Abstract
Key objectives in the treatment of multiple sclerosis (MS) include prevention of relapses, a reduction in the accumulation of disability and slowing of the brain volume loss that occurs from the earliest stages of the disease. Teriflunomide, a once-daily, oral immunomodulatory therapy, has demonstrated efficacy across multiple measures of disease activity and worsening in patients with relapsing forms of MS and in those with a first clinical episode suggestive of MS. In this review, the latest evidence relating to the proposed mechanism of action of teriflunomide in MS is explored, including novel insights provided from the recently completed Teri-DYNAMIC study. Key clinical and magnetic resonance imaging data from the completed long-term extensions of the phase II and III (TEMSO, TOWER and TOPIC) studies are highlighted, and the long-term safety profile of teriflunomide, as evidenced by data from these extension studies, is presented. Although randomized clinical trials represent the highest level of evidence to support the use of therapeutic interventions, it is also important to understand the performance of a particular treatment in the real-world setting. In this regard, the results of the recently completed, global, phase IV Teri-PRO study are of particular interest and provide further insights into the benefits of teriflunomide treatment from the patient perspective. Collectively, the data presented in this review demonstrate a favorable benefit–risk profile for teriflunomide, thereby supporting its long-term use for the treatment of patients with relapsing forms of MS.
Keywords: brain volume loss, clinical trials, disability, multiple sclerosis, relapsing, relapsing-remitting, teriflunomide, long-term safety outcomes
Introduction
Multiple sclerosis (MS) is a progressive and sometimes debilitating disease characterized by demyelination and axonal loss in the central nervous system (CNS). An estimated 2.3 million people worldwide are affected by MS, although prevalence rates vary greatly.1 MS affects more women than men (ratio of up to 3:1), and the majority of patients present during early adulthood with relapsing–remitting MS.2 Over time, relapsing–remitting MS often transitions into secondary progressive MS (SPMS), characterized by a progressive accumulation of disability in the absence of relapses.3 Approximately 10–15% of patients experience a progressive course from the outset.4
All forms of MS are characterized by an increased rate of brain volume loss (BVL) compared with the heathy population, primarily as a result of demyelination and axonal loss.5 An increased rate of BVL has been associated with long-term disease worsening in MS, in terms of both physical and cognitive decline.6,7 Although the mechanisms underlying this relationship remain unclear, minimizing BVL early in the disease course is likely to help delay accumulation of physical and cognitive disability. Thus, prevention of relapses, disability worsening and slowing BVL are important clinical outcomes to consider when evaluating treatment efficacy.
The current treatment landscape in MS mandates use of supplementary outcome measures that assess treatment effectiveness beyond established clinical and magnetic resonance imaging (MRI) endpoints. Regulatory agencies now recognize the importance of including patient-reported outcomes (PROs) in clinical trials.8,9 Methodologies such as number needed to treat (NNT) may also be useful for comparing treatment effectiveness in the absence of head-to-head trials.10–12 Additionally, real-world studies can provide useful data with regard to treatment effectiveness outside the highly controlled conditions of randomized clinical trials.
Since the introduction of the first disease-modifying therapy (DMT), interferon β-1b (IFNβ-1b, Bayer Healthcare Pharmaceuticals, Inc., Whippany, NJ, 2016) for the treatment of relapsing–remitting MS,13 the treatment landscape has broadened considerably, providing physicians and patients with an array of options with distinct mechanisms of action, administration routes and benefit–risk profiles.
Here, the efficacy and safety profile of teriflunomide, a once-daily oral immunomodulatory treatment approved for relapsing forms of MS (RMS) in 70 countries, is specifically evaluated. Approximately 74,000 patients are currently being treated with teriflunomide worldwide. References to date, included herein, include both published data and, when data are unpublished, congress presentations.
Teriflunomide mechanism of action
The proposed mechanism of action (MoA) of teriflunomide in MS has been reviewed and comprehensively discussed previously.14 In summary, teriflunomide acts via dihydro-orotate dehydrogenase (DHODH), a key mitochondrial enzyme in the de novo pyrimidine synthesis pathway, which is highly expressed in proliferating lymphocytes. The selective and reversible inhibition of DHODH by teriflunomide results in a reduction in proliferation of activated T and B lymphocytes in the periphery, thereby reducing their availability to cross the blood–brain barrier and contribute to damaging processes within the CNS. Resting lymphocytes rely on the pyrimidine salvage pathway and are therefore unaffected by teriflunomide and remain available to mount normal protective immune responses.
Further insights into the teriflunomide MoA have come from the recently completed Teri-DYNAMIC study [ClinicalTrials.gov identifier: NCT01863888], which evaluated the effects of teriflunomide on lymphocyte subsets, T-cell receptor repertoire and T-cell function in patients with relapsing–remitting MS compared with healthy individuals. Data from the Teri-DYNAMIC study suggest that teriflunomide treatment results in a shift in T-cell populations from proinflammatory to regulatory subtypes, with no adverse effects on proliferative and cytokine responses, consistent with an immunomodulatory MoA.15,16 Furthermore, patients with MS have significantly increased levels of unique CD4+ T-cell receptor clones, suggestive of immune dysregulation. Treatment with teriflunomide was shown to lower these levels to those comparable to levels observed in healthy individuals; this finding is suggestive of normalization of immune regulation. This effect has not been observed with other DMTs [IFNβ, dimethyl fumarate (DMF), mitoxantrone].16,17
The effect of teriflunomide on protective immunity has been investigated in two clinical studies that evaluated immune responses to vaccination.18,19 In TERIVA [ClinicalTrials.gov identifier: NCT01403376], patients with MS treated with teriflunomide were able to mount adequate immune responses to the seasonal influenza vaccine whereas, in a second study, teriflunomide did not impair the ability of healthy individuals to mount a protective immune response to rabies vaccine.18,19 Both studies demonstrated that immune responses are preserved in individuals receiving teriflunomide.14 Furthermore, pooled data from four clinical studies demonstrated that teriflunomide treatment resulted in reductions in lymphocyte and neutrophil counts of about 15% within the first 3 months, which stabilized thereafter. Values largely remained within normal range, and no increase in risk of serious infections or malignancy was observed.20,21
Although the bulk of evidence currently suggests that teriflunomide exerts its effects in MS via its impact on peripheral immune cell function, there has been growing interest in potential direct effects within the CNS. Recently, the direct effects of teriflunomide on rat and mouse CNS immune resident cells have been investigated in vitro.22 Pretreatment with teriflunomide did not affect microglia or astrocyte viability or microglial phagocytic activity. However, pretreatment with teriflunomide did result in decreased production of proinflammatory mediators [interleukin (IL)-6, IFNγ-induced protein 10, monocyte chemoattractant protein 1, IL-12, p40 and nitrite] in activated microglia and astrocytes, an increase in the anti-inflammatory cytokine IL-10 production from activated microglia and a reduction in tumor necrosis factor α, nitrite and IL-1β production from activated astrocytes. Activated astrocytes were also protected from hydrogen peroxide induced cytotoxicity. Together with a previous observation that teriflunomide crosses the blood–brain barrier and reaches clinically relevant concentrations in the CNS (~2.5–4.1 μm),23 these findings suggest teriflunomide may potentially have direct neuroprotective effects in the central compartment. Additional studies are required to confirm this hypothesis.
Clinical efficacy
Teriflunomide clinical development program: placebo-controlled studies
The efficacy of teriflunomide has been demonstrated in phase II and III randomized placebo-controlled studies of patients with RMS24–26 and with a first clinical episode suggestive of MS.27 Across these studies, teriflunomide has shown consistent effects on multiple markers of disease activity, including annualized relapse rates (ARRs), disability worsening and MRI outcomes. Teriflunomide is the only approved oral DMT to have demonstrated significant reductions in disability worsening in two phase III trials. These studies have been reviewed extensively in previous publications and are beyond the scope of this article; selected outcomes are summarized in Table 1. In addition to these studies, a smaller phase III study demonstrated that teriflunomide may be considered as an alternative therapy for patients with RMS for whom treatment with IFN is being considered. In the TENERE study [ClinicalTrials.gov identifier: NCT00883337], in which patients were treated with teriflunomide or subcutaneous IFNβ-1a for at least 48 weeks (maximum exposure ~115 weeks), effects on time to failure (primary composite endpoint defined as first occurrence of confirmed relapse or permanent treatment discontinuation for any cause) were comparable between teriflunomide and IFNβ-1a groups.28 However, patients reported greater satisfaction and less fatigue with teriflunomide than with IFNβ-1a.28
Table 1.
Summary of selected outcomes in phase II and III placebo-controlled teriflunomide studies.
| Phase II [ClinicalTrials.gov identifier: NCT01487096]24 |
TEMSO [ClinicalTrials.gov identifier: NCT00134563] 25 |
TOWER [ClinicalTrials.gov identifier: NCT00751881]26 |
TOPIC [ClinicalTrials.gov identifier: NCT00622700]27 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study duration: 36 weeks |
Study duration: 108 weeks |
Study duration: variable (48 weeks after the last patient was randomized) |
Study duration: ⩽108 weeks |
|||||||||
| Placebo | Teriflunomide |
Placebo | Teriflunomide |
Placebo | Teriflunomide |
Placebo | Teriflunomide |
|||||
| 7 mg | 14 mg | 7 mg | 14 mg | 7 mg | 14 mg | 7 mg | 14 mg | |||||
| Patients entering study, n | 61 | 61 | 57 | 363 | 365 | 358 | 388 | 407 | 370 | 197 | 203 | 214 |
| Clinical endpoints | ||||||||||||
| ARR | 0.81 | 0.58 | 0.55 | 0.54 | 0.37 | 0.37 | 0.50 | 0.39 | 0.32 | 0.284 | 0.190 | 0.194 |
| Relative risk reduction (versus control), % | 28 | 32 | 31.2 | 31.5 | 22.3 | 36.3 | 33.1 | 31.9 | ||||
| p value | NS | NS | p < 0.001 | p < 0.001 | p = 0.018 | p = 0.0001 | p = 0.054 | p = 0.058 | ||||
| Patients relapse free,*$ % | 62 | NR | 77 | 45.6 | 53.7 | 56.5 | 60.6 | 71.9 | 76.3 | – | – | – |
| p value (versus control) | p = 0.098 | p = 0.01 | p = 0.003 | p = 0.002 | p < 0.001 | |||||||
| Disability endpoints | ||||||||||||
| Patients with 12-week CDW,‡ % | 27.3 | 21.7 | 20.2 | 19.7 | 21.1 | 15.8 | 10§ | 10§ | 7§ | |||
| Risk reduction (versus placebo), % | 23.7 | 29.8 | 4.5 | 31.5 | 2.2 | 29.9 | ||||||
| p value (versus placebo) | p = 0.08 | p = 0.03 | p = 0.762 | p = 0.044 | p = 0.995 | p = 0.424 | ||||||
| Patients with increased EDSS score at endpoint versus baseline, % | 7.4 | – | 21.3 | |||||||||
| p value (versus placebo) | p < 0.04 | |||||||||||
| Change in EDSS score from baseline, mean | – | – | – | – | – | – | 0.09 | 0.04 | –0.05 | –0.056 | –0.250 | –0.265 |
| p value (versus placebo) | p = 0.482 | p = 0.043 | p = 0.033 | p = 0.044 | ||||||||
| MRI endpoints | ||||||||||||
| Change in total lesion volume from baseline, mean, ml | 2.21 | 1.31 | 0.72 | – | – | – | 0.044 | 0.023 | –0.028 | |||
| Relative reduction (versus placebo), % | 39.4 | 67.4 | – | – | ||||||||
| p value | p = 0.03 | p < 0.001 | p = 0.779 | p = 0.037 | ||||||||
| Gd-enhancing T1 lesions per scan, mean, n | 2.25 | 0.87 | 0.32 | 1.33 | 0.57 | 0.26 | 0.953 | 0.749 | 0.395 | |||
| Difference, mean | −1.38 | −1.39 | – | – | – | |||||||
| Relative risk | NR | NR | 0.43 | 0.20 | 21.4 | 58.5 | ||||||
| p value (versus placebo) | p < 0.04 | p < 0.02 | p < 0.001 | p < 0.001 | p = 0.444 | p = 0.001 | ||||||
| CU active lesions per scan, mean, n | 2.68 | 1.04 | 1.06 | 2.46 | 1.29 | 0.75 | ||||||
| Relative reduction, % | 61 | 61 | 69 | 48 | – | – | – | – | – | – | ||
| Relative risk | NR | NR | 0.52 | 0.31 | ||||||||
| p value (versus placebo) | p < 0.03 | p < 0.01 | p < 0.001 | p < 0.001 | ||||||||
In TEMSO and TOWER, defined as free from protocol-defined relapse at 48 weeks.
In TOPIC, a relapse was defined as a new neurologic abnormality separated by at least 30 days from a preceding clinical event, present for at least 24 h in the absence of fever or known infection.
Values in TOWER are those after 108 weeks of treatment.
Patients presenting with a first episode suggestive of MS do not have prolonged disease.
ARR, annualized relapse rate; CDW, confirmed disability worsening; CU, combined unique; EDSS, Expanded Disability Status Scale; Gd, gadolinium; MS, multiple sclerosis; NR, not recorded; NS, not significant.
Long-term outcomes: clinical trial extension studies
Long-term extension data from phase II [ClinicalTrials.gov identifier: NCT00228163], TEMSO [ClinicalTrials.gov identifier: NCT00803049], TOWER and TOPIC extension studies (up to 13, 10.5, 6 and 7 years of follow up, respectively) provide evidence in support of sustained long-term efficacy of teriflunomide for the treatment of MS.29–33 Nonetheless, the risk of attrition bias due to dropout rates should be considered; percentages of patients completing the extension studies ranged from about 40% in the phase II extension to 75% in the TOPIC extension.29–33
Disability worsening
Mean Expanded Disability Status Scale (EDSS) scores remained low and stable over the duration of the phase II TEMSO and TOWER extension studies.29–31,33 Large proportions of patients remained free of 12-week confirmed disability worsening (CDW) in the TEMSO extension (>50% for up to 9 years) and the TOPIC extension (⩾78%).32,33 Pooled data from the TEMSO and TOWER core and extension studies also showed that, after 5 years of treatment, very few patients receiving teriflunomide 14 mg had advanced to EDSS scores of at least 6 or at least 7 confirmed for 12 weeks or longer (3.9% and 0.4%, respectively).34 Furthermore, in a population of recently diagnosed patients (⩽1 year since diagnosis and previously untreated with DMTs), the vast majority of patients did not advance to EDSS scores of at least 4 (90.1%) or at least 6 (96.9%) confirmed for 12 weeks or longer, with treatment of up to 9 years.35 These observations are of interest as advancement to higher EDSS scores is associated with a substantial impact on patient health-related quality of life and increased healthcare burden.36,37
Relapses
ARR remained low, typically less than 0.3 at most time points for all treatment groups, throughout the extension studies29–31 and in those patients with a first clinical episode suggestive of MS (⩽0.163 across all groups).32 In the phase II extension, ARR was lower in the teriflunomide 14 mg group (0.188) than in the 7 mg group (0.256), and greater proportions of patients remained free from relapse (teriflunomide 14 mg, 51.5%; 7 mg, 39.5%).29 In the TEMSO extension, there was a decline in ARR in patients switching from placebo to teriflunomide on entering the extension. ARR remained low in all treatment groups (placebo/7 mg, placebo/14 mg, 7 mg/7 mg and 14 mg/14 mg) during the extension, and by the conclusion of the study ARR was numerically lower than at the end of the core study.30 In the TOWER extension, in which all patients received teriflunomide 14 mg regardless of core study treatment, no significant between-group differences in ARR were observed.31 In the TOPIC combined core and extension studies, most patients (⩾63% per group, 69.7% overall) did not experience relapse determining conversion to clinically definite MS.32
MRI outcomes
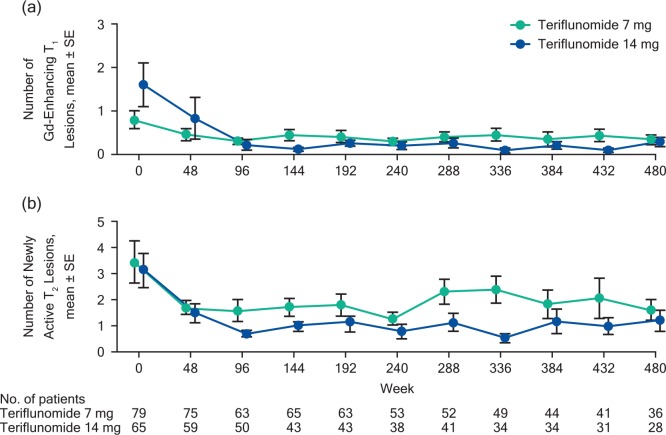
Consistent with the clinical outcomes, teriflunomide treatment was associated with favorable long-term MRI outcomes in extension studies (no MRI analysis was performed in TOWER). In the phase II extension, teriflunomide 14 mg was associated with a reduction in the number of active T2 and gadolinium-enhancing T1 lesions [Figure 1(a) and (b)] and a smaller change from baseline in T2 lesion volumes compared with teriflunomide 7 mg.29,38 In the TEMSO extension, total lesion volume (T1 and T2 volumes) remained relatively constant over the course of the study; most patients (⩾80%) did not have gadolinium-enhancing T1 lesions during the study.33
Figure 1.
Reductions in Gd-enhancing T1 lesions (a) and newly active T2 lesions (b) in the phase II extension.29,33 Gd, gadolinium; SE, standard error.
Long-term outcomes in patients switching to teriflunomide from other DMTs
In the TENERE extension [ClinicalTrials.gov identifier: NCT00883337] (up to 6 years of follow up), in which patients switched from subcutaneous IFNβ-1a to teriflunomide 14 mg, treatment satisfaction (assessed by the Treatment Satisfaction Questionnaire for Medication) increased to a score that was comparable to that reported by patients who were treated continuously with teriflunomide and was sustained for up 96 weeks.39,40 ARR remained low, with no evidence of rebound disease; despite no significant differences between groups, patients treated continuously with teriflunomide 14 mg had the lowest ARRs. EDSS scores also remained low and similar across all groups. These data support the use of teriflunomide as an effective, long-term option for managing disease activity in patients switching from subcutaneous IFNβ-1a.39 Interestingly, a post hoc analysis of pooled data from TEMSO and TOWER core studies demonstrated that switching to teriflunomide 14 mg was associated with numerically greater improvements in both ARR and disability worsening for patients having received previous DMTs compared with treatment-naïve patients.41
Long-term outcomes in patients with progressive MS with relapses
Data from up to 9 years of follow up in TEMSO and up to 5.5 years in TOWER indicate that teriflunomide may also have a beneficial effect in patients with progressive MS with relapses (SPMS or progressive relapsing MS). Using pooled TEMSO/TOWER data, the majority of the 122 patients with progressive MS with relapses did not experience either at least 12- or at least 24-week CDW (80.3% and 83.6%, respectively). Furthermore, although this patient subgroup had relatively high baseline EDSS scores, very few reached EDSS scores of at least 6 (12%) or at least 7 (5%) confirmed for 12 or longer or 24 weeks or longer.42
Brain volume loss
The effect of teriflunomide on BVL was assessed in patients with RMS in an investigator-blinded structural image evaluation using normalization of atrophy (SIENA) analysis of MRI scans from the TEMSO core study.43 In the primary analysis of the SIENA data, teriflunomide 14 mg significantly slowed BVL compared with placebo at both years 1 and 2 (relative reduction 36.9% and 30.6%, respectively, both p = 0.0001). Similar results were observed for teriflunomide 7 mg versus placebo. In addition, teriflunomide slowed the rate of BVL versus placebo irrespective of baseline disease activity (defined according to the number of relapses or the presence of gadolinium-enhancing T1 lesions at baseline), despite the fact that BVL was accelerated in patients with greater disease activity at baseline.44 Similarly, teriflunomide also significantly reduced BVL versus placebo regardless of the presence of on-study disability worsening, despite the fact that patients who experienced 12- or 24-week CDW had greater rates of BVL (as evidenced in the untreated placebo arm).43 These latter observations are important given that greater rates of BVL over 2 years have been shown to be predictive of long-term disability worsening as demonstrated in the TEMSO extension.45 The positive effects of teriflunomide on BVL were also evident in subgroups defined according to whether or not they had received treatment with a DMT before study entry. Although teriflunomide significantly reduced BVL versus placebo in both treatment-naïve and prior-treated patients, there was a more pronounced effect in the group with prior DMT exposure,46 consistent with another analysis that has demonstrated more pronounced effects of teriflunomide on ARR and disability worsening in this patient subgroup.47 Overall, the effects of teriflunomide on BVL are in accordance with the significant effects of teriflunomide on reducing the risk of disability worsening observed in TEMSO and TOWER.25,26
Additional efficacy measures
Considerable data exist for the use of DMTs in RMS; however, there are very few head-to-head clinical trials to enable direct comparisons of efficacy, and clinicians often make comparisons on the basis of relative reductions in a specific endpoint, commonly ARR.10,11 It has been recommended that both relative and absolute risk reduction (and its inverse, NNT) be used in any cross-trial comparison to provide a more reliable measure of comparative effectiveness and, thus, therapeutic gain.11,12
Using published data from the pivotal trials of oral DMTs, NNTs were calculated for a range of efficacy outcomes. NNTs for the prevention of one relapse and for the prevention of CDW were generally similar for teriflunomide 14 mg (consistent results observed in both TEMSO and TOWER), DMF 240 mg and fingolimod 0.5 mg (Table 2). NNTs for the prevention of CDW were higher for DMF in the CONFIRM [ClinicalTrials.gov identifier: NCT00451451] study and fingolimod in the FREEDOMS II study [ClinicalTrials.gov identifier: NCT00355134]. However, NNTs were similar between teriflunomide and DMF when pooled data were compared (Table 2).11,48,49 In addition, studies have shown that teriflunomide 14 mg is associated with lower NNTs for preventing one relapse and for preventing one patient from experiencing CDW compared with injectable DMTs, pegylated IFNβ-1a 125 μg and glatiramer acetate 40 mg (glatiramer acetate disability worsening data not available for comparison).50
Table 2.
NNT to prevent one relapse or one patient experiencing CDW in TEMSO, TOWER, DEFINE, CONFIRM, FREEDOMS and FREEDOMS II.
| Teriflunomide 14 mg once daily |
Dimethyl fumarate 240 mg twice daily |
Fingolimod 0.5 mg once daily |
||||||
|---|---|---|---|---|---|---|---|---|
| TEMSO25 (n = 721)* | TOWER26 (n = 758)* | TEMSO +TOWER pooled51 (n = 1479)* | DEFINE52 (n = 818)* | CONFIRM53 (n = 722)* | DEFINE + CONFIRM pooled54 (n = 1540)* | FREEDOMS55 (n = 843) | FREEDOMS II56 (n = 713) | |
| ARR | ||||||||
| Placebo | 0.54 | 0.50 | 0.534 | 0.36 | 0.40 | 0.37 | 0.40 | 0.40 |
| Intervention | 0.37 | 0.32 | 0.354 | 0.17 | 0.22 | 0.19 | 0.18 | 0.21 |
| Relative reduction versus placebo, % | 31.5 | 36.3 | 33.7 | 53 | 44 | 49 | 54 | 48 |
| Absolute reduction$ | 0.18 | 0.18 | 0.18 | 0.19 | 0.18 | 0.18 | 0.22 | 0.19 |
| NNT to prevent one relapse | 5.9 | 5.6 | 5.6 | 5.3 | 5.6 | 5.6 | 4.5 | 5.3 |
| Patients with CDW‡ | ||||||||
| Placebo | 27.3 | 19.7 | 24.0 | 27 | 17 | 22.2 | 24.1 | 29.0 |
| Intervention | 20.2 | 15.8 | 17.9 | 16 | 13 | 14.6 | 17.7 | 25.3 |
| Relative reduction versus placebo, % | 29.8§ | 31.5 | 30.5 | 38 | 21 | 32 | 30§ | 17§ |
| NNT to prevent CDW | 13.7 | 17.1 | 15.1 | 10.8 | 30.2 | 15.4 | 15.3 | 23.5 |
TEMSO: [ClinicalTrials.gov identifier: NCT00134563]; TOWER: [ClinicalTrials.gov identifier: NCT00751881]; DEFINE: [ClinicalTrials.gov identifier: NCT00420212]; CONFIRM: [ClinicalTrials.gov identifier: NCT00451451]; FREEDOMS: [ClinicalTrials.gov identifier: NCT00289978]; FREEDOMS II: [ClinicalTrials.gov identifier: NCT00355134].
The total number of patients includes those randomized and treated with dimethyl fumarate 240 mg twice daily, fingolimod 0.5 mg once daily or teriflunomide 14 mg once daily and the respective placebo groups in each study.
Absolute reductions were calculated as ARR for placebo-treated patients minus ARR for patients treated with intervention.
12-week CDW at 2 years.
Relative reduction versus placebo derived from hazard ratios reported in cited source.
ARR, annualized relapse rate; CDW, confirmed disability worsening; NNT, number needed to treat.
The MS Severity Score
The MS Severity Score integrates EDSS score and disease duration and provides a measure of disease severity and the rapidity of worsening, with higher scores reflecting faster-advancing MS.57 In a subgroup of 1184 (52%) patients with faster-advancing MS (defined by MS Severity Score results >5) derived from the pooled TEMSO/TOWER dataset, teriflunomide treatment was associated with significant reductions in ARR compared with placebo (relative risk reduction: 14 mg, 37.5%, p < 0.0001; 7 mg, 30.3%, p = 0.0002). Teriflunomide 14 mg also resulted in a significant reduction in risk of at least 12-week (40.3%, p = 0.0076) or at least 24-week (46.1%, p = 0.0110) CDW and risk of worsening to an EDSS score of at least 4 (35.5%, p = 0.0042) or at least 6 (38.4%, p = 0.0067) compared with placebo.58
Predictors of disability worsening
Risk assessments have been used to evaluate whether disease activity in the first year of teriflunomide treatment in the TEMSO study could predict treatment outcomes up to 7 years later.59 A simplified version of the Rio score, the modified Rio score, has previously shown that patients treated with IFNβ who had higher modified Rio scores after 1 year showed increased risk of CDW over 4–5 years.60 Using the modified Rio score to evaluate patients in the TEMSO study,60 after 1 year, patients were classified as having low, intermediate or high risk of 12-week CDW according to the occurrence of relapse (zero or at least two relapses) and the presence of active T2 lesions (up to three or more than three on 6- and 12-month MRI scans).59 The majority of patients (90.6%) were categorized as having low or intermediate risk of disability worsening after 1 year of treatment.59 Patients classified as being at intermediate risk of 12-week CDW were further classified into having low or high risk of disability worsening according to change from baseline in brain volume (up to −0.8% versus greater than −0.8%, respectively). Patients in the high-risk category had a significantly greater risk of 12-week CDW than those in the low-risk category (hazard ratio 1.69; p = 0.0022).59 Combined, these studies demonstrate that monitoring of relapses, T2 lesions and BVL in the first year of teriflunomide treatment can enable identification of patients who are likely to have an improved long-term response to therapy.
Real-world effectiveness data
Real-world studies provide an understanding of the effectiveness of a treatment within routine clinical practice and can capture the perspectives of both the healthcare professional and the patient. Teri-PRO [ClinicalTrials.gov identifier: NCT01895335] was a global, phase IV study that assessed treatment satisfaction, efficacy, safety and tolerability in 1000 patients treated with teriflunomide for 48 weeks. Patients treated with teriflunomide 14 mg reported sustained high levels of treatment satisfaction (as measured using the Treatment Satisfaction Questionnaire for Medication). Moreover, patients who were treated with another DMT within the 6 months prior to entering the Teri-PRO study demonstrated statistically significant increases in treatment satisfaction as early as week 4, which were sustained over the course of the study.61 Patient-reported disability (as measured by MS Performance Scale and Patient Determined Disease Steps scores) also remained stable over the 48-week treatment period. Patient Determined Disease Steps scores correlated strongly with physician-reported EDSS scores.61 Over the same period, patients also demonstrated improved or stable quality of life, as measured by the MS International Quality of Life (MusiQoL) questionnaire.61
Real-world switching
Data regarding treatment switching in clinical practice are invaluable to aid appropriate treatment sequencing decisions. Studies have shown that switching to teriflunomide from other DMTs is not associated with increased disease activity and any emerging safety issues. A prospective review of data from a single center of patients switching from natalizumab, due to an increased risk of progressive multifocal leukoencephalopathy (PML; n = 15), showed that most patients had stable disease for 6 months while on teriflunomide, with no new safety signals emerging and no PML cases observed during follow up.62 Furthermore, as discussed previously, switching from subcutaneous IFNβ-1a to teriflunomide can be an effective and well tolerated option,39 and switching from other DMTs to teriflunomide is associated with improvements in patient satisfaction, with a safety and tolerability profile consistent with the clinical development program.61
Safety and tolerability
Teriflunomide clinical development program: placebo-controlled studies
Teriflunomide has a well characterized safety and tolerability profile that is consistent across phase II and III studies in the teriflunomide clinical development program.24–27 Overall, adverse events (AEs) typically reported more frequently with teriflunomide than placebo included hair thinning, nausea and alanine aminotransferase (ALT) increase. In addition to these, among the most common AEs reported are nasopharyngitis, paresthesia, back pain, limb pain, diarrhea, headache, upper respiratory tract infection and arthralgia. These AEs were of mild to moderate intensity, self-limiting and infrequently resulted in treatment discontinuation (5–16% of patients). Treatment discontinuations in all groups were most frequently related to increased ALT concentration and were driven by protocol-mandated discontinuation in the event of increased ALT.20 Five deaths were reported in the core studies: three in the teriflunomide treatment groups in TOWER [n = 1, 7 mg (traffic accident); n = 2, 14 mg (suicide and septicemia)] and two in the placebo groups in TOWER (n = 1, respiratory infection) and TOPIC (n = 1, suicide); none were considered by the investigator to be causally related to teriflunomide treatment.
Long-term safety in extension studies
The safety and tolerability profile of teriflunomide remained consistent with continued long-term exposure for up to 13 years in extension studies.29–32,39 Table 3 provides a summary of outcomes from a pooled analysis of safety data derived from the phase II, TEMSO, TOWER and TOPIC core studies, as well as long-term extension data from the phase II and TEMSO studies. These data represent over 12 years of treatment duration with a cumulative exposure to teriflunomide of more than 6800 patients-years.20 Safety data from the individual TOWER, TOPIC and TENERE extensions (up to 7 years), which were not included in the above pooled analysis, also demonstrated a consistent safety profile relative to other teriflunomide studies.31,32,39
Table 3.
Overview of safety outcomes in teriflunomide-treated patients from the phase II, TEMSO, TOWER and TOPIC core studies and phase II and TEMSO long-term extensions.20
| Patients with adverse events, n (%)* | Teriflunomide 7 mg (n = 1204) | Teriflunomide 14 mg (n = 1134) |
|---|---|---|
| All adverse events | 1056 (87.7) | 1020 (89.9) |
| Serious adverse events | 246 (20.4) | 219 (19.3) |
| Events leading to permanent treatment discontinuation | 184 (15.3) | 172 (15.2) |
| Death | 4 (0.3) | 4 (0.4) |
| Intensity$ | ||
| Mild | 270 (22.4) | 252 (22.2) |
| Moderate | 559 (46.4) | 558 (49.2) |
| Severe | 227 (18.9) | 210 (18.5) |
| Common adverse events‡ | ||
| Nasopharyngitis | 278 (23.1) | 272 (24.0) |
| Headache | 256 (21.3) | 215 (19.0) |
| ALT increase | 205 (17.0) | 211 (18.6) |
| Diarrhea | 189 (15.7) | 192 (16.9) |
| Fatigue | 177 (14.7) | 170 (15.0) |
| Hair thinning§ | 127 (10.5) | 166 (14.6) |
| Back pain | 150 (12.5) | 157 (13.8) |
| Influenza | 136 (11.3) | 149 (13.1) |
| Upper respiratory tract infection | 153 (12.7) | 145 (12.8) |
| Nausea | 120 (10.0) | 142 (12.5) |
| Urinary tract infection | 136 (11.3) | 130 (11.5) |
| Paresthesia | 115 (9.6) | 129 (11.4) |
| Pain in extremity | 128 (10.6) | 123 (10.8) |
| Arthralgia | 138 (11.5) | 103 (9.1) |
Includes patients initially randomized to placebo in core studies.
Mild: no modification of daily activities and/or does not require symptomatic treatment; moderate: hinders normal daily activities and/or requires symptomatic treatment; severe: prevents daily activities and requires symptomatic treatment.
Events with a crude incidence rate of at least 10% in either teriflunomide group; listed in descending order in the teriflunomide 14 mg group.
MedDRA-preferred term: alopecia.
ALT, alanine aminotransferase; MedDRA, Medical Dictionary for Regulatory Activities.
AEs were generally mild to moderate in intensity. Across all studies, less than 23% of patients reported AEs that led to permanent treatment discontinuation;29,31,32,39 most were protocol-mandated discontinuations due to ALT elevations.20 An additional 15 deaths were reported in the phase II (n = 2; tachycardia and myocardial infarction), TEMSO (n = 7; colon cancer, cardiac arrest, malignant melanoma, acute heart failure, bleeding from duodenal ulcer and two deaths from an unknown cause) and TOWER (n = 6; pulmonary tuberculosis, pulmonary embolism, hematemesis, sepsis and two cases of suicide) extension studies. Two deaths (pulmonary tuberculosis and suicide; both in TOWER) were considered to be potentially related to treatment.29–32,39
Neutrophil and lymphocyte reductions were observed in the core phase II and III studies, although mean counts remained within normal ranges and stabilized on treatment; actual mean decrease was up to 15%.20 In the extensions, neutropenia and leukopenia were reported in less than 6% of patients receiving teriflunomide for up to 9 years and were not associated with serious or opportunistic infections.33 No hematologic malignancies were reported.31,33 These data demonstrate that long-term treatment with teriflunomide is not associated with any deleterious effects with respect to protective immunity and are consistent with the proposed immunomodulatory MoA discussed previously.15–19,63
In comparison with fingolimod or DMF, teriflunomide has a less pronounced effect on lymphocyte count. In FREEDOMS and FREEDOMS II, fingolimod reduced mean lymphocyte counts by over 70% from baseline during the first month of treatment, whereas DMF reduced lymphocytes by 28% to 32% in the DEFINE and CONFIRM studies.52,53,55,56 In long-term studies with fingolimod, lymphopenia was a commonly reported AE, occurring in 15.5% of patients (FREEDOMS extension, data to 54 months).64,65 This contrasts with 0.8% of patients experiencing lymphopenia in the teriflunomide clinical trials (data to 12 years).20 Although lymphopenia was an uncommon event in DMF trials, it was considered to be significantly associated with treatment [risk ratio (95% confidence interval), 5.69 (2.40–13.46); p < 0.0001 versus placebo].66 Lymphopenia is also believed to be a risk factor for opportunistic infections such as PML in patients receiving DMF. To date, there have been four instances of PML in DMF-treated patients (although not always in the context of prolonged marked lymphopenia).67 Nine postmarketing reports of PML have also been documented in patients receiving fingolimod with no prior natalizumab treatment.68 No cases of PML have yet been reported in teriflunomide-treated patients.20
Pregnancy
Embryo-fetal toxicity and malformations in rats and rabbits have been associated with teriflunomide exposure when administered at doses within the human therapeutic range.69 As a result of these observations, teriflunomide is contraindicated in women who are pregnant or of child-bearing potential and not using reliable contraception.70,71 Despite the requirement to use reliable contraception in the teriflunomide clinical development program, a number of pregnancies were reported in women receiving teriflunomide treatment and in the partners of men treated with teriflunomide. A summary of the pregnancy outcomes among female patients receiving teriflunomide is presented in Table 4. No structural or functional abnormalities were recorded in the 26 live births with teriflunomide exposure, and none of the induced abortions were due to defects or malformations.72
Table 4.
| Pregnancy outcome | Teriflunomide 7 mg or 14 mg |
Teriflunomide Dose blinded |
Placebo | IFNβ | Total | |
|---|---|---|---|---|---|---|
| Live birth* | 10 | 13$ | 3 | 2 | 2 | 30 |
| Induced abortion | 15 | 11‡ | 3 | 8 | 0 | 37 |
| Spontaneous abortion | 4 | 8 | 1 | 1 | 0 | 14 |
| Ongoing pregnancy§ | 0 | 1 | 0 | 0 | 0 | 1 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 30 | 33 | 7 | 11 | 2 | 83 |
Among the 25 female patients who gave birth to 26 newborns, 21 patients received an accelerated elimination procedure with cholestyramine (including the mother of twins) and 4 patients refused an accelerated elimination procedure; at least 1 of these patients had discontinued treatment prior to the pregnancy.
One patient had two live births during one pregnancy and therefore is counted twice in the table.
One patient had an induced abortion for two fetuses and therefore is counted twice in the table.
The ongoing pregnancy resulted in the birth of a baby boy at 39 weeks’ gestation, following data cutoff.
IFNβ, interferon β.
Interspecies differences may explain why similar or lower teriflunomide exposure results in teratogenicity in rats but not humans.73 The observed embryo-fetal malformations in animals could be related to noncompetitive DHODH enzyme inhibition in rats versus uncompetitive inhibition in humans. The difference in mechanism results in greater DHODH inhibition in rat lymphocytes, with a concomitant increase in antiproliferative activity.73–77
Pregnancy in partners of male patients
Teriflunomide is detectable in human semen, and although no animal studies have been performed to investigate potential male-mediated fetal risks, the US prescribing information indicates that men who wish to father a child should discontinue teriflunomide treatment and undergo an accelerated elimination procedure (AEP) to minimize any potential risk.70 This is not a requirement in the European Summary of Product Characteristics.71
Although administration of teriflunomide to male rats resulted in reduced epididymal sperm count at the mid and high doses tested, no adverse effects on fertility were observed.70,71,78 Data on the effects of teriflunomide on fertility in humans are lacking, but no effect on male and female fertility is anticipated.71
Accelerated elimination procedure
Should a patient become pregnant, wish to become pregnant or experience an AE, such that teriflunomide should be withdrawn,70,71 an AEP can be performed, which can rapidly reduce teriflunomide concentrations to a level considered to be of minimal risk to the fetus (<0.02 μg/ml). AEP involves administration of cholestyramine 8 g every 8 h for 11 days (4 g can be used if tolerability is an issue) or activated charcoal powder 50 g every 12 h for 11 days.70,71 Cholestagel (colesevelam HCl) may offer an additional option for accelerated elimination with improved tolerability.79 Following an AEP, teriflunomide plasma concentrations of less than 0.02 mg/liter should be confirmed and maintained for at least 14 days to mitigate any risk to the fetus.71 Currently, determination of teriflunomide plasma concentration requires liquid chromatography tandem mass spectrometry, which may not be practical for many clinics. The use of dried blood spot sampling may provide a more convenient method of accurately determining teriflunomide concentrations.80
Conclusion
In clinical trials, patients with MS treated with teriflunomide demonstrated improvements in clinical and MRI measures of disease activity that were maintained in long-term extension studies. Teriflunomide has a consistent and favorable safety profile over the long term, with no new or unexpected safety findings compared with the core studies. The positive MRI outcomes from the core studies are further extended by observations seen from the blinded SIENA analyses, which demonstrated significant reductions in the rate of BVL over 2 years with teriflunomide treatment compared with placebo. The benefits of teriflunomide treatment are further supported by supplementary efficacy measures, such as NNT, which demonstrated that teriflunomide has very similar efficacy compared to other oral DMTs and greater efficacy compared with injectable DMTs, with regard to preventing relapse or experiencing disability worsening. In addition, real-world studies demonstrate that teriflunomide is associated with high levels of patient treatment satisfaction, both in those initiating teriflunomide and in those switching from other DMTs, with a safety profile consistent with the clinical development program. The efficacy and safety data from clinical trials, coupled with real-world outcomes, support the use of teriflunomide in the treatment of patients with RMS, including in those who have previously received other DMTs.
Footnotes
Funding: Editorial support for this manuscript was provided by Jessica Donaldson, of Fishawack Communications Ltd, and was funded by Sanofi. The author was responsible for all content and editorial decisions and received no honoraria related to the preparation of this article.
Conflict of interest statement: AEM has received consulting fees [Accordant Health Services, Acorda Therapeutics, Alkermes, Biogen Idec, EMD Serono, Genentech/Roche, Genzyme, GSK, Mallinckrodt Pharmaceuticals (Questcor), Novartis, Roche, Teva] and fees for contracted research [Biogen Idec, Genentech, Novartis, Questcor, Roche, Sanofi].
References
- 1. Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83: 1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Multiple Sclerosis Society. Relapsing-remitting MS (RRMS), http://www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS (accessed 1 August 2016).
- 3. Plantone D, De Angelis F, Doshi A, et al. Secondary progressive multiple sclerosis: definition and measurement. CNS Drugs 2016; 30: 517–526. [DOI] [PubMed] [Google Scholar]
- 4. Goldenberg MM. Multiple sclerosis review. Pharmacol Ther 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- 5. De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 2014; 28: 147–156. [DOI] [PubMed] [Google Scholar]
- 6. Zivadinov R, Jakimovski D, Gandhi S, et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 2016; 16: 777–793. [DOI] [PubMed] [Google Scholar]
- 7. Sormani MP, Radue EW, Sprenger T, et al. Incorporating the TEMSO SIENA analysis improves correlation of brain atrophy and disability progression: P22119. Eur J Neurol 2016; 23: 546. [Google Scholar]
- 8. European Medicines Agency, Committee for Medicinal Products for Human Use: Reflection paper on the regulatory guidance for the use of health related quality of life (HRQL) measures in the evaluation of medicinal products, 2005. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001238.jsp&mid=WC0b01ac0580032ec4 (accessed 1 March 2017).
- 9. US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims, 2009.
- 10. Freedman MS, Hughes B, Mikol DD, et al. Efficacy of disease-modifying therapies in relapsing remitting multiple sclerosis: a systematic comparison. Eur Neurol 2008; 60: 1–11. [DOI] [PubMed] [Google Scholar]
- 11. Freedman MS, Montalban X, Miller A, et al. Comparing outcomes from clinical studies of oral disease-modifying therapies (dimethyl fumarate, fingolimod, and teriflunomide) in relapsing MS: assessing absolute differences using a number needed to treat analysis. Mult Scler Relat Disord 2016; 10: 204–212. [DOI] [PubMed] [Google Scholar]
- 12. Sormani MP, Bruzzi P. Estimating a treatment effect: choosing between relative and absolute measures. Mult Scler 2016; 23: 197–200. [DOI] [PubMed] [Google Scholar]
- 13. Bayer Healthcare Pharmaceuticals Inc. Betaseron prescribing information. 2016. Whippany, NJ. [Google Scholar]
- 14. Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014; 74: 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiendl H, Gross C, Lindner M, et al. Teri-DYNAMIC: exploring the impact of teriflunomide on immune cell population size, receptor repertoire, and function in patients with RRMS. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P5-282. [Google Scholar]
- 16. Klotz L, Lindner M, Gross C, et al. Effects of teriflunomide treatment on the CD4+ T-cell receptor repertoire in patients with relapsing-remitting MS. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P1646. [Google Scholar]
- 17. Wiendl H, Gross C, Lindner M, et al. Impact of teriflunomide on the CD4+ T-cell repertoire of patients with relapsing-remitting MS in the Teri-DYNAMIC study. Presented at 2nd Congress of the European Academy of Neurology, 28–31 May 2016, Copenhagen, Denmark, P32211. [Google Scholar]
- 18. Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013; 81: 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar-Or A, Wiendl H, Miller B, et al. Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol Neuroimmunol Neuroinflamm 2015; 2: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Comi G, Freedman MS, Kappos L, et al. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Mult Scler Relat Disord 2016; 5: 97–104. [DOI] [PubMed] [Google Scholar]
- 21. Leist TP, Freedman MS, Kappos L, et al. Pooled safety analyses from teriflunomide clinical studies. Presented at 67th Annual Meeting of the American Academy of Neurology, 18–25 April 2015, Washington, DC, P7-268. [Google Scholar]
- 22. Edling A, Woodworth L, Agrawal R, et al. Teriflunomide impacts primary microglia and astrocyte functions in vitro. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P670. [Google Scholar]
- 23. Kaplan J, Cavalier S, Turpault S. Biodistribution of teriflunomide in naïve rats vs rats with experimental autoimmune encephalomyelitis. Presented at 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 7–10 October 2015, Barcelona, Spain, P354. [Google Scholar]
- 24. O’Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006; 66: 894–900. [DOI] [PubMed] [Google Scholar]
- 25. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 26. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- 27. Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 977–986. [DOI] [PubMed] [Google Scholar]
- 28. Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014; 20: 705–716. [DOI] [PubMed] [Google Scholar]
- 29. Kremenchutzky M, Freedman MS, Bar-Or A, et al. Teriflunomide phase 2 extension study: 13 years of efficacy and safety results. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P3-027. [Google Scholar]
- 30. Freedman MS, Miller AE, Comi G, et al. Outcomes of the TEMSO extension study of teriflunomide: 10.5 years of clinical results. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P692. [Google Scholar]
- 31. Freedman MS, Kappos L, Comi G, et al. Final outcomes from the teriflunomide TOWER extension study: up to 6 years of follow-up (core plus extension) in patients with relapsing forms of MS. Presented at Annual Meeting of the Consortium of Multiple Sclerosis Centers, 1–4 June 2016, National Harbor, MD, DX48. [Google Scholar]
- 32. Miller AE, Kappos L, Comi G, et al. Outcomes of the TOPIC extension study of teriflunomide in patients with early multiple sclerosis: up to 7 years of clinical results. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P690. [Google Scholar]
- 33. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology 2016; 86: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lublin F, Freedman MS, Comi G, et al. Benefit of long-term treatment with teriflunomide on disability outcomes: results from TEMSO and TOWER. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P691. [Google Scholar]
- 35. Freedman MS, Comi G, Miller AE, et al. Long-term efficacy of teriflunomide in patients recently diagnosed with relapsing forms of MS. Presented at 2nd Congress of the European Academy of Neurology, 28–31 May 2016, Copenhagen, Denmark, P31138. [Google Scholar]
- 36. Ondrusova M, Psenkova M, Donath V. Health-care resource use and cost of multiple sclerosis in Slovakia: results from the national cross-sectional study. Presented at 17th Annual International Society for Pharmacoeconomics and Outcomes Research European Congress, 7–11 November 2015, Milan, Italy, PND37. [DOI] [PubMed] [Google Scholar]
- 37. Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry 2006; 77: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li DKB, Traboulsee AL, Truffinet P, et al. Long-term MRI outcomes from patients treated with teriflunomide: results from a phase 2 extension study. Presented at Joint Americas Committee for Treatment and Research in Multiple Sclerosis–European Committee for Treatment and Research in Multiple Sclerosis Meeting, 10–13 September 2014, Boston, MA, P079. [Google Scholar]
- 39. Vermersch P, Olsson TP, Czlonkowska A, et al. Safety and efficacy of teriflunomide in patients switching from subcutaneous interferon beta-1a. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P3-107. [Google Scholar]
- 40. Vermersch P, Hobart J, Dive-Pouletty C, et al. Measuring treatment satisfaction in MS: is the Treatment Satisfaction Questionnaire for Medication fit for purpose? Mult Scler 2016; 23: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freedman MS, Wolinsky JS, Comi G, et al. The efficacy of teriflunomide in patients who received prior disease-modifying treatments: subgroup analyses of the teriflunomide phase 3 TEMSO and TOWER studies. Mult Scler. Epub ahead of print 1 March 2017. DOI: 10.1177/1352458517695468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nelson F, Lebrun-Frenay C, Camu W, et al. Outcomes in patients with progressive MS: analysis of teriflunomide long-term extension data. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P3-038. [Google Scholar]
- 43. Radue EW, Sprenger T, Gaetano L, et al. Teriflunomide slows BVL in relapsing MS: A reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm 2017; 4: e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wuerfel J, Kappos L, Radue EW, et al. Teriflunomide slows brain volume loss: subgroup analysis of the SIENA TEMSO MRI dataset. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P3-052. [Google Scholar]
- 45. Kappos L, Sprenger T, Radue EW, et al. Brain volume loss correlates with long-term disability worsening in patients with MS: SIENA analysis of TEMSO MRI data. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P733. [Google Scholar]
- 46. Freedman MS, Sprenger T, Radue EW, et al. Teriflunomide is effective in reducing brain volume loss in previously treated patients: a subgroup analysis of TEMSO SIENA data. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P734. [Google Scholar]
- 47. Vermersch P, Thangavelu K, Benamor M, et al. Effect of teriflunomide across patient subgroups based on prior treatment: pooled analyses of the phase 3 TEMSO and TOWER studies. Presented at 9th World Congress on Controversies in Neurology, 26–28 March 2015, Budapest, Hungary. [Google Scholar]
- 48. Freedman MS, Miller AE, Thangavelu K, et al. Teriflunomide exhibits similar results to fingolimod in number needed to treat analysis. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P6-171. [Google Scholar]
- 49. Leist TP, Freedman MS, Miller AE, et al. Assessing comparative outcomes from teriflunomide and dimethyl fumarate studies in relapsing MS: use of ‘number needed to treat’ analysis. Presented at 67th Annual Meeting of the American Academy of Neurology, 18–25 April 2015, Washington, DC, P3-245. [Google Scholar]
- 50. Leist TP, Miller AE, Thangavelu K, et al. Number needed to treat analysis comparing teriflunomide and injectable disease-modifying therapies. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P6-173. [Google Scholar]
- 51. Kappos L, Comi G, Freedman MS, et al. Pooled efficacy data from two phase 3 placebo-controlled trials of oral, once-daily teriflunomide. Mult Scler 2013; 19: 74–558. [Google Scholar]
- 52. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 53. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 54. Fox RJ, Miller DH, Phillips JT, et al. Clinical efficacy of BG-12 (dimethyl fumarate) in relapsing–remitting multiple sclerosis (RRMS): an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Presented at 65th Annual Meeting of the American Academy of Neurology, 16–23 March 2013, San Diego, CA, P07-097. [Google Scholar]
- 55. Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 56. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 57. Roxburgh RH, Seaman SR, Masterman T, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology 2005; 64: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 58. Kappos L, Thangavelu K, Cavalier S, et al. Clinical outcomes in patients with faster advancing MS treated with teriflunomide in TEMSO and TOWER. Presented at 2nd Congress of the European Academy of Neurology, 28–31 May 2016, Copenhagen, Denmark, P11185. [Google Scholar]
- 59. Sormani MP, Wuerfel J, Thangavelu K, et al. Utilizing brain volume loss as an additional predictor of long-term treatment outcomes: analysis of TEMSO MRI SIENA data. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, P736. [Google Scholar]
- 60. Sormani MP, Rio J, Tintore M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 2013; 19: 605–612. [DOI] [PubMed] [Google Scholar]
- 61. Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: Real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord 2017; 7: 107–115. [DOI] [PubMed] [Google Scholar]
- 62. Edwards KR, Freedman MS. Safety and efficacy of transitioning to teriflunomide in patients switching from other disease-modifying therapies, including natalizumab. Presented at 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 7–10 October 2015, Barcelona, Spain, P1039. [Google Scholar]
- 63. Edling A, Woodworth L, Stockmann A, et al. Teriflunomide does not significantly affect primary and memory antibody responses to a viral antigen in mice. Presented at 66th Annual Meeting of the American Academy of Neurology, 26 April–3 May 2014, Philadelphia, PA, P1-215. [Google Scholar]
- 64. Kappos L, O’Connor P, Radue EW, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology 2015; 84: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khatri BO. Fingolimod in the treatment of relapsing-remitting multiple sclerosis: long-term experience and an update on the clinical evidence. Ther Adv Neurol Disord 2016; 9: 130–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu Z, Zhang F, Sun F, et al. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst Rev 2015; 4: CD011076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fiore K. Will 4th PML case change Tecfidera risk profile?, http://www.medpagetoday.com/Neurology/MultipleSclerosis/54704 (accessed 20 April 2017).
- 68. Clark SJ, Wang Q, Mao-Draayer Y. Switching from natalizumab to fingolimod: case report and review of literature. J Immunol Clin Res 2016; 3: 1030. [Google Scholar]
- 69. Davenport L, Edling A, Finn P, et al. Teriflunomide mechanism of action: linking species’ sensitivities to pregnancy outcomes. Presented at 68th Annual Meeting of the American Academy of Neurology, 15–21 April 2016, Vancouver, BC, Canada, P2-068. [Google Scholar]
- 70. Genzyme Corporation. A Sanofi Company. (2016) AUBAGIO Prescribing information: Cambridge. [Google Scholar]
- 71. Genzyme Corporation. A Sanofi Company. (2016) AUBAGIO Summary of product characteristics: Paris. [Google Scholar]
- 72. Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther 2014; 3: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davenport L, Beyer YJ, Truffinet P, et al. Nonclinical data demonstrate high sensitivity of rats versus humans to embryo-fetal toxicity when exposed to teriflunomide. Presented at 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 14–17 September 2016, London, UK, EP1420. [Google Scholar]
- 74. Knecht W, Loffler M. Species-related inhibition of human and rat dihydroorotate dehydrogenase by immunosuppressive isoxazol and cinchoninic acid derivatives. Biochem Pharmacol 1998; 56: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 75. Knecht W, Bergjohann U, Gonski S, et al. Functional expression of a fragment of human dihydroorotate dehydrogenase by means of the baculovirus expression vector system, and kinetic investigation of the purified recombinant enzyme. Eur J Biochem 1996; 240: 292–301. [DOI] [PubMed] [Google Scholar]
- 76. Cherwinski HM, Mccarley D, Schatzman R, et al. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J Pharmacol Exp Ther 1995; 272: 460–468. [PubMed] [Google Scholar]
- 77. Ullrich A, Knecht W, Fries M, et al. Recombinant expression of N-terminal truncated mutants of the membrane bound mouse, rat and human flavoenzyme dihydroorotate dehydrogenase. A versatile tool to rate inhibitor effects? Eur J Biochem 2001; 268: 1861–1868. [PubMed] [Google Scholar]
- 78. Davenport L, Czich A, Turpault S. Teriflunomide: no effects on sperm DNA. Presented at 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 2–5 October 2013, Copenhagen, Denmark, P1187. [Google Scholar]
- 79. Lunven C, Guo Z, Turpault S, et al. Investigation of the effectiveness and tolerability of colesevelam HCl for accelerated elimination of teriflunomide in healthy subjects. Presented at Annual Meeting of the Consortium of Multiple Sclerosis Centers, 27–30 May 2015, Indianapolis, IN, DX50. [Google Scholar]
- 80. Filali-Ansary A, Lunven C, Turpault S, et al. Dried blood spot methodology in combination with liquid chromatography/tandem mass spectrometry facilitates the monitoring of teriflunomide. Ther Drug Monit 2016; 38: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]