Abstract
The sweetpotato whitefly Bemisia tabaci (Gennadius) is one of the most invasive pest species worldwide. Q and B biotypes are the two most devastating species within the B. tabaci complex. Bemisia tabaci can vector hundreds of plant viruses that seriously threaten crop production. Endoparasitoid, Encarsia formosa Gahan, is widely used to control whiteflies, however, little is known about the effects of virus-infected plants on E. formosa parasitism of B. tabaci. Here, we reported that tomato, which was infected with Tomato Yellow Leaf Curl Virus (TYLCV), altered the host selection of E. formosa between B. tabaci Q and B biotypes. On healthy tomato plants, parasitism and host selection by E. formosa did not differ between the 3rd-instar nymphs of B. tabaci Q and B biotypes. On TYLCV-infected tomato plants, however, B. tabaci Q biotype were significantly more attractive to E. formosa than B biotype. When TYLCV-infected tomato plants were infested with B. tabaci Q or B biotype, volatile profiles differed quantitatively but not qualitatively. Olfactometer assays suggested that the preference of E. formosa to Q over B biotype was associated with an elevated level of β-Myrcene, β-Ocimene, β-Caryophyllene, and α-Humulene from TYLCV-infected tomato plants.
Keywords: Encarsia formosa, Bemisia tabaci, tomato yellow leaf curl virus, host selection, plant volatiles
Introduction
Parasitoid wasps, during foraging, may encounter various and multiple situations or factors, such as insect species and these insect-borne pathogens; this would make the parasitoids foraging more complicated and difficult (Ponzio et al., 2013, 2016a; Li et al., 2014; Martini et al., 2014; Mauck et al., 2015). Hence, finding a reliable and efficient way to locate their suitable host is important for their propagation. Numerous evidences have indicated that the pathogen, herbivory or oviposition-induced plant volatiles are important infochemicals and reliable indicators to many parasitoid species for seeking and locating their hosts (McCormick et al., 2012; Li et al., 2014; Martini et al., 2014; Mauck et al., 2015; Ponzio et al., 2016a,b). These chemical volatiles thus have an important role for the host searching, location and discrimination behaviors of carnivores (McCormick et al., 2012; Li et al., 2014; Ponzio et al., 2016a,b).
The sweetpotato whitefly, Bemisia tabaci is an important insect pest on many vegetables and other agricultural crops. The Q and B biotypes are two most invasive and destructive cryptic species of B. tabaci in China (De Barro et al., 2011; Pan et al., 2012; Zheng et al., 2017). Serious damage by B. tabaci results not only from its removal of plant sap and secretion of honeydew (which causes sooty mold) but also from its transmission of various begomoviruses (De Barro et al., 2011). The only known vector of Tomato yellow leaf curl virus (TYLCV), which is a typical begomovirus, is B. tabaci, and B. tabaci transmits TYLCV in a persistent manner (Brown and Czosnek, 2002; Jones, 2003). A recent study found that TYLCV transmission efficiency is higher for the Q biotype than for the B biotype (Ning et al., 2015). Although the Q biotype has replaced the B biotype in many areas of China over the past decades (Pan et al., 2011), the two biotypes continue to coexist in some locations (Chu et al., 2010a,b; Teng et al., 2010; Pan et al., 2012; Zheng et al., 2017).
Encarsia formosa Gahan is a cosmopolitan and commercially important parasitoid of B. tabaci and other whiteflies, and has been widely used to reduce whitefly damage to crops (Gerling et al., 2001; Grille et al., 2012; Liu et al., 2015, 2016). Parasitism of B. tabaci by E. formosa is affected by the developmental stage of Q and B biotypes (Liu et al., 2016) and may also be affected by infection of host plants by TYLCV (Liu et al., 2014). In the latter case, parasitism of Q biotype nymphs was higher on TYLCV-infected tomato plants than on TYLCV-free plants, but parasitism of B biotype nymphs did not significantly differ on TYLCV-infected vs. TYLCV-free tomato plants (Pan et al., 2013, 2014; Liu et al., 2014). These studies suggested that the selection of B. tabaci biotype Q and B as hosts by E. formosa may differ and may be affected by TYLCV infection of plants. Additional research is needed to determine how parasitism and host selection by E. formosa differs between the two important biotypes of B. tabaci and how such selection and parasitism is affected by TYLCV infection of host tomato plants.
The first objective of the present study was to further describe how host selection by E. formosa differs between B. tabaci Q and B biotypes and how host selection is affected by TYLCV infection of tomato plants. A second objective was to determine potential mechanisms underlying the effects of biotype and TYLCV infection of tomato on such host selection.
Materials and methods
Plant and insect materials
Lycopersicon esculentum (variety “No. 9 Zhongza”) tomato plants were grown in plastic pots (7 cm diameter) containing a mixture of peat moss, vermiculite, organic fertilizer, and perlite in a 10:10:10:1 ratio by volume (Shi et al., 2013). Tomato plants with three to four expanded leaves were inoculated with a TYLCV infectious clone kindly provided by Professor Xueping Zhou (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China); infection by TYLCV was confirmed as previously described (Ghanim et al., 2007; Shi et al., 2013; Liu et al., 2014). TYLCV-free plants were not inoculated with the virus. Plants with six to eight fully expanded leaves were used for the assays and experiments described in the following sections.
Laboratory colonies of B. tabaci biotype Q and B, whose identities was periodically confirmed based on the cleavage amplified polymorphic sequence (CAPS) and mitochondrial cytochrome oxidase I genes (mtCOI) (Chu et al., 2010a), were separately maintained on virus-free tomato plants in insect-proof cages in a greenhouse. Encarsia formosa was provided by the Beneficial Insects Research Center, Shandong Academy of Agricultural Sciences in China; the parasitoid had been reared on nymphs of Trialeurodes vaporariorum (Westwood) for >10 years.
Encarsia formosa's parasitism on B. tabaci Q and B biotypes under in Vitro and semi-natural conditions
Selection and oviposition of E. formosa on HQ vs. HB or VQ vs. VB were compared under in vitro conditions in the laboratory. HQ and HB refer to virus-free plants infested with 3rd-instar nymphs of B. tabaci Q or B. VQ and VB refer to virus-infected plants infested with 3rd-instar nymphs of B. tabaci Q or B. TYLCV-infected or TYLCV-free tomato leaves were infested with 30 3rd-instar nymphs of B. tabaci biotype Q or B, and in vitro parasitism was assessed as previously described (Liu et al., 2016). In brief, the leaves infested by 3rd-instar nymphs of B. tabaci biotype Q or B were detached from the TYLCV-free or TYLCV-infected plants. The leaf petioles were wrapped in cotton wool saturated with distilled water. Each treatment pair (HQ vs. HB and VQ vs. VB) was placed in plastic Petri dishes (diameter 8.5 cm, height 1.5 cm, two leaves per dish). A filter paper disk saturated with distilled water was also placed beneath each leaf. Newly emerged E. formosa females (1 day old; wasps: nymphs = 1: 30) were subsequently released in the center of each Petri dishes between the two leaves and were carefully removed after 24 h. About 8–10 days later, the number of nymphs parasitized by wasps was recorded. Each comparison was carried out for 19 replicates.
To confirm the results obtained from the in vitro experiment, a greenhouse experiment that simulated field conditions was carried out as described by Zhang et al. (2013b). Bemisia tabaci biotype Q or B adults were placed in clip-cages on the leaves of TYLCV-infected or TYLCV-free plants (four clip-cages per plant, 20 adults per clip-cage) and allowed to oviposit; after 24 h, all of the adults were removed. About 20 days later, when most eggs had hatched and developed into 3rd-instar nymphs, one plant of each treatment pair (HQ vs. HB and VQ vs.VB) was placed in a ventilated cage (88 × 60 × 79 cm); the two plants in each cage were 50 cm apart. Newly emerged E. formosa females (1 day old; wasps: nymphs = 1: 30) were subsequently released in the center of each cage between the two plants. After 24 h, we carefully checked the tomato leaves and removed the parasitoids by using a small suction trap. The viruliferous and TYLCV-free tomato plants carrying nymphs were separately maintained in a climate chamber at 26 ± 1°C with 70 ± 5% relative humidity and a 16 h/8 h light/dark photoperiod. About 8–10 days later, the number of nymphs parasitized by wasps was recorded. Each comparison was replicated four times, and replications were conducted on different days to account for day-to-day variation (Zhang et al., 2013b).
Olfactometer assays
A Y-tube olfactometer was used to assess the behavioral responses of E. formosa to the following pairs of odor sources: TYLCV-free tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q (HQ) or B (HB); TYLCV-infected tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q (VQ) or B (VB). The following combinations were also carried out to verify the absence of differences within each treatment: TYLCV-infected tomato plants without B. tabaci infestation (V) vs. V; TYLCV-free tomato plants without B. tabaci infestation (H) vs. H; VQ vs. VQ; VB vs.VB; HQ vs. HQ; and HB vs. HB. In all cases involving infestation, plants were infested with approximately 20 nymphs per leaf.
The olfactometer assays were carried out as previously described (Zhang et al., 2013b). In brief, an adult female of E. formosa (1.0–2.0 days old) was released at the base of the Y-tube and then observed continuously for 5 min. Each female was tested only once. The selection of the two odor sources was recorded when the female had moved into one arm of the Y-tube for at least one-third of the arm's length and remained for at least 15 s; a female that walked to the far end of the arm was also recorded as a selection. If the female did not choose within 5 min, “no choice” was recorded. After five females were tested, odor sources were interchanged to avoid any influence of asymmetries in the set-up system. The Y-tube was replaced with a new one after it had been used to test 10 wasps. To avoid the influence of residual odor, each used tube was rinsed with 75% alcohol and kept in an oven at 180°C overnight. Each comparison was replicated three times, with 20 females per replicate.
Collection and analysis of plant volatiles
Volatiles emitted from TYLCV-infected or TYLCV-free tomato plants infested by nymphs of B. tabaci biotype Q or B were collected and analyzed as described previously (Wei et al., 2007; Chen et al., 2017). The potted plants, which were carefully wrapped in aluminum foil with only the above-ground green part exposed, were placed in collection bags (one plant per bag) with a gas inlet and a gas outlet. Purified and humidified air at 300 mL min−1 was pumped into each bag through the inlet. A glass tube filled with 0.1 g of PoraPak Q (80/100-mesh; Waters, USA) was used to trap plant volatiles for 4 h at the outlet under continuous high-intensity sodium-halide light. Volatiles were collected from four replicate plants per treatment.
The trapped volatile samples were eluted from the PoraPak Q with 800 μL of high-performance liquid chromatography (HPLC)-grade methylene chloride (Tedia Company, Fairfield, Ohio, USA); an internal standard (10 μL of 20 ng/μL of n-octane) was added to each sample for quantification of relative compound amounts. A 1-μL sample of the solution was subjected to gas chromatography–mass spectrometry (GC-MS); the gas chromatograph (GC-2010 Shimadzu, Japan) was equipped with an Agilent Technologies capillary column DB-5MS (30 m × 0.25 mm ID × 0.25 μm film thickness). The temperature program was as follows: the initial oven temperature was kept at 40°C for 4 min and was then increased to 200°C at a programmed rate of 5°C min−1, followed by a rate at 20°C min−1 to 280°C. The injector temperature was maintained at 250°C with a constant flow rate of 1.0 mL min−1. Compounds were identified by comparison of retention times and mass spectra (NIST database and synthetic standards). The peak area of the volatile expressed as a percentage of the peak area of the internal standard was used to analysis the change of volatiles (Chen et al., 2017).
Attraction verification of E. formosa to synthetic volatiles
A Y-tube olfactometer was used to assess the attractions of candidate volatile compounds to E. formosa females as previously described (Zhang et al., 2013b). The candidate compounds (β-Myrcene, β-Ocimene, β-Caryophyllene, α-Humulene, and β-Elemene) were selected based on data obtained with TYLCV-infected tomato plants infested with B. tabaci nymphs (see Results). Nine pairs of treatments were compared: Paraffin oil (CK) vs. CK, β-Myrcene (4.350 ng/μl) vs. CK, β-Ocimene (3.525 ng/μl) vs. CK, β-Caryophyllene (3.350 ng/μl) vs. CK, α-Humulene (1.625 ng/μl) vs. CK, β-Elemene (0.675 ng/μl) vs. CK, and mixture of monoterpenes (β-Myrcene and β-Ocimene) vs. CK, mixture of sequiterpenes (β-Caryophyllene, α-Humulene, and β-Elemene) vs. CK, mixture of total candidate compounds vs. CK. As indicated, paraffin oil (CK) was used as the solvent. Two streams of purified and humidified air at 300 ml min−1 were separately passed through two glass containers containing the test volatile in paraffin oil or containing only paraffin oil as a control and into the olfactometer arms (Chen et al., 2017). The experimental method and data collection were the same as described earlier for the olfactometer assays.
Statistical analysis
Chi-squared tests were used to analyze the Y-tube olfactometer assays (significance level: P < 0.05) (Martini et al., 2014; Mauck et al., 2015). Parasitoids that did not make a choice were excluded from the analysis. Independent-sample t-tests were used to compare the percentage of B. tabaci nymphs parasitized by E. formosa in each treatment pair and the quantities of volatile compounds collected from TYLCV-infected or TYLCV-free tomato plants infested with nymphs of B. tabaci biotype Q or B. All proportional data were first checked for normality and were transformed when necessary to meet the assumption of normal distribution before analysis. SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis (Liu et al., 2016).
Results
Encarsia formosa' s parasitism on B. tabaci Q and B biotypes under laboratory and semi-natural conditions
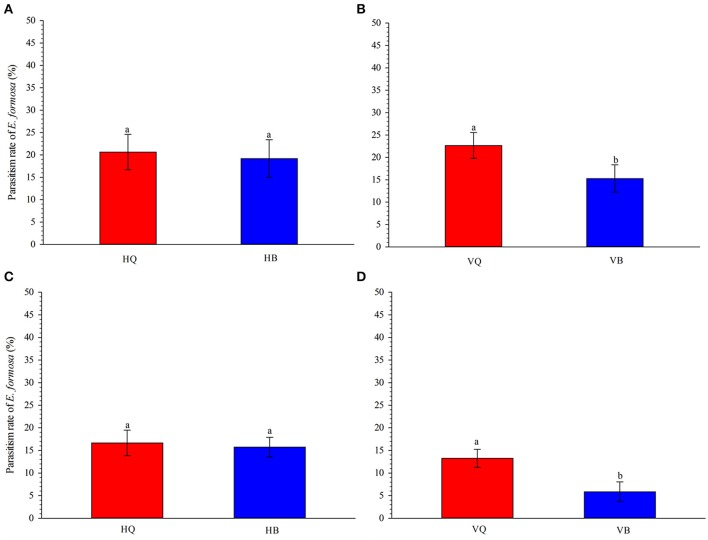
On tomato leaves in the laboratory, parasitism of 3rd-instar nymphs of B. tabaci by E. formosa females did not differ between biotype Q and B on TYLCV-free tomato plants (P = 0.834, Figure 1A). On TYLCV-infected tomato plants, however, parasitism was significantly higher for biotype Q than B (P = 0.031, Figure 1B).
Figure 1.
Parasitism of 3rd-instar nymphs of Bemisia tabaci biotype Q vs. B by E. formosa on TYLCV-free (A) and TYLCV-infected (B) tomato plants under in vitro conditions and on TYLCV-free (C) and TYLCV-infected (D) plants under semi-natural conditions. HQ and HB refer to TYLCV-free plants infested with biotype Q or B. VQ and VB refer to virus-infected plants infested with biotype Q or B. Values are means ± SE. In each panel, means with different letters are significantly different according to the Tukey test at P < 0.05.
The results of the semi-natural experiment showed the same trends as that in the laboratory experiment. The E. formosa did not show a preference for Q or B biotype on TYLCV-free tomato plants (P = 0.797, Figure 1C) but preferred biotype Q to biotype B on TYLCV-infected tomato plants (P = 0.027, Figure 1D).
Olfactometer assays
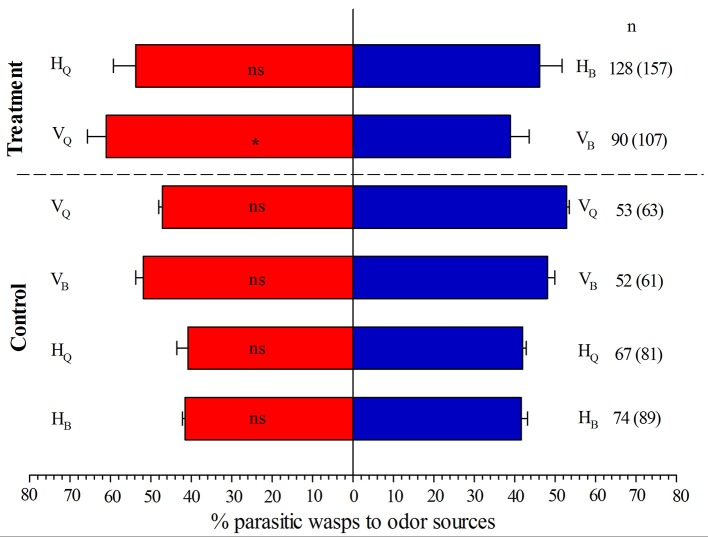
When tomato plants were not infected with TYLCV in Y-tube olfactometer assays, the naive E. formosa females showed no significant preference for plants infested with 3rd-instar nymphs of biotype Q vs. biotype B of B. tabaci (P = 0.424, Figure 2). When tomato plants were infected by TYLCV, however, E. formosa significantly preferred tomato plants infested with biotype Q rather than biotype B (P = 0.046, Figure 2).
Figure 2.
Responses of Encarsia formosa to odor sources in a Y-tube olfactometer. Bars (means + SE) indicate the percentages of wasps choosing either of the odor sources. HQ /HB, TYLCV-free tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q/B; VQ/VB, TYLCV-infected tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q/B. The numbers to the right of bars indicate the number of wasps making a choice, and the total number of wasps used in the assay is indicated in parentheses. Asterisks indicate significant differences between the treatment pairs (χ2 test; *P < 0.05; ns, not significant).
Analysis of plant volatiles
According to the analysis of the plant headspace volatiles by gas chromatography-mass spectrometry (GC-MS), all treatments produced the same 32 compounds, including monoterpenes, sequiterpenes, aldehydes, alcohols, and esters (Table 1), and differences between treatments were quantitative rather than qualitative. Quantitative analysis revealed that emissions of the monoterpenes including β-Myrcene and β-Ocimene, and the sesquiterpenes including β-Caryophyllene, α-Humulene, and β-Elemene were higher from TYLCV-infected plants infested with 3rd-instar nymphs of B. tabaci Q than with nymphs of B. tabaci B. The amounts of these compounds emitted from TYLCV-free plants did not significantly differ when the plants were infested with 3rd-instar nymphs of B. tabaci Q vs. B (Table 1).
Table 1.
The peak area ratios of volatile components released from tomato plants infested by the 3rd-instar nymphs of B. tabaci.
| Compound | Retention time (min) | HQ (mean ± SE) | HB (mean ± SE) | P | VQ (mean ± SE) | VB (mean ± SE) | P |
|---|---|---|---|---|---|---|---|
| Methyl-Benzene | 5.046 | 2.67 ± 0.16a | 2.11 ± 0.24a | 0.099 | 2.18 ± 0.11a | 1.84 ± 0.16a | 0.145 |
| Hexanal | 6.104 | 0.21 ± 0.33a | 0.29 ± 0.05a | 0.183 | 0.20 ± 0.03a | 0.14 ± 0.02a | 0.101 |
| 1-Octene | 7.322 | 0.37 ± 0.02a | 0.37 ± 0.01a | 0.721 | 0.26 ± 0.03a | 0.22 ± 0.02a | 0.260 |
| Mthyl-Benzene | 7.994 | 0.50 ± 0.07a | 0.47 ± 0.08a | 0.755 | 0.28 ± 0.03a | 0.25 ± 0.02a | 0.369 |
| O-Xylene | 9.08 | 0.45 ± 0.06a | 0.43 ± 0.07a | 0.836 | 0.25 ± 0.02a | 0.22 ± 0.01a | 0.203 |
| α-Pinene | 10.53 | 9.96 ± 0.38a | 9.75 ± 1.44a | 0.733 | 12.35 ± 2.77a | 9.31 ± 0.76a | 0.238 |
| β-Cymene | 11.875 | 11.61 ± 0.54a | 10.13 ± 1.17a | 0.235 | 10.26 ± 1.37a | 9.47 ± 1.49a | 0.703 |
| β-Pinene | 12.081 | 0.43 ± 0.03a | 0.33 ± 0.06a | 0.109 | 0.52 ± 0.10a | 0.32 ± 0.04a | 0.086 |
| β-Myrcene | 12.545 | 1.15 ± 0.11a | 0.87 ± 0.10a | 0.087 | 1.79 ± 0.15a | 1.10 ± 0.05b | 0.003 |
| (+)-4-Carene | 12.84 | 50.19 ± 2.89a | 43.17 ± 6.76a | 0.314 | 44.25 ± 9.16a | 41.45 ± 3.49a | 0.072 |
| l-Phellandrene | 13.088 | 16.98 ± 1.22a | 14.00 ± 2.41a | 0.259 | 11.61 ± 0.83a | 14.75 ± 1.30a | 0.069 |
| (+)-2-Carene | 13.453 | 3.36 ± 0.25a | 2.87 ± 0.55a | 0.392 | 2.79 ± 0.49a | 3.10 ± 0.33a | 0.607 |
| A | 13.719 | 1.34 ± 0.07a | 1.11 ± 0.13a | 0.105 | 1.54 ± 0.23a | 1.02 ± 0.10a | 0.080 |
| β-Phellandrene | 13.926 | 210.95 ± 15.22a | 187.68 ± 28.69a | 0.457 | 189.09 ± 14.63a | 158.75 ± 18.55a | 0.228 |
| β-Ocimene | 14.469 | 0.20 ± 0.02a | 0.19 ± 0.03a | 0.707 | 0.21 ± 0.05a | 0.098 ± 0.01b | 0.037 |
| B | 14.843 | 0.73 ± 0.06a | 0.58 ± 0.11a | 0.237 | 0.47 ± 0.04a | 0.55 ± 0.03a | 0.147 |
| α-Terpinolene | 15.731 | 0.74 ± 0.07a | 0.65 ± 0.14a | 0.517 | 0.82 ± 0.16a | 0.49 ± 0.07a | 0.083 |
| Tridecane | 16.227 | 0.33 ± 0.01a | 0.31 ± 0.02a | 0.393 | 0.15 ± 0.04a | 0.06 ± 0.03a | 0.109 |
| Nonanal | 16.381 | 0.40 ± 0.02a | 0.36 ± 0.01a | 0.053 | 0.43 ± 0.07a | 0.24 ± 0.07a | 0.068 |
| Naphthalene | 18.829 | 1.47 ± 0.14a | 1.05 ± 0.16a | 0.066 | 0.58 ± 0.04a | 0.51 ± 0.04a | 0.323 |
| Decanal | 19.503 | 0.10 ± 0.01a | 0.08 ± 0.01a | 0.09 | 0.07 ± 0.01a | 0.06 ± 0.02a | 0.923 |
| C | 21.818 | 0.07 ± 0.01a | 0.06 ± 0.01a | 0.327 | 0.42 ± 0.04a | 0.42 ± 0.05a | 0.963 |
| Methyl-naphthalene | 22.085 | 0.29 ± 0.01a | 0.22 ± 0.03a | 0.075 | 0.19 ± 0.02a | 0.14 ± 0.01a | 0.053 |
| D | 22.353 | 0.17 ± 0.03a | 0.12 ± 0.02a | 0.2 | 0.18 ± 0.04a | 0.09 ± 0.02a | 0.078 |
| Tridecanol | 22.678 | 0.09 ± 0.01a | 0.1 ± 0.01a | 0.114 | 0.11 ± 0.01a | 0.08 ± 0.01a | 0.590 |
| β-Elemene | 23.206 | 0.16 ± 0.02a | 0.18 ± 0.03a | 0.62 | 0.27 ± 0.05a | 0.15 ± 0.02b | 0.035 |
| Tetradecane | 24.934 | 0.23 ± 0.01a | 0.22 ± 0.02a | 0.906 | 0.25 ± 0.03a | 0.31 ± 0.04a | 0.221 |
| β-Caryophyllene | 25.494 | 0.84 ± 0.06a | 0.72 ± 0.13a | 0.421 | 1.34 ± 0.12a | 0.34 ± 0.17b | 0.001 |
| α-Humulene | 26.416 | 0.39 ± 0.03a | 0.35 ± 0.06a | 0.574 | 0.65 ± 0.06a | 0.33 ± 0.04b | 0.001 |
| Dibutyl phthalate | 37.637 | 1.70 ± 0.07a | 1.51 ± 0.05a | 0.053 | 0.66 ± 0.06a | 0.53 ± 0.04a | 0.880 |
| E | 43.442 | 0.36 ± 0.03a | 0.32 ± 0.03a | 0.444 | 0.34 ± 0.06a | 0.25 ± 0.04a | 0.234 |
| Squalene | 46.037 | 0.33 ± 0.05a | 0.38 ± 0.03a | 0.374 | 0.35 ± 0.05a | 0.31 ± 0.05a | 0.877 |
HQ /HB, TYLCV-free tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q/B; VQ /VB, TYLCV-infected tomato plants infested with 3rd-instar nymphs of B. tabaci biotype Q/B. A, Methyl-(1-methylethyl)-Benzene; B, 1-methyl-4-(1-methylethyl)-1,4-Cyclohexadiene; C, [1,1′-Bicyclopentyl]-2-One; D, 1-methyl-4-(1-methylethyl)-2,3-Dioxabicyclo-5-ene; E, 1,2-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester. Values are means ± SE. Means with different letters are significantly different according to the Tukey test at P < 0.05. Significantly different pairs are in bold.
Attraction verification of E. formosa to synthetic volatiles
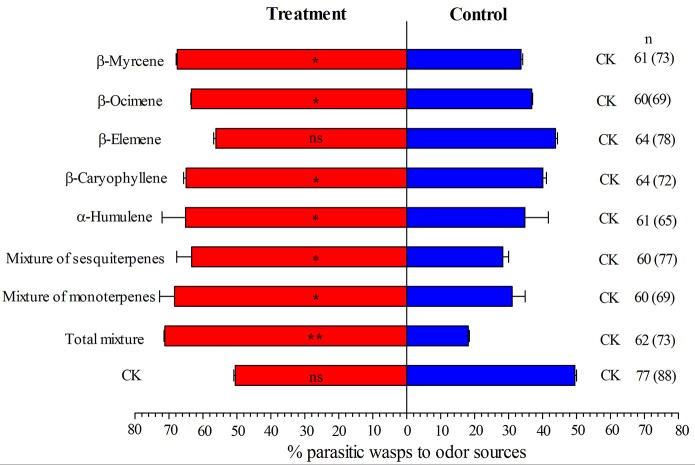
Results from olfactory test indicated that the mixture of monoterpenes, mixture of sesquiterpenes and mixture of all synthetic volatiles were all strongly attractive for E. formosa. In the meanwhile, synthetic β-Myrcene, β-Ocimene, β-Caryophyllene, and α-Humulene attracted E. formosa females (Figure 3). β-Elemene did not attract significantly E. formosa females (Figure 3).
Figure 3.
Responses of Encarsia formosa to synthetic volatiles and paroline oil (CK) in a Y-tube olfactometer. Bars (means + SE) indicate the percentages of wasps choosing either of the odor sources. The numbers to the right of bars indicate the number of wasps making a choice, and the total number of wasps used in the assay is indicated in parentheses. Asterisks indicate significant differences between the treatment pairs (chi-square test; *P < 0.05; **P < 0.001; ns, not significant).
Discussion
In addition to the parasitoid E. formosa, the current study included two major threats on the tomato plant: biotypes Q or B of B. tabaci and the virus TYLCV. In the experiments in which the parasitoid could choose between B. tabaci Q and B on tomato leaves in the laboratory and on tomato plants in the greenhouse, the results proved to follow the same trend, i.e., E. formosa parasitized significantly more whitefly Q nymphs than B nymphs on TYLCV-infected plants but not on TYLCV-free tomato plants. The results of Y-tube olfactometer assays also showed that naive females of E. formosa exhibited a stronger olfactory preference for tomato plants infested with 3rd-instar nymphs of biotype Q than B when the plants were infected with TYLCV but not for the TYLCV-free plants. This would be helpful for the control of whiteflies populations and virus spread in the field, because the parasitoids, the higher trophic level, prefer to parasitize B. tabaci Q biotype (the prominent species in China and vectoring TYLCV widely) on TYLCV-infected plants.
Results from other systems also indicated that parasitoid attraction or parasitism rate increased when the herbivore-infested plant was infected by a pathogen (Cardoza et al., 2003; Tack et al., 2012; Ponzio et al., 2016b). For instance, the parasitoid Cotesia glomerata was more attracted to Brassica nigra plants that were both infested by Pieris brassicae larvae and infected by Xanthononas campestris than to plants that were only infested with the larvae (Ponzio et al., 2016b). However, other reports indicated that simultaneous attack by an herbivorous insect and a pathogenic fungus did not affect parasitoid foraging (Rostas et al., 2006), indicating that such effect on parasitism foraging may differ with different research systems (Ponzio et al., 2013).
The different responses of E. formosa to B. tabaci Q vs. B on TYLCV-infected tomato plants vs. TYLCV-free tomato plants was related to quantitative (but not qualitative) differences in certain components of the volatiles emitted by the tomato plants; such differences were not evident in the volatiles emitted from TYLCV-free plants infested with B. tabaci Q vs. B. There are also other studies indicated that quantitative differences in volatiles released from plant could alter parasitoid behavior (McCormick et al., 2012; Ponzio et al., 2016b). For example, the olfactometer differences of parasitoids C. glomerata on different plant treatments are due to quantitative differences of released volatile compounds of Brussels sprout plants (Ponzio et al., 2016b).
In this present study, the main components of the volatiles that exhibited quantitative changes in response to simultaneous attack by TYLCV and B. tabaci were the terpenoids β-Myrcene, β-Ocimene, β-Caryophyllene, α-Humulene, and β-Elemene. The quantities of these components were higher in TYLCV-infected tomato plants, but not TYLCV-free tomato plants, were infested with B. tabaci Q rather than B. In subsequent olfactometer assays with synthetic compounds, E. formosa was attracted to β-Myrcene, β-Ocimene, β-Caryophyllene, and α-Humulene. β-Myrcene has been proved to strongly attract E. formosa adult (Zhang et al., 2013b). Because odors emitted by plants are mixtures of volatile compounds, a complete behavioral response of associated organisms is more likely to be elicited by multiple compounds than by single compounds (Li et al., 2014). Consistent with that view, E. formosa was strongly attracted to the mixture of β-Myrcene and β-Ocimene, the mixture of β-Caryophyllene, α-Humulene, and β-Elemene, and the total mixture.
In addition, plants will attempt to defend themselves by activating the salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) signaling pathways, when threatened by herbivores or pathogens (Ponzio et al., 2016b; Su et al., 2016). Feeding by B. tabaci B biotype nymphs could induce SA defenses and suppress JA defenses of Arabidopsis (Arabidopsis thaliana) plants (Zarate et al., 2007; Zhang et al., 2013a). Previous studies found that challenges by B. tabaci Q or B adults have different effects on plant defenses, and that TYLCV infection alters the plant's defense response to B. tabaci (Shi et al., 2013, 2014). Moreover, TPS7 encoding an enzyme that catalyzes the formation of β-Myrcene, TPS12 encoding an enzyme that catalyzes the formation of the sesquiterpenes β-Caryophyllene and α-Humulene, and TPS5 (which encodes monoterpenes) are important terpene synthase (TPS) genes in tomato and are induced by JA or SA treatment (Vasiliki Falara et al., 2011; Simon Zebelo et al., 2014). Therefore, it is speculated that feeding by B. tabaci Q vs. B nymphs on TYLCV-infected tomato plants may activate different defense singling pathways, altering the expression of SA-regulated and JA-regulated genes that encode volatile terpenes and thereby affecting E. formosa attraction and parasitism (Bruce et al., 2008; Zhang et al., 2013a,b; Wu et al., 2017). This possibility needs more additional and deeper studies.
In conclusion, TYLCV-infected plants infested with the B. tabaci Q nymphs were significantly more attractive to E. formosa than TYLCV-infected plants infested with B. tabaci B nymphs. The difference in attraction was associated with increased quantities of β-Myrcene, β-Ocimene, β-Caryophyllene, and α-Humulene in TYLCV-infected plants infested with B. tabaci Q nymphs.
Author contributions
XL designed and performed the experiments, wrote and revised the manuscript. GC conceived the idea, and performed the experiments. SW conceived the idea and reviewed the manuscript. YZ, WX, and QW coordinated and designed the study. All the authors have read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are greatly grateful to Dr Xuguo Zhou (University of Kentucky) for the invaluable advice on the a previous version of the manuscript, Dr Xueping Zhou (Institute of Biotechnology, Zhejiang University, Hangzhou, China) for providing the infectious TYLCV clone.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (31572014), National Key Research and Development Plan (2017YFD0200400), and the China Agriculture Research System (CARS-25), and the Beijing Key Laboratory for Pest Control and Sustainable Control. The granting agencies had no role in study design, data collection, analysis and interpretation, decision to publish, or manuscript preparation.
References
- Brown J. K., Czosnek H. (2002). Whitefly transmission of plant viruses. Adv. Bot. Res. 36, 65–76. 10.1016/S0065-2296(02)36059-2 [DOI] [Google Scholar]
- Bruce T. J. A., Matthes M. C., Chamberlain K., Woodcock A. M., Mohib A., Webster B., et al. (2008). Cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. U.S.A. 105:4553–4558. 10.1073/pnas.0710305105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoza Y. J., Teal P. E. A., Tumlinson J. H. (2003). Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ. Entomol. 32, 970–976. 10.1603/0046-225X-32.5.970 [DOI] [Google Scholar]
- Chen G., Su Q., Shi S. B., Liu X., Peng Z. K., Zheng H. X., et al. (2017). Odor, Not performance, dictates Bemisia tabaci's selection between healthy and virus infected plants. Front. Physiol. 8:146. 10.3389/fphys.2017.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D., Wan F. H., Zhang Y. J., Brown J. K. (2010a). Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ. Entomol. 39, 1028–1036. 10.1603/EN09161 [DOI] [PubMed] [Google Scholar]
- Chu D., Zhang Y. J., Wan F. H. (2010b). Cryptic invasion of the exotic Bemisia tabaci biotype Q occurred widespread in Shandong province of China. Fla. Entomol. 93, 203–207. 10.1653/024.093.0209 [DOI] [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M., Dinsdale A. B. (2011). Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19. 10.1146/annurev-ento-112408-085504 [DOI] [PubMed] [Google Scholar]
- Falara V., Akhtar T. A., Nguyen T. T. H., Spyropoulou E. A., Bleeker P. M., Schauvinhold I., et al. (2011). The tomato terpene synthase gene family. Plant Physiol. 157, 770–789. 10.1104/pp.111.179648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling D., Alomar O., Arno J. (2001). Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 20, 779–799. 10.1016/S0261-2194(01)00111-9 [DOI] [Google Scholar]
- Ghanim M., Sobol I., Ghanim M., Czosnek H. (2007). Horizontal transmission of begomoviruses between Bemisia tabaci biotypes. Arthropod Plant Interact. 1, 195–204. 10.1007/s11829-007-9018-z [DOI] [Google Scholar]
- Grille G., Lorenzo M. E., Burla J. P., Franco J., Basso C. (2012). Parasitoid niches of Encarsia formosa and Encarsia lycopersici (Hymenoptera: Aphelinidae) exploiting Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Fla. Entomol. 95, 1024–1030. 10.1653/024.095.0431 [DOI] [Google Scholar]
- Jones D. R. (2003). Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 109, 195–219. 10.1023/A:1022846630513 [DOI] [Google Scholar]
- Li S. J., Ren S. L., Xue X., Ren S. X., Cuthbertson A. G. S., van Dam N. M., et al. (2014). Efficiency of plant induced volatiles in attracting Encarsia formosa and Serangium japonicum, two dominant natural enemies of whitefly Bemisia tabaci in China. Pest Manag. Sci. 70, 1604–1610. 10.1002/ps.3749 [DOI] [PubMed] [Google Scholar]
- Liu T. X., Stansly P. A., Gerling D. (2015). Whitefly parasitoids: distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 60, 273–292. 10.1146/annurev-ento-010814-021101 [DOI] [PubMed] [Google Scholar]
- Liu X. Y., Xiang W. S., Jiao X. G., Zhang Y. J., Xie W., Wu Q. J., et al. (2014). Effects of plant virus and its insect vector on Encarsia formosa, a biocontrol agent of whiteflies. Sci. Rep. 4:5926. 10.1038/srep05926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Y. J., Xie W., Wu Q. J., Wang S. L. (2016). The suitability of biotypes Q and B of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) at different nymphal instars as hosts for Encarsia formosa Gahan (Hymenoptera: Aphelinidae) PeerJ. 4:e1863. 10.7717/peerj.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini X., Pelz-Stelinski K. S., Stelinski L. L. (2014). Plant pathogen-induced volatiles attract parasitoids to increase parasitism of an insect vector. Front. Ecol. Evol. 2:8 10.3389/fevo.2014.00008 [DOI] [Google Scholar]
- Mauck K. E., De Moraes C. M., Mescher M. C. (2015). Infection of host plants by Cucumber mosaic virus increases the susceptibility of Myzus persicae aphids to the parasitoid Aphidius colemani. Sci. Rep. 5:10963. 10.1038/srep10963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C. A., Unsicker S. B., Gershenzon J. (2012). The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 17:303 10.1016/j.tplants.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Ning W. X., Shi X. B., Liu B. M., Pan H. P., Wei W. T., Zeng Y., et al. (2015). Transmission of tomato yellow leaf curl virus by Bemisia tabaci as affected by whitefly sex and biotype. Sci. Rep. 5:10744. 10.1038/srep10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Li Y. X., Luan J. B., Liu S. S., Liu Y. Q. (2014). Olfactory responses of the whitefly Bemisia tabaci and its parasitoid Eremocerus hayati to tobacco infected by the Tomato yellow leaf curl China virus. Chin. J. Appl. Entomol. 51, 60–66. 10.7679/j.issn.2095-1353.2014.007 [DOI] [Google Scholar]
- Pan D., Wang L. L., Liu S. S., Li Y. X., Liu Y. Q. (2013). Effects of begomovirus infection of tomato plants on leaf trichome densityz and foraging performance and fitness of Eretmocerus hayati (Hymenoptera: Aphelinidae), a parasitoid of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Acta Entomol. Sin. 56, 644–651. 10.16380/j.kcxb.2013.06.006 [DOI] [Google Scholar]
- Pan H. P., Chu D., Ge D. Q., Wang S. L., Wu Q. J., Xie W., et al. (2011). Further spread of and domination by Bemisia tabaci biotype Q on field crops in China. J. Econ. Entomol. 104, 978–985. 10.1603/EC11009 [DOI] [PubMed] [Google Scholar]
- Pan H. P., Chu D., Yan W. Q., Su Q., Liu B. M., Wang S. L., et al. (2012). Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 7:e34817. 10.1371/journal.pone.0034817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio C., Cascone P., Cusumano A., Weldegergis B. T., Fatouros N. E., Guerrieri E., et al. (2016a). Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim. Behav. 111, 197–206. 10.1016/j.anbehav.2015.10.024 [DOI] [Google Scholar]
- Ponzio C., Gols R., Pieterse C. M. J., Dicke M. (2013). Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 27, 587–598. 10.1111/1365-2435.12035 [DOI] [Google Scholar]
- Ponzio C., Weldegergis B. T., Dicke M., Gols R. (2016b). Compatible and incompatible pathogen-plant interactions differentially affect plant volatile emissions and the attraction of parasitoid wasps. Funct. Ecol. 30, 1779–1789. 10.1111/1365-2435.12689 [DOI] [Google Scholar]
- Rostas M., Ton J., Mauch-Mani B., Turlings T. (2006). Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naive parasitoids. J. Chem. Ecol. 32, 1897–1909. 10.1007/s10886-006-9147-3 [DOI] [PubMed] [Google Scholar]
- Shi X. B., Pan H. P., Xie W., Wu Q. J., Wang S. L., et al. (2013). Plant virus differentially alters the plant's defense response to its closely related vectors. PLoS ONE 8:e83520. 10.1371/journal.pone.0083520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. B., Pan H. P., Zhang H. Y., Jiao X. G., Xie W., Wu Q. J., et al. (2014). Bemisia tabaci Q carrying tomato yellow leaf curl virus strongly suppresses host plant defenses. Sci. Rep. 4:5230. 10.1038/srep05230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q., Mescher M. C., Wang S. L., Chen G., Xie W., Wu Q. J., et al. (2016). Tomato yellow leaf curl virus differentially influences plant defence responses to a vector and a non-vector hervibore. Plant Cell Environ. 39, 597–607. 10.1111/pce.12650 [DOI] [PubMed] [Google Scholar]
- Tack A. J. M., Gripenberg S., Roslin T. (2012). Cross-kingdom interactions matter: fungal-mediated interactions structure an insect community on oak. Ecol. Lett. 15, 177–185. 10.1111/j.1461-0248.2011.01724.x [DOI] [PubMed] [Google Scholar]
- Teng X., Wan F. H., Chu D. (2010). Bemisia tabaci biotype Q dominates other biotypes across China. Fla. Entomol. 93, 363–368. 10.1653/024.093.0307 [DOI] [Google Scholar]
- Wei J. L., Wang L. Z., Zhu J. W., Zhang S. F., Nandi O. I., Kang L. (2007). Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 9:e852 10.1371/journal.pone.0000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. W., Qi T. C., Li W. L., Tian H. X., Gao H., Wang J. J., et al. (2017). Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 27, 402–415. 10.1038/cr.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate S. I., Kempema L. A., Walling L. L. (2007). Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143, 866–875. 10.1104/pp.106.090035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebelo S., Piorkowski J., Disi J., Fadamiro H. (2014). Secretions from the ventral eversible gland of Spodoptera exigua caterpillars activate defense related genes and induce emission of volatile organic compounds in tomato, Solanum lycopersicum. BMC Plant Biol. 14:140. 10.1186/1471-2229-14-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. J., Li W. D., Huang F., Zhang J. M., Xu F. C., Lu Y. B. (2013a). Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 39, 612–619. 10.1007/s10886-013-0283-2 [DOI] [PubMed] [Google Scholar]
- Zhang P. J., Xu C. X., Zhang J. M., Lu Y. B. (2013b). Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct. Ecol. 27, 1304–1312. 10.1111/1365-2435.12132 [DOI] [Google Scholar]
- Zheng H. X., Xie W., Wang S. L., Wu X., Zhou Z., Zhang Y. (2017). Dynamic monitoring (B vs. Q) and further resistance status of Bemisia tabaci in China. Crop Prot. 94, 115–122. 10.1016/j.cropro.2016.11.035 [DOI] [Google Scholar]