Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a disease process wherein tumor cells are thought to embolize to the pulmonary circulation causing pulmonary hypertension (PH) and death from right heart failure. Presented herein are clinical, laboratory, radiographic, and histologic features across seven cases of PTTM. Highlighted in this publication are also involvement of pulmonary venules and clinical features distinguishing PTTM from clinical mimics.
We conducted a retrospective chart review of seven cases of PTTM from hospitals in the greater Los Angeles metropolitan area.
Patients in this series exhibited: symptoms of cough and progressive dyspnea; PH and/or heart failure on physical exam; laboratory abnormalities of anemia, thrombocytopenia, elevated LDH, and elevated D-dimer; chest computed tomography (CT) showing diffuse septal thickening, mediastinal and hilar lymphadenopathy and nodules; elevated pulmonary artery pressures on transthoracic echocardiogram and/or right heart catheterization; and presence of malignancy. Tumor emboli and fibrocellular intimal proliferation were seen in pulmonary arterioles, while two patients had pulmonary venopathy.
PTTM is a devastating disease occurring in patients with metastatic carcinoma. An early diagnosis is challenging. Understanding the clinical presentation of PTTM and distinguishing PTTM from clinical mimics may help achieve an early diagnosis and allow time for initiation of treatment.
Keywords: pulmonary pathology, chest imaging, pulmonary arterial hypertension
Pulmonary tumor thrombotic microangiopathy (PTTM) is a disease entity that is seen most commonly in patients with metastatic adenocarcinoma.1 The pathogenesis is hypothesized to begin with tumor cell embolization to the pulmonary vasculature leading to activation of the coagulation cascade, fibrocellular intimal proliferation, and subsequent stenosis of pulmonary arterioles, resulting in severe pulmonary hypertension (PH) and right heart failure.1
Patients with PTTM often present with: symptoms of cough and progressive dyspnea on exertion;2 signs of PH and/or decompensated heart failure;3,4 intra- and interlobular septal thickening, mediastinal lymphadenopathy, and nodules on chest computed tomography (CT);4 and elevated pulmonary artery pressures on transthoracic echocardiogram and/or right heart catheterization (RHC).4 PTTM may be the first manifestation of malignancy in some patients and portends a poor prognosis, with death occurring within a few weeks of presentation to a hospital, usually due to right heart failure.1–3
A significant challenge in the management of PTTM is obtaining an early diagnosis. The delay in diagnosis is potentially attributable to diagnostic confounders, such as variable presentation and duration of symptoms, lack of knowledge of PTTM, and disease mimics with similar clinical presentation but different underlying pathophysiology. Two disease entities with different underlying pathophysiology, pulmonary veno-occlusive disease (PVOD) and chronic thromboembolic pulmonary hypertension (CTEPH), pose as diagnostic mimics of PTTM. We describe the clinical, laboratory, radiographic, and histologic features of PTTM in seven cases. We note, in particular, pulmonary venular involvement in PTTM that has only once been described previously.3 Finally, we highlight some key clinical distinctions between PTTM, PVOD, and CTEPH.
Methods
A retrospective chart review was conducted of seven cases of PTTM from three teaching hospitals in the greater Los Angeles metropolitan area. The cases were collected between 2014 and 2015 by approaching clinical faculty members who had experience with the diagnosis of PTTM and had saved clinical information for review. The chart review was approved by the University of California Los Angeles Institutional Review Board.
We obtained the following information from each patient chart at the initial clinical evaluation: age; gender; ethnicity; symptoms and duration of symptoms; physical examination findings; laboratory abnormalities; radiographic findings; diagnosis of the primary malignancy; method of diagnosis of the primary malignancy; history of prior exposure to chemotherapy; method of diagnosis of PTTM; time required to establish the diagnosis of both the primary malignancy and PTTM; and time from the initial clinical evaluation to death (Tables 1–5). Initial clinical evaluation was defined as the first point of contact with the health system with symptoms attributable to PTTM that led to a further work-up.
Table 1.
Demographic characteristics, clinical findings, and RVSP on echocardiography of patients with PTTM.
| Case no. | Age (years) | Gender | Ethnicity | Prior chemo | Cough | Dyspnea | Heart failure* or PH† on exam | Hypoxemia | RVSP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | M | Hispanic | N | Y | Y | Y | Y | 75 |
| 2 | 45 | M | Hispanic | N | Y | Y | Y | Y | 140 |
| 3 | 65 | M | Caucasian | Y | Y | Y | N | N | 66 |
| 4 | 63 | M | Caucasian | N | N/A‡ | N/A‡ | N | Y | 51 |
| 5 | 23 | M | Hispanic | N | Y | Y | N | Y | 58 |
| 6 | 48 | M | Caucasian | N | Y | Y | Y | Y | 60 |
| 7 | 58 | F | Asian | N | Y | Y | N | Y | 86 |
Heart failure on exam includes jugular venous distension, right-sided S3, lower extremity edema, and pulsatile liver at right upper quadrant.
PH on exam includes wide and fixed splitting of S2 with loud P2 component.
History could not be obtained due to altered mentation.
PH, pulmonary hypertension; RVSP, right ventricular systolic pressure.
Table 2.
Radiographic features of patients with PTTM.
| Case no. | CXR Abnormal (Y/N) | Nodules* | Septal thickening | Mediastinal LAD | Pleural effusion | GGO | Pulmonary embolism |
|---|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | Y | N | N | N |
| 2 | Y | N | Y | Y | Y | N | N/A† |
| 3 | Y | Y | Y | Y | Y | Y | N |
| 4 | Y | N | N | Y | Y | N | N |
| 5 | Y | Y | Y | Y | N | Y | N |
| 6 | Y | Y | Y | Y | N | N | N |
| 7 | Y | Y | Y | N | Y | Y | N |
Nodules were most commonly < 4 mm and in centrilobular distribution; the largest nodule seen in this series was 1 cm.
This patient had a CT chest without contrast and pulmonary embolism was not evaluated.
GGO, ground-glass opacification; CXR, chest X-ray; LAD, lymphadenopathy.
Table 3.
Histologic features and methods of diagnosis of PTTM.
| Case no. | Primary malignancy | Method of diagnosis of primary malignancy | Method of diagnosis of PTTM | Pulmonary arteriopathy* | Pulmonary venopathy† | Lymphatic involvement |
|---|---|---|---|---|---|---|
| 1 | Gastric adenocarcinoma‡ | CT-guided para-aortic lymph node biopsy | Clinical + lung histology | Y | Y | Y |
| 2 | Gastric adenocarcinoma‡ | Autopsy | Clinical + lung histology | Y | Y | Y |
| 3 | Gastric adenocarcinoma‡ | Diagnosed 2 years prior (endoscopy) | Clinical + lung histology | Y | N | Y |
| 4 | Lung adenocarcinoma, neuroendocrine tumor | Autopsy | Clinical + lung histology | Y | N | Y |
| 5 | Gastric adenocarcinoma‡ | Transbronchial biopsy | Clinical + lung histology | Y | N/A§ | Y |
| 6 | Gastric adenocarcinoma | CT-guided para-aortic node biopsy | Clinical | N/A** | N/A** | N/A** |
| 7 | Gastric adenocarcinoma | Upper endoscopy with biopsy | Clinical | N/A** | N/A** | N/A** |
Pulmonary arteriopathy: defined as fibrocellular intimal thickening, thrombus formation with or without recanalization in pulmonary arterioles.
Pulmonary venopathy: defined as fibrocellular intimal thickening, thrombus formation with or without recanalization in pulmonary venules.
Indicates Signet ring cell morphology of tumor cells noted on histology.
Insufficient tissue to evaluate pulmonary venules.
No lung tissue available.
CT, computed tomography.
Table 4.
Clinical timelines in patients with PTTM.
| Case no. | Symptom duration* (days) | Time to establish diagnosis of primary malignancy (days) | Time to death (days) |
|---|---|---|---|
| 1 | 7 | 7 | 14 |
| 2 | 186 | N/A | 13 |
| 3 | 14 | N/A† | 18 |
| 4 | 1 | N/A | 1 |
| 5 | 42 | 5 | 10 |
| 6 | 21 | 16 | 23 |
| 7 | 56 | 45 | 56 |
*Times start from initial clinical evaluation when patient first presented and work-up was begun that led to the diagnosis of PTTM.
Primary malignancy diagnosed two years prior to presentation.
Table 5.
Clinical, echocardiographic, laboratory, radiographic, and histologic features of PTTM.
| Symptoms of cough and progressively worsening dyspnea |
| Physical examination notable for PH* and/or congestive heart failure† |
| Hypoxemia |
| PH on echocardiography or right heart catheterization (elevated mean PAP; normal PAOP; increased PVR) |
| Anemia, thrombocytopenia, elevated LDH, elevated D-dimer on laboratory analysis |
| Chest CT findings of inter- and intra-lobular septal thickening, lymphadenopathy, nodules, and GGO |
| Pulmonary tissue histology revealing pulmonary arteriopathy +/– pulmonary venopathy +/– involvement of lymphatics |
| Presence of malignancy (carcinoma most common) |
PH on exam includes split S2 with a loud P2 component; LDH, lactate dehydrogenase.
Heart failure on exam includes jugular venous distension, S3 heart sound, lower extremity edema, and pulsatile liver at right upper quadrant.
PAP, pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; PVR, pulmonary vascular resistance; GGO, ground-glass opacification.
Results
There were seven patients in this series (age range = 23–65 years, median age = 48 years). The most common symptom was cough and progressively worsening dyspnea. Two patients complained of night sweats and two complained of weight loss. One patient noted hemoptysis. The average duration of symptoms prior to the initial clinical evaluation was 46.5 days (median = 21 days, range = 1–186). Tachycardia and scattered rales were the most common physical examination findings, noted in four patients. Features of right ventricular dysfunction (right-sided S3 heart sound, jugular venous distension, and lower extremity edema) were described in three patients while fixed and wide splitting of S2 with loud P2 component, attributable to PH, was noted in one. Resting hypoxemia was observed in all but one patient at the initial clinical evaluation. Transthoracic echocardiography demonstrated moderate-to-severe PH in all seven patients. The average right ventricular systolic pressure (RVSP) was 77 mmHg (range = 51–140 mmHg). Moderate to severe right ventricular dilatation was also noted in all patients (Table 1).
On laboratory evaluation, five patients had an anemia, three had thrombocytopenia, two had acute renal failure, and all seven had elevated lactate dehydrogenase (LDH). D-dimer was obtained in four patients and was elevated in three. Disseminated intravascular coagulation (DIC) was noted in one patient. Results for beta natriuretic peptide (BNP) were available for six patients and were elevated in three. Serum level of platelet-derived growth factor (PDGF), which has been used as a marker of response to treatment with the tyrosine kinase inhibitor, imatinib, was not tested in this series.5 None of our patients received chemotherapy or imatinib after the diagnosis of PTTM was established.
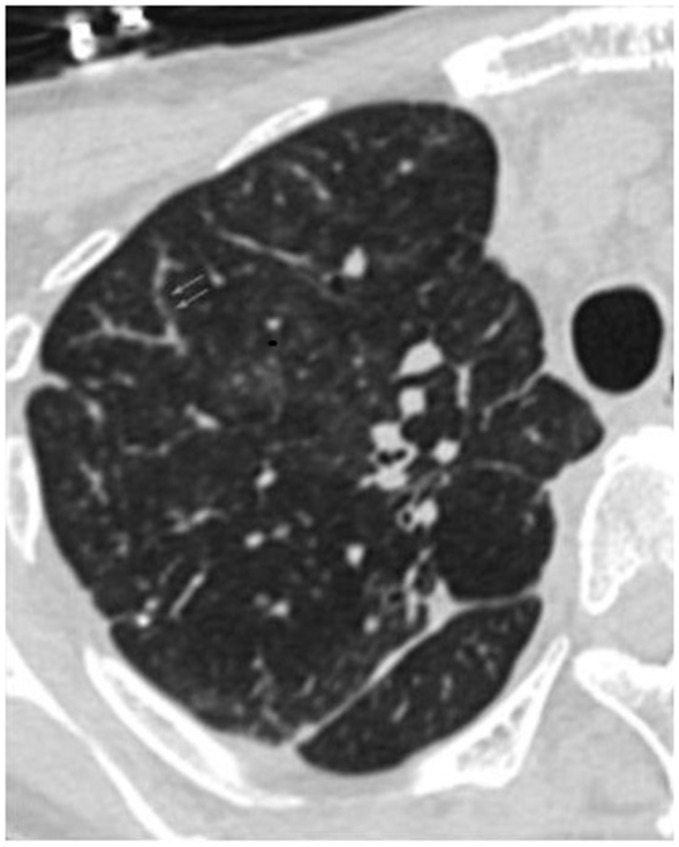
All patients had a chest radiograph, including chest CT. On chest roentgenogram, the most common findings were diffuse nodular opacities, followed by prominent hila and/or pulmonary artery enlargement, and diffuse reticular opacities (Fig. 1, Table 2). The most common findings on chest CT were intra- and interlobular septal thickening, mediastinal and hilar lymphadenopathy, nodules, pleural effusion, and ground-glass opacification (GGO) (Fig. 2; Table 2). CT pulmonary angiography was performed in all but one case and no pulmonary embolism was detected. One patient underwent RHC, which was notable for the following hemodynamic values: right atrial (RA) pressure = 12 mmHg; right ventricular (RV) pressure = 129/12 mmHg; PA pressure =129/34 mmHg (mean = 67 mmHg); pulmonary artery wedge pressure (PAWP) = 15 mmHg; cardiac output (CO) = 3.5 L/min; and pulmonary vascular resistance (PVR) = 15 Wood units (WU). Only one patient underwent a ventilation-perfusion lung scan, which showed multiple small wedge-shaped defects in perfusion. Pulmonary function testing and six-minute walking test were not performed in any of these patients.
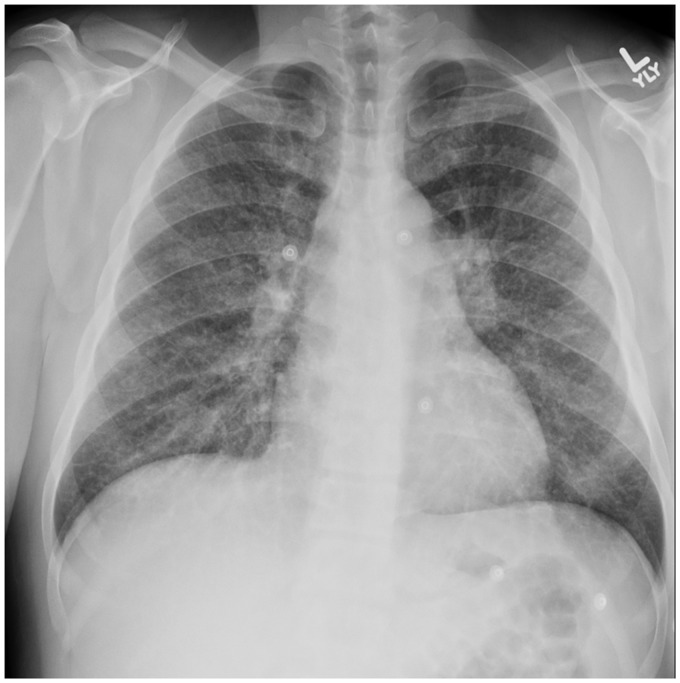
Fig. 1.
Representative chest radiograph (PA view) shows diffuse fine reticulonodular opacities and hilar prominence. Image from Case 1.
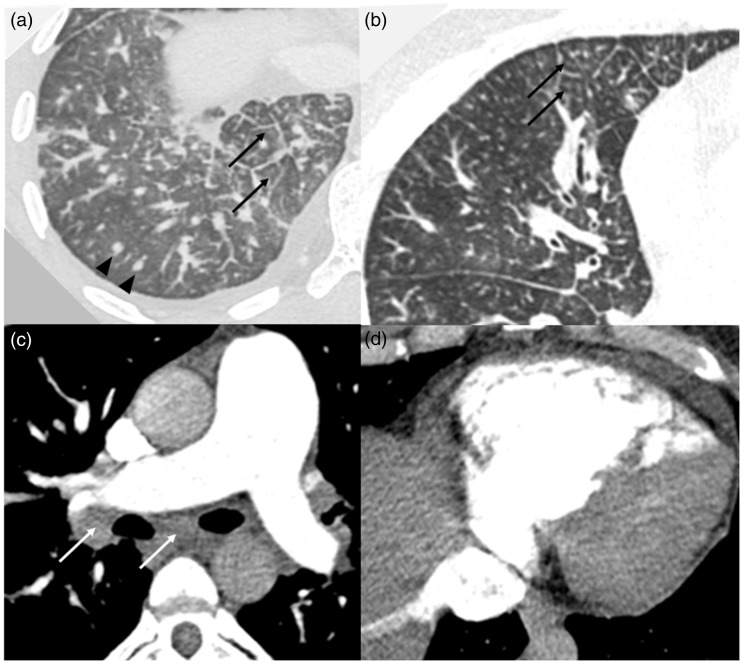
Fig. 2.
Representative chest CT images. (a) Lung window: shows interlobular septal thickening (black arrows), random hematogenous micronodules (black arrowheads), and a small pleural effusion; (b) lung window: shows multifocal tree-in-bud centrilobular micronodules caused by tumor emboli (arrows); (c) soft tissue window: shows an enlarged main pulmonary artery and mediastinal and hilar lymphadenopathy (white arrows); (d) soft tissue window: shows right ventricular enlargement and reflux of contrast into the inferior vena cava. All images from Case 1.
All seven patients were diagnosed with an adenocarcinoma. In two patients, the primary malignancy was established post-mortem, while in the remaining five patients, the primary malignancy was diagnosed ante-mortem. In all patients except one, the symptoms of PH and the clinical diagnosis of PTTM preceded the diagnosis of cancer. Six patients were diagnosed with gastric adenocarcinoma. The remaining patient was diagnosed with a combined lung neuroendocrine tumor and lung adenocarcinoma. One patient was diagnosed with gastric adenocarcinoma based on a CT-guided biopsy of an enlarged para-aortic lymph node (Table 3).
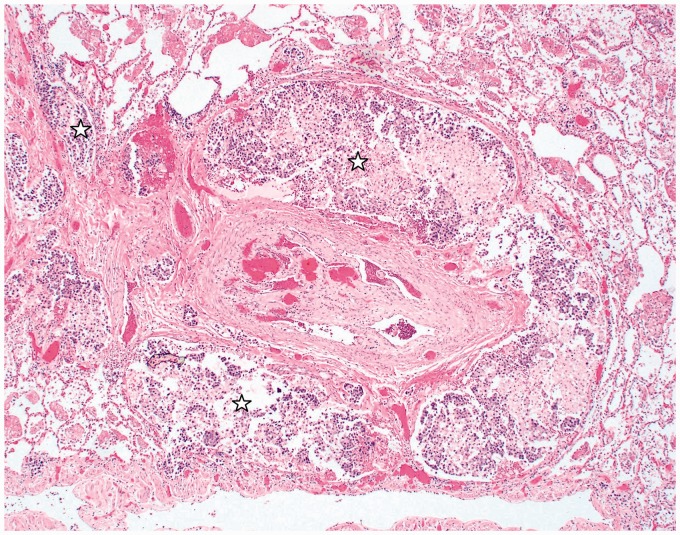
Lung tissue specimens from five patients were examined, four at the post-mortem examination and one ante-mortem via transbronchial biopsy. Tumor emboli within the pulmonary arterioles along with fibrocellular intimal proliferation were noted in all cases, consistent with a diagnosis of PTTM.1 Complete obstruction and recanalization of thrombi was observed within the arteries and arterioles of most patients (Fig. 4). Within the post-capillary venules of two patients, thrombus formation and arterialization of venules with fibrocellular intimal thickening were noted (Fig. 5). In one patient, there was diffuse involvement of pulmonary venules, whereas in the other the involvement was patchy. Septal thickening was seen in patients with and without pulmonary venule involvement. Smooth septal lines were seen in four patients while nodular septal lines were noted in two; the latter two cases also exhibited pulmonary venule involvement on histology (Fig. 3). Lymphangitic involvement was noted in all five cases where lung tissue was available (Fig. 6). Tissue staining for various markers in PTTM including tissue factor (TF), vascular endothelial growth factor (VEGF), osteopontin, and platelet-derived growth factor (PDGF) has been reported previously but were not assessed in this series.6,12,13
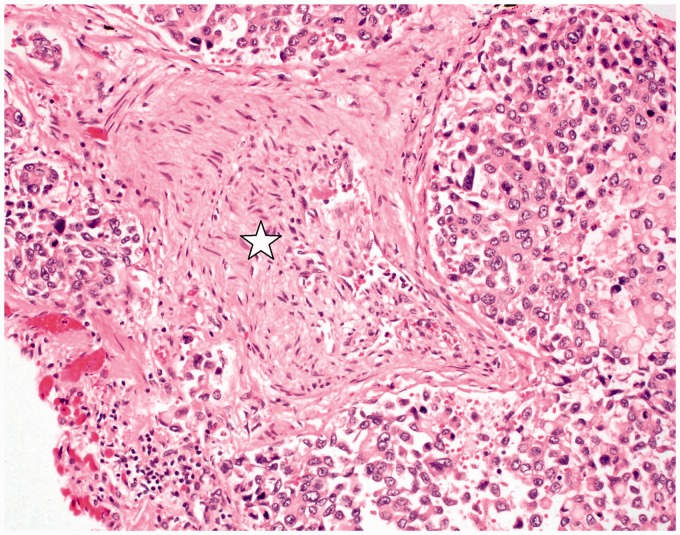
Fig. 4.
Small pulmonary artery with near luminal obliteration from organized thrombus with recanalization (star). The adjacent lymphatic channels are markedly dilated by tumor cells (hematoxylin and eosin (H&E) stain; original magnification 400×). Image from Case 1.
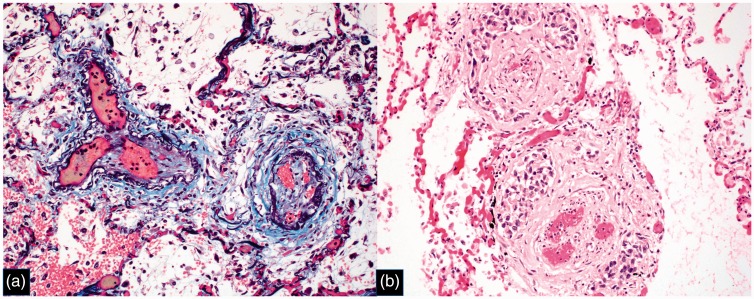
Fig. 5.
Pulmonary venules with intimal fibrosis and recanalization, consistent with PVOD pattern. (a) Combined trichrome/EVG stain, original magnification 200×; (b) H&E stain, original magnification 200×. Image from Case 1.
Fig. 3.
Chest CT lung window: beading is seen within peripheral pulmonary venules of a patient with histologic evidence of pulmonary venopathy (blue arrows). Image from Case 2.
Fig. 6.
Pulmonary artery with organized and recanalized thrombus and surrounding lymphatic spaces markedly dilated by tumor cells (stars) (H&E stain; original magnification 40×). Image from Case 1.
The median time from the initial clinical evaluation to the diagnosis of the primary malignancy was 11.5 days (range = 5–45 days, mean = 18 days). The median time from the initial clinical evaluation to death was 14 days (range = 10–56 days, mean = 19 days). All of the patients died before any specific treatment directed towards malignancy could be initiated (Table 4).
Discussion
In 1990, von Herbay et al coined the term PTTM and hypothesized the pathophysiology of the disease process. Tumor cell embolization to the pulmonary arterioles, activation of the coagulation cascade, thrombus formation, and fibrocellular intimal proliferation lead to arteriolar obstruction and, subsequently, PH.1 First, we note the clinical, laboratory, radiographic, and histologic features across seven cases of PTTM. We discuss the previously reported involvement of pulmonary venules.3 Lastly, we highlight information that could help distinguish PTTM from clinically similar but pathophysiologically distinct disease processes: PVOD and CTEPH.
There are certain features of PTTM commonly noted in this series and across the literature (Table 5). Cough and progressively worsening dyspnea are two commonly encountered symptoms with an onset ranging from days to months prior to presentation.2,5,6,9 The pulmonary arteriolar occlusion and increase in dead space, resultant hypoxemia, and PH likely explain the dyspnea. The mechanism for cough is not clearly understood. Symptoms other than cough and dyspnea include hemoptysis, fatigue, weight loss, and night sweats.6–8 A thorough history is key in determining benignity of presenting symptoms and guiding further work-up.
A detailed physical examination may accelerate a diagnostic work-up. In three cases where signs of PH or heart failure were noted, a chest CT and transthoracic echocardiogram were obtained within 48 h for two of those patients and within nine days for the third patient.
On laboratory analysis anemia, thrombocytopenia, elevated LDH, and elevated D-dimer were seen in this series. DIC was noted in one case. In PTTM, anemia, specifically microangiopathic hemolytic anemia (MAHA), is thought to result from shearing of erythrocytes passing through stenosed vessels.1 Thrombocytopenia, elevated LDH, and elevated D-dimer are also described in PTTM.2,3,7 These same laboratory abnormalities have been described in patients with cancer before, in a condition termed cancer-associated thrombotic microangiopathy, where DIC, tumor lysis syndrome, and severe acute illness occurred.15,16 Whether PTTM represents a pulmonary-specific process and is a subset of cancer-associated thrombotic microangiopathy can be speculated. In their autopsy case series, von Herbay et al. found no other organ exhibiting the pathologic arteriolar changes besides the lungs. The current hypothesis postulates the presence of procoagulants, TF being an example, released by tumor cells, that activates coagulation and promotes the development of microvascular thrombi, MAHA, and thrombocytopenia.1,7 Why it only potentially affects the pulmonary circulation is unknown. Assessing for MAHA, thrombocytopenia, elevated LDH, and elevated D-dimer may elevate the index of suspicion of PTTM.
Ventilation-perfusion scans in PTTM may show small, peripheral, subsegmental perfusion defects, which can mimic findings noted in patients with CTEPH. The radiographic features noted on chest CT in patients PTTM are variable. The most common findings in this series included intra- and interlobular septal thickening, mediastinal and hilar lymphadenopathy, and nodules, which are also reported in the literature of patients with PTTM due to different primary malignancies.4,5,7,11,14 One report noted the absence of all abovementioned findings in a patient with breast adenocarcinoma.13 Other findings on CT imaging include RV and PA enlargement, GGO with variable distribution, and small centrilobular micronodules which can have a tree-in-bud morphology.4,11 The histologic correlates of septal thickening may be extrapolated from reports on lymphangitic carcinomatosis. In a series of 20 patients with proven lymphangitic carcinomatosis, 17 had interlobular septal thickening and 18 had hilar lymphadenopathy.17 All five patients in this series with lung tissue available for analysis had concomitant lymphangitic carcinomatosis. Therefore, radiographic septal thickening may histologically be due to lymphangitic engorgement with tumor cells or edema. Two patients (Cases 1 and 2) that had pulmonary venular involvement had septal thickening that was nodular in appearance, but given the small number of patients in this series, no definitive statement can be made regarding this finding. The heterogeneity of radiographic features may reflect the point in time when imaging was obtained along a continuum of disease progression. For improved histologic correlation, future studies should note the distribution of any abnormality, interval changes occurring on repeat imaging, and in terms of septal thickening, whether they are smooth or nodular in appearance.
Histologically, PTTM is characterized by the presence of tumor emboli in pulmonary arterioles along with fibrocellular intimal proliferation, a theoretical consequence of activation of coagulation.1 PTTM is seen in any type of adenocarcinoma and in our series most patients had gastric adenocarcinoma. The pathophysiology of PTTM may involve the release in serum of TF and PDGF by tumor cells.6,10 Other markers that have been detected on tissue staining include VEGF and osteopontin.2,11,13 Tissue factor might play an important role in the pathogenesis of PTTM. Additionally, VEGF has been reported to be involved in a variety of forms of PH and to be upregulated by TF. These findings suggest that VEGF and TF may be involved in the pathogenesis of PTTM. The exact mechanism involving these markers is unknown; however, therapeutic options targeting such factors have been attempted. Imatinib, an inhibitor of the PDGF receptor, has been used successfully in a few case reports, with one case reporting an improvement in PH and survival of nine months.5
This case series is the second report that describes pulmonary venule involvement in PTTM.3 Pulmonary venule involvement was either diffuse or patchy in distribution in two patients. At this time, the significance of the pulmonary venopathy is unclear. It is hypothesized that local factors released by the tumor cells are involved, much like in the arterioles.3,4 The venopathy could also be a consequence of excessive shunting of blood across neo-vessels, formed to bypass the tumoral obstruction and the capillary system, a mechanism similar to that theorized to exist in CTEPH.21
PH due to PTTM can be classified under either World Health Organization (WHO) PH Group 4 or 5. As in CTEPH, pulmonary arteriolar obstruction in PTTM leads to shunting of blood through non-affected arterioles or neo-vessels, inducing compensatory changes in those arterioles and vessels, a mechanism dubbed “secondary arteriopathy.”21 Tumoral obstruction that occurs in PTTM enables classification under Group 5.3
Despite the distinct pathophysiology between each disease entity, PTTM, PVOD, and CTEPH, may be difficult to distinguish clinically. However, some clinical distinctions can be made. All three disease entities are associated with dyspnea, but in PTTM and PVOD, there is progressively worsening dyspnea, whereas prior to the development of CTEPH, an improvement in dyspnea may be seen after the initial thromboembolism, during the well-recognized “honeymoon period.”19,20,22 Cough is commonly noted in cases of PTTM but is not typical of PVOD.2,20 Though cough has been reported in CTEPH, it is not noted as the most common symptom.22 While MAHA, thrombocytopenia, elevated LDH, and elevated D-dimer are noted in PTTM, they are attributable to a wide range of etiologies limiting their utility in distinguishing PTTM from the other disease processes. On chest imaging, septal thickening, GGO, and mediastinal adenopathy can be seen in PTTM, PVOD, and CTEPH.4,20,24 In cases of suspected PTTM, distinguishing between the three diagnoses requires a high index of suspicion, especially given the short duration of time from presentation to death.
PTTM is a devastating disease entity occurring in patients with metastatic carcinoma. An early diagnosis is challenging. Understanding the clinical, laboratory, radiographic, and histologic features of PTTM and distinguishing PTTM from clinical mimics may help achieve an early diagnosis and allow time for the initiation of treatment.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990; 66: 587–592. [DOI] [PubMed] [Google Scholar]
- 2.Uruga H, Fujii T, Kurosaki A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med 2013; 52(12): 1317–1323. [DOI] [PubMed] [Google Scholar]
- 3.Kumar N, Price LC, Montero MA, et al. Pulmonary tumour thrombotic microangiopathy: unclassifiable pulmonary hypertension? Eur Respir J 2015; 46: 1214–1217. [DOI] [PubMed] [Google Scholar]
- 4.Price LC, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med 2016; 22(5): 421–428. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa A, Yamadori I, Matsubara O, et al. Pulmonary tumor thrombotic microangiopathy with circulatory failure treated with imatinib. Intern Med 2013; 52(17): 1927–1930. [DOI] [PubMed] [Google Scholar]
- 6.Godbole R, Ghatol A, Betancourt J, et al. Pulmonary tumor thrombotic microangiopathy: clinical, radiology, and histologic correlation. J Clin Imaging Sci 2015; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voigtlaender M, Holstein K, Leuenroth S, et al. Clinical evidence that coagulation activation drives cancer progression – a report of 2 cases. Oncol Res Treat 2015; 38: 449–452. [DOI] [PubMed] [Google Scholar]
- 8.Demirag F, Cakir E, Yazici U, et al. Pulmonary tumor thrombotic microangiopathy from metastatic epithelioid angiosarcoma. J Thorac Dis 2013; 5(3): E107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimaraes M, Almeida M, Brelinger, et al. Diffuse bronchiolitis pattern on a computed tomography scan as a presentation of pulmonary tumor thrombotic microangiopathy: a case report. J Med Case Rep 2011; 5: 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCabe JM, Bhave PD, McGlothlin D, et al. Running from her past: a case of rapidly progressive dyspnea on exertion. Circulation 2011; 124(21): 2355–2361. [DOI] [PubMed] [Google Scholar]
- 11.Okubo Y, Wakayama M, Kitahara K, et al. Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: morphometric and immunohistochemical analysis of six autopsy cases. Diagn Pathol 2011; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokomine T, Hirakawa H, Ozawa E, et al. Pulmonary thrombotic microangiopathy caused by gastric carcinoma. J Clin Pathol 2010; 63(4): 367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi F, Kumasaka T, Nagaoka T, et al. Osteopontin expression in pulmonary tumor thrombotic microangiopathy caused by gastric carcinoma. Pathol Int 2009; 59(10): 752–756. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura A, Nishimura N, Jinta T, et al. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep Pulmonol 2013; 2013: 259080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis KK, Kalyanam N, Terrell DR, et al. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: A report of 10 patients and a systemic review of published cases. Oncologist 2007; 12(1): 11–19. [DOI] [PubMed] [Google Scholar]
- 16.Elliott MA, Letendre L, Gastineau DA, et al. Cancer-associated microangiopathic hemolytic anemia with thrombocytopenia: an important diagnostic consideration. Eur J Haematol 2010; 85(1): 43–50. [DOI] [PubMed] [Google Scholar]
- 17.Johkoh T, Ikezoe J, Tomiyama N, et al. CT findings in lymphangitic carcinomatosis of the lung: correlation with histologic findings and pulmonary function tests. AJR Am J Roentgenol 1992; 158(6): 1217–1222. [DOI] [PubMed] [Google Scholar]
- 18.Resten A, Maitre S, Humbert M, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol 2004; 183(1): 65–70. [DOI] [PubMed] [Google Scholar]
- 19.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. [DOI] [PubMed] [Google Scholar]
- 20.Montani D, Kemp K, Dorfmuller P, et al. Idiopathic pulmonary arterial hypertension and pulmonary veno-occlusive disease: similarities and differences. Semin Respir Crit Care Med 2009; 30(4): 411–420. [DOI] [PubMed] [Google Scholar]
- 21.Lang IM, Dorfmuller P, Noordegraaf AV. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc 2016; 13 Suppl 3: S215–221. [DOI] [PubMed] [Google Scholar]
- 22.Lang IM, Klepetko W. Chronic thromboembolic pulmonary hypertension: an updated review. Curr Opin Cardiol 2008; 23(6): 555–559. [DOI] [PubMed] [Google Scholar]
- 23.Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990; 81(6): 1735–1743. [DOI] [PubMed] [Google Scholar]
- 24.Castaner E, Gallardo X, Ballesteros E, et al. CT diagnosis of chronic pulmonary thromboembolism. Radiographics 2009; 29(1): 31–50. [DOI] [PubMed] [Google Scholar]