Abstract
Pulmonary hypertension (PH) is often associated with cardiopulmonary co-morbidities, especially in older adults. A multispecialty approach to suspected PH is recommended, but there are few data on adherence to guidelines or outcomes in such patients. This was a single-center retrospective study of consecutively evaluated Veteran patients with suspected PH evaluated in a multispecialty PH clinic at a Veterans Affairs Medical Center, evaluating clinical characteristics, workup outcomes, and prognosis. The referral population (n = 125) was older (mean ± SD age = 73.6 ± 9.8 years) with frequent co-morbidities (e.g. COPD 60%) and obesity (mean ± SD BMI = 32.8 ± 8.1 kg/m2). Of 94 patients undergoing right heart catheterization (RHC), 73 (78%) had confirmed PH (mean pulmonary artery pressure ≥ 25 mmHg). PH was associated with higher BMIs (odds ratio [95% CI] for PH per 1 unit increase = 1.10 [1.02–1.19]) and brachial pulse pressures (odds ratio per 1 mmHg increase = 1.07 [1.02–1.13]). Seventy out of 73 were classifiable by WHO PH groupings. Most patients underwent guideline-recommended PH evaluation. Observed one-year mortality was high (17.8%); the one-year hospitalization rate was 34.2%. These results compare favorably to observations from the VA Clinical Assessment, Reporting, and Tracking cohort of Veterans with PH by RHC (19.1% and 60.9% one-year mortality and hospitalization rates, respectively). Multispecialty PH clinic evaluation revealed a high prevalence of co-morbidities in veterans with suspected PH; PH was prevalent in this referral population. PH patients had significant morbidity and mortality but supportive care measures improved following PH evaluation. Further prospective randomized study is needed to determine if a multispecialty clinic approach improves PH morbidity and mortality in veterans.
Keywords: pulmonary hypertension, veterans, multispecialty clinic
Pulmonary hypertension (PH) is a condition in which elevated pulmonary artery pressures (PAPs) can lead to right ventricular dysfunction, dilation and hypertrophy, and eventually heart failure and death. Elevated PAPs are prevalent in the community and are relatively common in older adults.1,2 Many medical conditions are associated with PH,3 including conditions commonly found in veterans, such as chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, heart failure, and liver disease. As a result, in veterans, about 14% of echocardiograms, a well-established screening test for PH, show evidence of PH.4
However, despite evidence of PH being a frequent finding on echocardiograms, studies have shown that PH is often mis- or underdiagnosed in both veterans4,5 and non-veterans.6 To address this issue at our VA medical center, we undertook efforts to establish a referral clinic for veterans with known or suspected PH. Because veterans with PH often have multiple cardiopulmonary co-morbidities associated with PH,7 we utilized a multispecialty model for the clinic, with staffing by both pulmonary and cardiology physicians. In the current report, we describe the development of a multispecialty PH clinic in the VA healthcare system, and report on the evaluation and outcomes of patients referred to our PH clinic.
Methods
Study design
This report includes narrative description of a multispecialty PH clinic as well as results and outcomes of patients referred to the PH clinic. Patient data were collected prospectively in clinic as part of clinical care and are retrospectively reviewed. This study was approved by the IRB of the Providence VA Medical Center (IRB no. 2014-009).
Study population
The study population consisted of the first 125 consecutive patients referred to the Multispecialty Pulmonary Hypertension clinic at the Providence VA Medical Center who attended at least one clinic visit.
Outcomes
The main study outcomes are: (1) the results of diagnostic workup for PH, including right heart catheterization (RHC); (2) the adequacy of diagnostic workup and prognostic assessment for PH in patients confirmed to have PH by RHC; (3) clinical outcomes including hospitalizations and mortality; and (4) changes in treatment and functional status for patients with confirmed PH and at least one follow-up visit. PH was defined as a mean pulmonary artery pressure (mPAP) ≥ 25 mmHg on RHC.
Exposure
The main exposure in the study was referral to the multispecialty PH clinic at the Providence VA Medical Center.
Statistical analysis
Complete statistical methods are included in the Supplement. Baseline characteristics were stratified by presence of PH on RHC (PH by RHC group, mPAP ≥ 25 mmHg), absence of PH on RHC (No PH by RHC group, mPAP < 25 mmHg), and by patients who did not undergo or had a technically inadequate RHC without measurement of mPAP (Unknown PH status group). The baseline characteristics of the PH by RHC group were compared to the No PH by RHC group. The association between baseline characteristics that were significantly different in the PH by RHC and No PH by RHC groups and PH was further assessed using univariate logistic regression. The RHC hemodynamics were described in the PH by RHC and No PH by RHC groups. Differences in hemodynamic values were compared between the two groups.
Adequacy of PH workup was described as the frequency with which guideline appropriate testing (included in Supplemental Methods) was performed in patients confirmed to have PH.8
Survival from time of first clinic visit was assessed through the final follow-up date of 31 May 2016, and time to death for patients who died was calculated; one-year mortality from time of first clinic visit was calculated. A Kaplan–Meier survival curve for patients with confirmed PH by RHC was drawn.
Changes in treatment and functional status for patients with at least one follow-up visit after initial clinic visit were described. Prescription for new or changes in existing supportive medications and other treatments (loop diuretics, including dosage in furosemide equivalents; warfarin; digoxin; pulmonary arterial hypertension [PAH]-specific therapies; oxygen; CPAP or other non-invasive ventilation), as well as changes in mMRC dyspnea scale, six-minute walk testing (6MWT), and functional status from first visit to most recent clinic visit (through 31 May 2016) are presented. A follow-up visit was defined as a return visit to the PH clinic and/or a visit for RHC. Furosemide equivalents were defined as follows: 40 mg furosemide = 1 mg bumetanide = 20 mg torsemide =50 mg ethacrynic acid. Paired t-test for continuous variables, Wilcoxon signed ranks sums test for ordinal variables, or McNemar’s chi-square testing for categorical variables was performed as appropriate to assess for changes in these characteristics between the first and most recent follow-up visit.
All analyses were performed using Stata/SE version 11.2 software (StataCorp LP). A two-sided P value < 0.05 was considered significant.
Results
The multispecialty PH clinic
The Supplement contains a complete description of the multispecialty PH clinic. The clinic is staffed by an attending cardiologist and attending pulmonologist with interests in PH clinical care and research. Patients are jointly seen and discussed by the PH attendings, data are reviewed, and a diagnostic and/or care plan established for each patient. Catheterization results are reviewed jointly by the PH attending team.
Baseline characteristics of patients in the multispecialty PH clinic
The baseline characteristics of the patients referred to PH clinic are described in Table 1. The overall patient population referred to the PH clinic was older (mean age = 73.6 ± 9.8 years) and predominantly male (94.4%). The patients reported significant dyspnea (median modified Medical Research Council dyspnea score was 3, interquartile range [IQR] = 2–3). World Health Organization (WHO)/New York Heart Association (NYHA) functional class (FC) was also impaired in most patients (median WHO/NYHA FC = 3, IQR = 2–3). Co-morbidities were common, with the most commonly reported being systemic hypertension (61.6%) and COPD (60.0%). Impairments in pulmonary function were prevalent, including obstruction (65.2%) and restriction (37.3%); diffusion capacity was impaired (mean DLco ± SD = 52 ± 21%). Most patients referred to the clinic had elevated right ventricular systolic pressure (RVSP) on echocardiogram (78.9% had an RVSP ≥ 40 mmHg) and right atrial enlargement was present in about half of patients. Reasons for referral in the minority of patients without elevated RVSP on echocardiogram included evidence of RV dysfunction on echocardiogram, clinical right heart failure, or otherwise unexplained dyspnea.
Table 1.
Baseline characteristics of patient referred to the PH clinic.
| All patients seen in PH clinic (n = 125) | Patients with confirmed PH by RHC (n = 73) | Patients without PH by RHC (n = 21) | Patients who did not undergo RHC (n = 30) or had technically limited RHC (n = 1) (n = 31 total) | |
|---|---|---|---|---|
| Age (years) | 73.6 (9.8) | 72.8 (9.0) | 73 (11.3) | 75.7 (10.5) |
| Male sex | 118/125 (94.4%) | 68/73 (93.2%) | 21/21 (100%) | 29/31 (93.6%) |
| BMI (kg/m2) | 32.8 (8.1) | 34.3 (8.3) | 29.3 (5.6) | 31.4 (8.5) |
| BMI categories | ||||
| AHA ideal health (BMI < 25) | 21/125 (16.8%) | 10/73 (13.7%) | 3/21 (14.3%) | 8/31 (25.8%) |
| AHA intermediate health (BMI ≥ 25, < 30) | 33/125 (26.4%) | 18/73 (24.7%) | 8/21 (38.1%) | 7/31 (22.6%) |
| AHA poor health (BMI ≥ 30) | 71/125 (56.8%) | 45/73 (61.6%) | 10/21 (47.6%) | 16/31 (51.6%) |
| Systemic hemodynamics | ||||
| Heart rate (bpm) | 75 (14) | 76 (14) | 76 (9) | 74 (18) |
| Systolic blood pressure (mmHg) | 125 (19) | 126 (18) | 113 (13) | 130 (19) |
| Diastolic blood pressure (mmHg) | 71 (9) | 71 (9) | 69 (8) | 70 (10) |
| Pulse pressure (mmHg) | 54 (16) | 55 (15) | 44 (11) | 60 (19) |
| Oxyhemoglobin saturation (%) | 95 (4) | 94 (4) (n = 70) | 96 (2) | 95 (4) |
| Signs and symptoms | ||||
| mMRC dyspnea score, initial visit | 3 (2, 3)* (n = 122) | 3 (2, 3)* (n = 72) | 2 (1.5, 3)* (n = 20) | 2 (1, 3)* (n = 30) |
| mMRC dyspnea score 0 | 9/122 (7.4%) | 2/72 (2.8%) | 2/20 (10.0%) | 5/30 (16.7%) |
| mMRC dyspnea score 1 | 13/122 (10.7%) | 6/72 (8.3%) | 3/20 (15.0%) | 4/30 (13.3%) |
| mMRC dyspnea score 2 | 30/122 (24.6%) | 17/72 (23.6%) | 6/20 (30.0%) | 7/30 (23.3%) |
| mMRC dyspnea score 3 | 50/122 (41.0%) | 32/72 (44.4%) | 8/20 (40.0%) | 10/30 (33.3%) |
| mMRC dyspnea score 4 | 20/122 (16.4%) | 15/72 (20.8%) | 1/20 (5.0%) | 4/30 (13.3%) |
| NYHA FC, initial visit | 3 (2, 3)* (n = 85) | 3 (2, 3)* (n = 51) | 2 (1, 2.5)* (n = 12) | 2.5 (2, 3)* (n = 22) |
| NYHA FC 0 | 1/85 (1.2%) | 0/51 (0%) | 0/12 (0%) | 0/22 (4.6%) |
| NYHA FC 1 | 8/85 (9.4%) | 3/51 (5.9%) | 4/12 (33.3%) | 1/22 (4.6%) |
| NYHA FC 2 | 28/85 (32.9%) | 14/51 (27.4%) | 5/12 (41.7%) | 9/22 (40.9%) |
| NYHA FC 3 | 37/85 (43.5%) | 27/51 (52.9%) | 3/12 (25.0%) | 7/22 (31.8%) |
| NYHA FC 4 | 11/85 (12.9%) | 7/51 (13.7%) | 0/12 (0%) | 4/22 (18.2%) |
| Jugular venous distention, initial visit | 42/125 (33.6%) | 25/73 (34.2%) | 6/21 (28.6%) | 11/31 (35.5%) |
| Peripheral edema | 62/125 (49.6%) | 40/73 (54.8%) | 8/21 (38.1%) | 14/31 (45.2%) |
| Medical history | ||||
| History of anemia | 41/125 (32.8%) | 25/73 (34.2%) | 3/21 (14.3%) | 13/31 (41.9%) |
| History of congenital heart disease | 4/125 (3.2%) | 2/73 (2.7%) | 1/21 (4.8%) | 1/31 (3.2%) |
| History of valvular heart disease | 24/125 (19.2%) | 15/73 (20.6%) | 7/21 (33.3%) | 2/31 (6.5%) |
| History of diabetes | 42/125 (33.6%) | 30/73 (41.1%) | 8/21 (38.1%) | 4/31 (12.9%) |
| History of systemic hypertension | 77/125 (61.6%) | 48/73 (65.8%) | 12/21 (57.1%) | 17/31 (54.8%) |
| History of anorexigen use | 7/125 (5.6%) | 5/73 (6.8%) | 1/21 (4.8%) | 1/31 (3.2%) |
| History of bleeding disorder, GI bleeding, or epistaxis | 27/125 (21.6%) | 16/73 (21.9%) | 3/21 (14.3%) | 8/31 (25.8%) |
| History of cancer | 35/125 (28%) | 25/73 (34.2%) | 6/21 (28.6%) | 4/31 (12.9%) |
| History of heart failure | 55/1125 (44%) | 37/73 (51%) | 7/21 (33.3%) | 11/31 (35.5%) |
| History of connective tissue disease | 9/125 (7.2%) | 5/73 (6.8%) | 4/21 (19%) | 0/31 (0%) |
| History of CAD | 61/125 (48.8%) | 43/73 (58.9%) | 7/21 (33.3%) | 11/31 (35.5%) |
| History of COPD | 75/125 (60%) | 47/73 (64.4%) | 9/21 (43%) | 19/31 (61.3%) |
| History of DVT/PE | 21/125 (16.8%) | 12/73 (16.4%) | 3/21 (14.3%) | 6/31 (19.4%) |
| History of heart surgery/CABG | 23/125 (18.4%) | 16/73 (21.9%) | 4/21 (19%) | 3/21 (9.7%) |
| History of HIV infection | 1/125 (0.8%) | 0/73 (0%) | 1/21 (4.8%) | 0/31 (0%) |
| History of liver disease | 13/125 (10.4%) | 9/73 (12.3%) | 2/21 (9.5%) | 2/31 (6.4%) |
| History of OSA | 59/125 (47.2%) | 41/73 (56.2%) | 9/21 (42.9%) | 9/31 (29%) |
| History of pulmonary fibrosis | 13/125 (10.4%) | 8/73 (11.0%) | 3/21 (14.3%) | 2/31 (6.4%) |
| History of rheumatic fever/heart disease | 3/125 (2.4%) | 2/73 (2.7%) | 0/21 (0%) | 1/31 (3.2%) |
| History of sickle cell trait or disease, or thalassemia | 0/125 (0%) | 0/73 (0%) | 0/21 (0%) | 0/31 (0%) |
| History of thyroid disease | 8/125 (6.4%) | 7/73 (9.6%) | 1/21 (4.8%) | 0/31 (0%) |
| History of splenectomy | 1/125 (0.8%) | 1/73 (1.4%) | 0/21 (0%) | 0/31 (0%) |
| History of myeloproliferative disease | 1/125 (0.8%) | 1/73 (1.4%) | 0/21 (0%) | 0/31 (0%) |
| Ever smokers | 108/125 (86.4%) | 66/73 (90.4%) | 16/21 (76.2%) | 26/31 (83.9%) |
| Current smokers | 19/125 (15.2%) | 16/73 (21.9%) | 1/21 (4.8%) | 2/31 (6.5%) |
| Former smokers | 89/125 (71.2%) | 50/73 (68.5%) | 15/21 (71.4%) | 24/31 (77.4%) |
| Never smokers | 17/125 (13.6%) | 7/73 (9.6%) | 5/21 (23.8%) | 5/31 (16.1%) |
| Functional capacity | ||||
| 6MWD (feet) | 906 (339) (n = 71) | 897 (281) (n = 53) | 1220 (458) (n = 9) | 645 (301) (n = 9) |
| 6MWD (m) | 309 (115) (n = 71) | 306 (96) (n = 53) | 416 (156) (n = 9) | 220 (103) (n = 9) |
| Pulmonary function tests | ||||
| Forced expiratory volume in 1 s (FEV1), % | 67 (23) (n = 116) | 65 (21) (n = 69) | 76 (20) (n = 19) | 67 (30) (n = 28) |
| Forced vital capacity (FVC), % | 80 (20) (n = 116) | 78 (19) (n = 69) | 89 (17) (n = 19) | 77 (24) (n = 28) |
| FEV1/FVC ratio | 62 (15) (n = 115) | 61 (14) (n = 69) | 64 (18) (n = 19) | 62 (17) (n = 27) |
| Total lung capacity (TLC), % | 85 (17) (n = 110) | 83 (16) (n = 66) | 94 (18) (n = 18) | 84 (20) (n = 26) |
| Diffusion capacity of the lungs for carbon monoxide, % | 52 (21) (n = 111) | 48 (20) (n = 66) | 61 (24) (n = 19) | 55 (22) (n = 26) |
| Airflow obstruction (FEV/FVC < 70%) | 75/115 (65.2%) | 49/69 (71%) | 10/19 (52.6%) | 16/27 (59.3%) |
| Restriction (TLC < 80%) | 41/110 (37.3%) | 28/66 (42%) | 3/18 (16.7%) | 10/26 (38.5%) |
| Mixed obstruction and restriction | 21/110 (19.1%) | 14/66 (21.2%) | 1/17 (5.6%) | 6/26 (23.1%) |
| Echocardiography | ||||
| Right atrial enlargement | 60/123 (48.8%) | 41/73 (56.2%) | 9/20 (45%) | 10/30 (33.3%) |
| Right ventricular hypertrophy | 9/123 (7.3%) | 6/73 (8.2%) | 0/20 (0%) | 3/30 (10%) |
| Systolic septal flattening | 24/123 (19.5%) | 19/73 (26%) | 1/20 (5%) | 4/30 (13.3%) |
| Tricuspid plane annular systolic excursion (cm) | 2.0 (0.6) (n = 108) | 1.9 (0.6) (n = 63) | 2.1 (0.6) (n = 16) | 2.1 (0.6) (n = 29) |
| Left atrial size volume index | 28.9 (13.4) (n = 105) | 29.8 (15.2) (n = 61) | 26.3 (9.5) (n = 17) | 28.6 (11.1) (n = 27) |
| Left ventricular ejection fraction (%) | 57 (7) (n = 125) | 56 (8) (n = 73) | 58 (4) (n = 21) | 58 (6) (n = 31) |
| Reduced left ventricular ejection fraction (<50%) | 11/125 (8.8%) | 9/73 (12.3%) | 0/21 (0%) | 2/31 (6.4%) |
| Left ventricular diastolic dysfunction present | 46/123 (37.4%) | 25/73 (34.2%) | 8/20 (40%) | 13/30 (43%) |
| Pericardial effusion present | 4/123 (3.2%) | 3/73 (4.1%) | 0/20 (0%) | 1/30 (3.3%) |
| Aortic stenosis | 16/123 (13.0%) | 12/73 (16.4%) | 1/20 (5%) | 3/30 (10%) |
| Aortic stenosis severity† | 1 4/16 (25%) | 1 4/12 (33.3%) | 1 0/1 (0%) | 1 0/3 (0%) |
| 2 2/16 (12.5%) | 2 2/12 (16.7%) | 2 0/1 (0%) | 2 0/3 (0%) | |
| 3 5/16 (31.2%) | 3 3/12 (25%) | 3 1/1 (100%) | 3 1/3 (33%) | |
| 4 2/16 (12.5%) | 4 0/12 (0%) | 4 0/1 (0%) | 4 2/3 (33%) | |
| 5 3/16 (18.8%) | 5 3/12 (25%) | 5 0/1 (0%) | 5 0/3 (0%) | |
| Aortic regurgitation | 37/123 (30.1%) | 24/73 (32.9%) | 6/14 (30%) | 7/30 (23.3%) |
| Aortic regurgitation severity† | 1 33/38 (86.8%) | 1 21/24 (87.5%) | 1 6/7 (85.7%) | 1 6/7 (85.7%) |
| 2 3/38 (7.9%) | 2 2/24 (8.3%) | 2 1/7 (14.3%) | 2 0/7 (0%) | |
| 3 2/38 (5.3%) | 3 1/24 (4.2%) | 3 0/7 (0%) | 3 1/7 (14.3%) | |
| Mitral stenosis | 2/123 (1.6%) | 0/73 (0%) | 0/20 (0%) | 2/30 (6.7%) |
| Mitral stenosis severity† | 1 2/2 (100%) | NA | NA | 1 2/2 (100%) |
| Mitral regurgitation | 58/123 (47.2%) | 35/73 (48%) | 10/20 (50%) | 13/30 (43.3%) |
| Mitral regurgitation severity† | 1 50/60 (83.3%) | 1 30/37 (81.1%) | 1 9/10 (90%) | 1 11/13 (84.6%) |
| 2 3/60 (5.0%) | 2 3/37 (8.1%) | 2 0/10 (0%) | 2 0/13 (0%) | |
| 3 3/60 (5.0%) | 3 2/37 (5.4%) | 3 1/10 (10%) | 3 0/13 (0%) | |
| 4 1/60 (1.7%) | 4 1/37 (2.7%) | 4 0/10 (0%) | 4 0/13 (0%) | |
| 5 3/60 (5.0%) | 5 1/37 (2.7%) | 5 0/10 (0%) | 5 2/13 (15.4%) | |
| Tricuspid regurgitation | 90/123 (73.2%) | 52/73 (71.2%) | 18/20 (90%) | 20/30 (66.7%) |
| Tricuspid regurgitation severity† | 1 53/91 (58.2%) | 1 31/53 (58.5%) | 1 12/18 (66.7%) | 1 10/20 (50%) |
| 2 6/91 (6.6%) | 2 3/53 (5.7%) | 2 0/18 (0%) | 2 3/20 (15%) | |
| 3 13/91 (14.3%) | 3 9/53 (17.0%) | 3 2/18 (11.1%) | 3 2/20 (10%) | |
| 4 6/91 (6.6%) | 4 4/53 (7.6%) | 4 0/18 (22.2%) | 4 2/20 (10%) | |
| 5 13/91 (14.3%) | 5 6/53 (11.3%) | 5 4/18 (22.2%) | 5 3/20 (15%) | |
| Estimated right ventricular systolic pressure (mmHg) | 47 (13) (n = 90) | 49 (13) (n = 56) | 43 (10) (n = 15) | 44 (12) (n = 19) |
| Right ventricular systolic pressure ≥ 40 mmHg | 71/90 (78.9%) | 49/56 (87.5%) | 9/15 (60.0%) | 13/19 (68.4%) |
| Right ventricular systolic pressure ≥ 60 mmHg | 20/90 (22.2%) | 16/56 (28.6%) | 0/15 (0%) | 4/19 (21.0%) |
Mean (SD) or ratio (%), unless otherwise noted.
Median (IQR)
1 = mild; 2 = mild to moderate; 3 = moderate; 4 = moderate to severe; 5 = severe.
Characteristics of patients with and without PH by RHC
Of the 125 patients referred to clinic, 95 patients underwent or had had a RHC prior to referral; in one patient, the RHC was technically limited and a mPAP was not measured.
Of the 94 patients with technically adequate RHC, 73 (77.7%) had confirmed PH. Patients with PH by RHC had higher body mass index (BMI), systolic blood pressures, and brachial pulse pressures than patients without PH by RHC (Supplemental Table 1). They had higher dyspnea scores and worse functional class on average, and were more likely to have a history of coronary artery disease (CAD). They also had lower lung volumes and lower diffusion capacity of the lungs for carbon monoxide. On univariate logistic regression analysis (Table 2), factors significantly associated with PH on RHC included BMI, systolic blood pressure, pulse pressure, mMRC dyspnea score, WHO/NYHA FC, history of CAD, FVC% predicted, total lung capacity, diffusion capacity, and estimated RVSP ≥ 40 mmHg.
Table 2.
Association of baseline factors significantly different in subjects with and without PH by RHC with PH by RHC.
| Variable | Odds ratio for PH (95% CI) | P value |
|---|---|---|
| BMI (kg/m2) | 1.10 (1.02–1.19) | 0.015 |
| Systolic blood pressure (mmHg) | 1.05 (1.02–1.09) | 0.005 |
| Pulse pressure (mmHg) | 1.07 (1.02–1.13) | 0.005 |
| mMRC dyspnea score, initial (n = 92) | 1.69 (1.05–2.74) | 0.032 |
| NYHA FC, initial (n = 63) | 3.73 (1.51–9.21) | 0.004 |
| History of CAD | 2.87 (1.03–7.95) | 0.043 |
| FEV1% predicted (n = 88) | 0.98 (0.95–1.00) | 0.053 |
| FVC% predicted (n = 88) | 0.97 (0.94–1.00) | 0.031 |
| Total lung capacity, % (n = 84) | 0.96 (0.93–1.00) | 0.027 |
| Diffusion capacity, % (n = 85) | 0.97 (0.95–1.00) | 0.030 |
| Restriction (n = 84) | 3.05 (0.80–11.59) | 0.102 |
| Systolic septal flattening on echo | 6.69 (0.84–53.4) | 0.073 |
| Estimated right ventricular systolic pressure, mmHg (n = 71) | 1.05 (1.00–1.10) | 0.069 |
| Estimated right ventricular systolic pressure ≥ 40 mmHg | 4.67 (1.27–17.15) | 0.020 |
Patients with and without PH by RHC had similar cardiac outputs, but pulmonary vascular resistance (PVR) was higher in patients with PH (3.6 ± 1.9 Wood units [WU]) than no PH (2.0 ± 0.9 WU) (Table 3). Of patients with PH by RHC, 58% had a pulmonary artery occlusion pressure (PAOP) greater than 15 mmHg compared to 9.5% of patients without PH.
Table 3.
Hemodynamic characteristics and PH groups of patients with confirmed PH by RHC and hemodynamic characteristics of patients without confirmed PH by RHC.
| Hemodynamic variables | Patients with confirmed PH by RHC (n = 73) | Patients without PH by RHC (n = 21) |
|---|---|---|
| Mean right atrial pressure (mmHg) | 12 (6) | 6 (3) |
| Right ventricular systolic pressure (mmHg) | 56 (13) | 31(8) |
| Right ventricular diastolic pressure (mmHg) | 7 (6)* | 5 (3) |
| Pulmonary artery systolic pressure (mmHg) | 57 (13) | 32 (7) |
| Pulmonary artery diastolic pressure (mmHg) | 24 (7) | 13 (4) |
| Pulmonary artery pulse pressure (mmHg) | 33 (11) | 19 (6) |
| Mean pulmonary artery pressure (mmHg) | 35 (8) | 20 (4) |
| Pulmonary artery occlusion pressure (mmHg) | 18 (6) (n = 72†) | 11 (4) |
| Pulmonary artery diastolic – pulmonary artery occlusion pressure gradient (mmHg) | 7 (7) (n = 72†) | 3 (4) |
| Transpulmonary gradient (mmHg) | 7 (7) (n = 72†) | 3 (4) |
| Cardiac output, thermodilution (lpm) | 5.6 (1.7)* (n = 55) | 5.5 (2.2) (n = 18) |
| Cardiac index, thermodilution | 2.5 (0.6)* (n = 55) | 2.6 (0.8) (n = 18) |
| Cardiac output, Fick (lpm) | 4.8 (1.4)* (n = 68) | 4.2 (1.1) (n = 19) |
| Cardiac index, Fick | 2.2 (0.5)* (n = 67) | 2.0 (0.4) (n = 19) |
| Pulmonary vascular resistance, WU | 3.6 (1.9) (n = 67) | 2.0 (0.9) (n = 20) |
| Systemic vascular resistance | 1204 (752)* (n = 61) | 1278 (685) (n = 20) |
| Systemic arterial oxygen saturation, % | 94 (3)* (n = 62) | 96 (2) (n = 19) |
| Mixed venous oxygen saturation, % | 64 (8)* (n = 62) | 64 (5) (n = 19) |
| Mean arterial blood pressure (mmHg) | 95 (20)* (n = 64) | 91 (17) (n = 19) |
| Heart rate (bpm) | 69 (13)* (n = 59) | 67 (13) (n = 18) |
| Elevated pulmonary artery occlusion pressure > 15 mmHg | 42/72 (58%) | 2/21 (9.5) |
| Elevated pulmonary artery occlusion pressure > 18 mmHg |
27/72 (37.5%) |
1/21 (4.8%) |
| PH groups | ||
| 1 | 6/73 (8.2%) | |
| 2 | 40/73 (54.8%) | |
| 3 | 23/73 (31.5%) | |
| 4 | 1/73 (1.4%) | |
| 5 | 0/73 (0%) | |
| Group undefined or multiple after evaluation | 3/73 (4.1%) | |
Mean (SD) or ratio (%).
P value for comparison not significant.
In one patient, a PAOP could not be obtained.
Adequacy of workup and prognostic assessment in patients with confirmed PH
Of the 73 patients with proven PH by RHC, 70/73 were classified according to WHO PH groupings after evaluation (Table 3); 3/73 (4.1%) could not be classified or were felt to have characteristics of more than one PH group. Most patients fell into WHO group 2 (54.8%); group 1 (8.2%) and group 4 PH (1.4%) were less common.
Table 4 details the adequacy of PH workup in patients with PH by RHC. As demonstrated, most patients underwent guideline-recommended assessments. While frequency of assessment of left ventricular function, liver function, and pulmonary function were very high, assessment of HIV status was only completed in about two-thirds of patients.
Table 4.
Adequacy of PH workup in patients with confirmed PH.
| Guideline-recommended assessment | Frequency performed (% of total PH patients) | Frequency performed in PH patients with PAOP ≤ 15 mmHg (% of PH patients with PAOP ≤ 15) |
|---|---|---|
| Echocardiography | 73/73 (100%) | 30/30 (100%) |
| Left ventricle functional assessment | 73/73 (100%) | 30/30 (100%) |
| Laboratory assessment | ||
| ANA | 51/73 (69.9%) | 25/30 (83.3%) |
| HIV serology | 49/73 (67.1%) | 20/30 (66.7%) |
| Liver function testing | 73/73 (100%) | 30/30 (100%) |
| Pulmonary function testing | 69/73 (94.5%) | 29/30 (96.7%) |
| Chest CT | 61/73 (83.6%) | 27/30 (90%) |
| V/q scan or chest CT angiography | 55/73 (75.3%) | 25/30 (83.3%) |
| Sleep testing or overnight oximetry | 58/73 (79.4%) | 24/30 (80%) |
Mortality
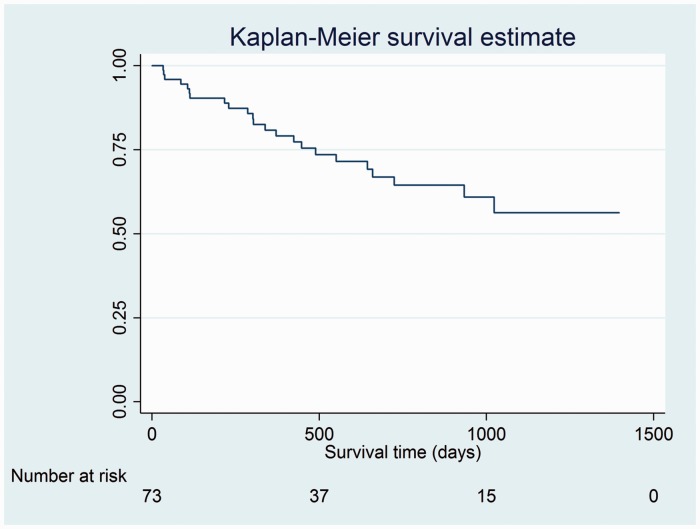
The observed one-year mortality in patients with PH by RHC was 13/73 patients (17.8%) (Table 5). Total mortality during the study period was 31.5%, with a median follow-up time for mortality of 303 days (IQR = 112–551 days). A Kaplan–Meier survival curve for the patients with RHC-confirmed PH is illustrated in Fig. 1.
Table 5.
Follow-up and clinical outcomes in patients with confirmed PH.
| n (%) | |
|---|---|
| Total mortality during study period | 23/73 (31.5%) |
| Median follow-up time for mortality (IQR) | 303 (112, 551) |
| Mean follow-up time for mortality (SD) | 368 (283) |
| One-year mortality | 13/73 (17.8%) |
| (One-year mortality VA CART database) | (19.1%) |
| (One-year mortality REVEAL database) | (9.0%) |
| Hospitalization within one year of initial clinic visit | 25/73 (34.25%) |
| (One-year hospitalizations, VA CART database) | (60.9%) |
| Median number of hospitalizations within one year (IQR) | 0 (0, 1) |
| Mean number of hospitalizations within one year (SD) | 0.6 (1.1) |
| One-year hospitalizations, range | 0–6 |
| Clinic follow-up | 68/73 (93.2%) |
| Median clinic follow-up time, days (IQR) | 270 (91–466) |
| Mean clinic follow-up time, days (SD) | 366 (339) |
| Median total study follow-up time, all patients, days (IQR) | 545 (286–858) |
| Mean total study follow-up, all patients, days (SD) | 593 (386) |
Fig. 1.
Kaplan–Meier survival estimate for patients with PH by RHC. Analysis time is in days.
Hospitalizations, follow-up, treatment changes, and functional outcomes
Sixty-eight of the 73 patients (93.2%) with PH by RHC attended at least one follow-up visit, with a median clinic follow-up time of 270 days (IQR = 91–466 days). Overall, 25 patients were hospitalized within one year of initial PH clinic visit (34.25%) (Table 5). The number of hospitalizations within the first year after PH clinic visit was in the range of 0–6.
Of the 68 patients who attended at least one follow-up, the proportion receiving loop diuretics increased from 61.8% to 83.8% (P = 0.0001) (Supplemental Table 3). Loop diuretic dosage increased on average from 41 ± 53 mg furosemide equivalents to 66 ± 59 mg furosemide equivalents (P < 0.001). Significantly more patients were prescribed oxygen and CPAP at follow-up than had been prescribed these interventions at their initial clinic assessment (Supplemental Table 2). Six-minute walk distance (6MWD) did not change significantly in the limited number (n = 26) with more than one 6MWT, while dyspnea scores (n = 57) were slightly worse on average at follow-up.
Discussion
Joint pulmonary-cardiology collaboration on PH management is more likely to be reported by non-U.S. clinicians9 than U.S. clinicians. A prior report of a multidisciplinary care team for PH consisting of rheumatology, pulmonology, cardiology, and specialized nurses described a diagnostic algorithm for PH assessment.10 Guidelines suggest that the interpretation of complex testing in PH “may best be discussed at a multidisciplinary team meeting,”11 and this type of multidisciplinary approach is being increasingly advocated.12 However, there are few data to inform this approach.
We now show that veteran patients undergoing PH evaluation at a multispecialty PH clinic within the VA generally undergo comprehensive guideline-based assessments. In a prior study of veterans with abnormally high PAPs on echocardiogram, only a small fraction (5/227, 2.2%) ultimately underwent RHC.5 In another study of veterans with a pulmonary artery systolic pressure ≥ 60 mmHg on echocardiogram, only a minority underwent further diagnostic testing such as pulmonary function testing with diffusion capacity measurement, V/Q scan or chest computed tomography angiography, or RHC.4 Referral to a multidisciplinary PH clinic resulted in completion of guideline-recommended assessments8 in the majority of our patients with PH.
In our PH clinic population, 95/125 (76%) underwent RHC. This percentage is identical to that in the Multicenter RePHerral study, in which 106/140 patients (76%) underwent RHC (47 pre-referral and 59 post-referral).6 In the RePHerral study, 14% of patients undergoing RHC were found not to have PH,6 while in our study the percentage was about 22%. Therefore, the percentage of patients undergoing RHC in our study and the percentage being found to have/not have PH was in line with this prior published report from several specialty PH centers. We note that about 10% of our PH patients were placed in WHO groups 1 or 4 following evaluation, and these patients would not have been managed appropriately had they not undergone comprehensive evaluation, including RHC. Our results suggest that the evaluation of patients with PH in a multispecialty PH clinic can result in performance of an appropriate diagnostic workup in veterans with PH. We were also able to describe changes in care that were made in response to the diagnosis of PH in our patient population, which suggest that this evaluation leads to more appropriate supportive measures.
Mortality in patients with RHC-confirmed PH was significant in our study, with a one-year mortality of 17.8% and total mortality of over 30% during the study follow-up period. The one-year mortality rate in our PH patients was higher than was reported in the REVEAL cohort, in which one-year survival following enrollment was 91.0%.13 However, the REVEAL cohort included patients with PAH, who were notably younger (mean age ± SD = 50.4 ± 16.8 years versus 72.8 ± 9.0 years in patients with PH in our cohort) and had longer 6MWDs than our cohort.13 Furthermore, most of the PH patients in our cohort had non-PAH PH. Therefore, comparisons of this veteran PH population with the REVEAL registry of patients with PAH may not be appropriate.
Data from the VA Clinical Assessment, Reporting, and Tracking (CART) program may provide a better basis for comparison for our multispecialty PH clinic population. The CART program captures hemodynamic and outcome data from 76 VA catheterization centers.14 Patients with PH by RHC in our cohort had comparable hemodynamics to veterans (n = 12,490) with a mPAP ≥ 25 mmHg on RHC in the CART cohort,14 with similar mPAPs (mean 35 ± 8 mmHg versus median 34 mmHg [IQR = 29–41] in CART), slightly higher PVRs (mean 3.6 ± 1.9 WU versus median 2.5 WU [IQR = 1.7–3.8] in CART), and somewhat lower PAOPs (18 ± 6 mmHg versus 21 mmHg [IQR = 16–26] in CART).
While the CART report provided comprehensive hemodynamic data on a large number of veterans who had undergone RHC, our current study differs significantly from the prior report. The CART population was referred for RHC for a variety of indications, mainly valvular heart disease, cardiomyopathy, and heart failure,14 whereas our patient population underwent catheterization principally for suspicion of PH. The lower PAOPs and higher PVRs in our patients may reflect the fact that they were undergoing catheterization for suspected PH. Because these patients were assessed in a systematic fashion for PH-related conditions, we are able to describe in detail the clinical, functional, and echocardiographic factors associated with PH in our cohort, data that are not available in CART. This information may help in future risk-stratification efforts focused on suspected PH in the veteran population or in other older cohorts. We were also able to classify patients by PH grouping following a comprehensive diagnostic assessment as summarized above. These details were not available in the prior report.
The prior report of RHC data from the VA CART database was focused on the association between PAP level and mortality in this population and reported outcomes data.14 Veterans with PH in the CART cohort had a one-year mortality rate of 19.1%14 compared to the 17.8% observed in our study population. The one-year hospitalization rate in PH patients in the CART cohort was 60.9%,14 while in our cohort the one-year hospitalization rate was 34.2%. The differences between our results and the outcomes in the CART cohort may reflect confounding factors. Whether PH care in a multispecialty clinic setting improves morbidity and/or mortality in the veteran population with PH requires further study.
Our study has several limitations. Given the retrospective nature of the study and the utilization of clinical data that were collected during routine care, some missing data were inevitable. However, test results and outcomes data were available for most patients. We have noted the above studies reporting potential historical controls for our patient group. However, to our knowledge, the multispecialty model for PH care is novel within the VA healthcare system and we lack directly comparable data on PH-specific care from other VA sites or an internal control group. We have reported information on morbidity and mortality in our patient population, but we lacked an appropriate prognostic model with specificity for the veteran and/or older PH population.
In summary, our report indicates that a comprehensive diagnostic approach to PH in veteran patients with a high prevalence of cardiopulmonary co-morbidities is feasible and results in a high rate of completion of guideline-based testing as well as improved supportive care measures for PH patients in follow-up. This approach may reduce morbidity in this patient population, but confirmation will require further research.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 2009; 119: 2663–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in African-Americans. PLoS One 2013; 8: e84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SJ. Pulmonary hypertension. JAMA 2012; 308: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 4.Maron BA, Choudhary G, Khan UA, et al. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail 2013; 6: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingrey JF, Panos RJ, Ying J, et al. Provider recognition and response to echocardiographic findings indicating pulmonary hypertension in the Veterans affairs medical center population. Pulm Circ 2013; 3: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deano RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013; 173: 887–893. [DOI] [PubMed] [Google Scholar]
- 7.Steiner J, Wu WC, Jankowich M, et al. Echocardiographic predictors of mortality in patients with pulmonary hypertension and cardiopulmonary comorbidities. PLoS One 2015; 10: e0119277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JJ, Butrous G, Maron BA. The heterogeneity of clinical practice patterns among an international cohort of pulmonary arterial hypertension experts. Pulm Circ 2014; 4: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonk MC, van Dijk AP, Heijdra YF, et al. Pulmonary hypertension: its diagnosis and management, a multidisciplinary approach. Neth J Med 2005; 63: 193–198. [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 12.Zhai ZG, Wang C. [Multidisciplinary discrimination and comprehensive management of pulmonary hypertension]. Zhonghua Yi Xue Za Zhi 2016; 96: 1233–1235. [DOI] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 14.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016; 133: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.