Thus, a better understanding of how Depo-Provera modulates immune responses in women with diverse backgrounds and health states is critical for developing an effective strategy for HIV prevention and treatment.
Supplementary Material
Acknowledgments
We thank Sally Hodder, Gerson Weiss, and Laura Goldsmith for their suggestions on the study design, subjects who participated in the study, Jeanette Rio and Jennifer Winter for their assistance on patient recruitment.
Footnotes
The authors have declared that no conflict of interest exists.
References
- 1.United Nations, D. o. E. a. S. A., Population Division. Trends in Contraceptive Use Worldwide 2015. 2015. [Google Scholar]
- 2.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 3.McCoy S, Zheng W, Montgomery ET, Blanchard K, van der Straten A, de Bruyn G, Padian N. Oral and injectable contraception use and risk of HIV acquisition among women: MIRA study. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. p. 20LB. [DOI] [PubMed] [Google Scholar]
- 4.Morrison CS, Skoler-Karpoff S, Kwok C, Chen PL, van de Wijgert J, Gehret-Plagianos M, Patel S, Ahmed K, Ramjee G, Friedland B, Lahteenmaki P. Hormonal contraception and the risk of HIV acquisition among women in South Africa. Aids. 2012;26:497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 5.Heffron R, Mugo N, Ngure K, Celum C, Donnell D, Were E, Rees H, Kiarie J, Baeten JM. Hormonal contraceptive use and risk of HIV-1 disease progression. Aids. 2012 doi: 10.1097/QAD.0b013e32835ad473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012;26:375–380. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 8.Lutalo T, Musoke R, Polis C, Serwadda D, Makumbi F, Nalugoda F, Bwanika J, Sekasanvu J, Wawer M, Gray R. Effects of hormonal contraceptive use on HIV acquisition in women and transmission to men among HIV-discordant couples, Rakai, Uganda. 19th Conference on Retroviruses and opportunistic infections; Seattle, WA. 2012. Abstract #563. [Google Scholar]
- 9.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkus JE, Brown ER, Hillier SL, Coletti A, Ramjee G, Mgodi N, Makanani B, Reid C, Martinson F, Soto-Torres L, Abdool Karim SS, Chirenje ZM. Oral and injectable contraceptive use and HIV acquisition risk among women in four African countries: a secondary analysis of data from a microbicide trial. Contraception. 2016;93:25–31. doi: 10.1016/j.contraception.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crook AM, Ford D, Gafos M, Hayes R, Kamali A, Kapiga S, Nunn A, Chisembele M, Ramjee G, Rees H, McCormack S. Injectable and oral contraceptives and risk of HIV acquisition in women: an analysis of data from the MDP301 trial. Hum Reprod. 2014;29:1810–1817. doi: 10.1093/humrep/deu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutalo T, Musoke R, Kong X, Makumbi F, Serwadda D, Nalugoda F, Kigozi G, Sewankambo N, Sekasanvu J, Wawer M, Gray R. Effects of hormonal contraceptive use on HIV acquisition and transmission among HIV-discordant couples. AIDS. 2013;27(Suppl 1):S27–34. doi: 10.1097/QAD.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 13.Blish CA, Baeten JM. Hormonal contraception and HIV-1 transmission. Am J Reprod Immunol. 2011;65:302–307. doi: 10.1111/j.1600-0897.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy SI, Zheng W, Montgomery ET, Blanchard K, van der Straten A, de Bruyn G, Padian NS. Oral and injectable contraception use and risk of HIV acquisition among women in sub-Saharan Africa. AIDS. 2013;27:1001–1009. doi: 10.1097/QAD.0b013e32835da401. [DOI] [PubMed] [Google Scholar]
- 15.Ralph LJ, McCoy SI, Shiu K, Padian NS. Does hormonal contraceptive use increase women’s risk of HIV acquisition? A meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–189. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison CS, Chen PL, Kwok C, Baeten JM, Brown J, Crook AM, Van Damme L, Delany-Moretlwe S, Francis SC, Friedland BA, Hayes RJ, Heffron R, Kapiga S, Karim QA, Karpoff S, Kaul R, McClelland RS, McCormack S, McGrath N, Myer L, Rees H, van der Straten A, Watson-Jones D, van de Wijgert JH, Stalter R, Low N. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med. 2015;12:e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, Westreich DJ, Steyn PS. An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS. 2016;30:2665–2683. doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall KM, Kilembe W, Vwalika B, Htee Khu N, Brill I, Chomba E, Johnson BA, Haddad L, Tichacek A, Allen S. Hormonal contraception does not increase women’s HIV acquisition risk in Zambian discordant couples, 1994–2012. Contraception. 2015;91:480–487. doi: 10.1016/j.contraception.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall KM, Kilembe W, Vwalika B, Ravindhran P, Khu NH, Brill I, Chomba E, Johnson BA, Haddad LB, Tichacek A, Allen S. Hormonal Contraceptive Use Among HIV-Positive Women and HIV Transmission Risk to Male Partners, Zambia, 1994–2012. J Infect Dis. 2016;214:1063–1071. doi: 10.1093/infdis/jiw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Govender Y, Avenant C, Verhoog NJD, Ray RM, Grantham NJ, Africander D, Hapgood JP. The Injectable-Only Contraceptive Medroxyprogesterone Acetate, Unlike Norethisterone Acetate and Progesterone, Regulates Inflammatory Genes in Endocervical Cells via the Glucocorticoid Receptor. PLoS One. 2014:9. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Africander D, Louw R, Hapgood JP. Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem Biophys Res Commun. 2013;433:305–310. doi: 10.1016/j.bbrc.2013.02.086. [DOI] [PubMed] [Google Scholar]
- 23.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2008;61:171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Hapgood JP. Immunosuppressive biological mechanisms support reassessment of use of the injectable contraceptive medroxyprogesterone acetate. Endocrinology. 2013;154:985–988. doi: 10.1210/en.2013-1066. [DOI] [PubMed] [Google Scholar]
- 25.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, Hel Z. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampah MES, Laird GM, Blankson JN, Siliciano RF, Coleman JS. Medroxyprogesterone acetate increases HIV-1 infection of unstimulated peripheral blood mononuclear cells in vitro. AIDS. 2015;29:1137–1146. doi: 10.1097/QAD.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassiliadou N, Tucker L, Anderson DJ. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol. 1999;162:7510–7518. [PubMed] [Google Scholar]
- 28.Sciaranghella G, Wang C, Hu H, Anastos K, Merhi Z, Nowicki M, Stanczyk FZ, Greenblatt RM, Cohen M, Golub ET, Watts DH, Alter G, Young MA, Tsibris AM. CCR5 Expression Levels in HIV-Uninfected Women Receiving Hormonal Contraception. J Infect Dis. 2015;212:1397–1401. doi: 10.1093/infdis/jiv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Effect of Hormonal Contraception on the Function of Plasmacytoid Dendritic Cells and Distribution of Immune Cell Populations in the Female Reproductive Tract. J Acquir Immune Defic Syndr. 2015;65:511–518. doi: 10.1097/QAI.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg A, Park JG, Bosch R, Cho A, Livingston E, Aweeka F, Cramer Y, Watts DH, Luque AE, Cohn SE. Effect of Depot Medoxyprogesterone Acetate on Immune Functions and Inflammatory Markers of HIV-Infected Women. J Acquir Immune Defic Syndr. 2016;71:137–145. doi: 10.1097/QAI.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishell DR. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996;41:381–390. [PubMed] [Google Scholar]
- 32.Jeppsson S, Gershagen S, Johannson EDB, Rannevik G. Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera®) as a contraceptive agent. Acta Endocrinol. 1982;99:339–343. doi: 10.1530/acta.0.0990339. [DOI] [PubMed] [Google Scholar]
- 33.Ding J, Tasker C, Lespinasse P, Dai J, Fitzgerald-Bocarsly P, Lu W, Heller D, Chang TL. Integrin a4b7 Expression Increases HIV Susceptibility in Activated Cervical CD4+ T Cells by an HIV Attachment-Independent Mechanism. J Acquir Immune Defic Syndr. 2015;69:9. doi: 10.1097/QAI.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang K, Albert PS. Models for Longitudial Data: A Generalize Estimating Equation Approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 35.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4:11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. 2009;6:e1000199. doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus:host interactions? Aids. 2000;14:1079–1089. doi: 10.1097/00002030-200006160-00004. [DOI] [PubMed] [Google Scholar]
- 38.Malim MH, Bieniasz PD. HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb Perspect Med. 2012;2:a006940. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris RS, Hultquist JF, Evans DT. The restriction factors of human immunodeficiency virus. J Biol Chem. 2012;287:40875–40883. doi: 10.1074/jbc.R112.416925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voigt EA, I, Ovsyannikova G, Haralambieva IH, Kennedy RB, Larrabee BR, Schaid DJ, Poland GA. Genetically defined race, but not sex, is associated with higher humoral and cellular immune responses to measles vaccination. Vaccine. 2016;34:4913–4919. doi: 10.1016/j.vaccine.2016.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haralambieva IH, Salk HM, Lambert ND, Ovsyannikova IG, Kennedy RB, Warner ND, Pankratz VS, Poland GA. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946–1953. doi: 10.1016/j.vaccine.2014.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Division of HIV/AIDS Prevention, N. C. f. H. A., Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, Georgia. Diagnoses of HIV Infection in the United States and Dependent Areas. 2015;2015:27. [Google Scholar]
- 43.Rosenberg ES, Millett GA, Sullivan PS, Del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modeling study. Lancet HIV. 2014;1:e112–e118. doi: 10.1016/S2352-3018(14)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WLt, Wilson PA, Rourke SB, Heilig CM, Elford J, Fenton KA, Remis RS. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380:341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 45.Tillerson K. Explaining racial disparities in HIV/AIDS incidence among women in the U.S.: a systematic review. Stat Med. 2008;27:4132–4143. doi: 10.1002/sim.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achilles SL, Mhlanga F, Meyn LA, Stoner KA, Matubu AT, Chirenje ZM, Hillier SL. ZIM-CHIC: Impact of Contraceptive Initiation on Immune Cells in the Cervix and PBMCs. Abstract - Conference on Retroviruses and Opportunistic Infections (CROI); 2017; Seattle, WA. 2017. [Google Scholar]
- 47.Smith-McCune KK, Hilton JF, Shanmugasundaram U, Critchfield JW, Greenblatt RM, Seidman D, Averbach S, Giudice LC, Shacklett BL. Effects of depot-medroxyprogesterone acetate on the immune microenvironment of the human cervix and endometrium: implications for HIV susceptibility. Mucosal Immunol. 2017 doi: 10.1038/mi.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.