Abstract
A 26-year-old female presented with vision loss accompanied by migraine-like headaches. A contrast-enhanced magnetic resonance imaging of the brain was performed which revealed findings suggestive of stroke-like migraine attacks after radiation therapy (SMART) syndrome. SMART syndrome is a delayed complication of brain radiation characterized by neurologic symptoms including migraine-like headaches, seizures, and hemispheric impairment. The purpose of this article is to make the readers aware of this rare complication of brain irradiation. Appropriate diagnosis of SMART syndrome is essential to avoid invasive tests.
Keywords: Delayed complication, brain radiation, gyriform enhancement, SMART syndrome, stroke-like migraine attacks after radiation therapy
Introduction
Stroke-like migraine attacks after radiation therapy (SMART) syndrome is a delayed complication of brain irradiation which is not as well known as the other radiation effects such as leukoencephalopathy or radiation necrosis. The diagnosis of SMART syndrome can be based on typical clinical presentation of migraine-like headaches, stroke-like symptoms and seizures and characteristic magnetic resonance imaging (MRI) findings. It is essential to make this diagnosis to avoid an unnecessary brain biopsy.
Case report
A 26-year-old old female presented with five-day history of vision loss in both eyes. There was a gradual loss of color vision five days previously with complete loss of vision on the next day. These symptoms were also accompanied by headaches over the entire head but predominantly in the frontal and the vertex region. No diplopia, floaters, or photophobia were noted. She described similar episodes three months ago but less severe in nature with spontaneous resolution after eight days. She also described numbness in the hands with these episodes. There was no history of fever or weight loss. She had a single episode of seizure on day 3 of hospitalization with left head version, left gaze preference and full body shaking followed by myoclonic jerking of the left arm. Her past medical history is significant for surgical resection of an unknown brain tumor on the left side followed by chemotherapy and radiation at the age of seven years. She had chronic right homonymous hemianopsia after the treatment of brain tumor. Current medications are atorvastatin, metformin and levothyroxine. On physical examination, she only had light perception in both eyes. Pupils were slightly dilated but symmetric and reactive. The fundoscopic examination was unremarkable. Cranial nerves, sensory, and motor examinations were unremarkable. Cerebrospinal fluid (CSF) analysis was negative for infection. Tests for Angiotensin converting enzyme (ACE), anti-nuclear antibody (ANA), Lyme disease, rapid plasma reagin (RPR), human immunodeficiency virus (HIV), Sjogren's syndrome type A antigen (SSA) and Sjogren's syndrome type B antigen (SSB) were negative. MRI of the brain was performed with contrast. Her vision was slightly improved with steroids. She was discharged five days later on Keppra. Her symptoms were completely resolved on a follow-up visit at three months.
Imaging findings
Brain MRI at the time of admission showed pial/gyriform enhancement in the right parieto-occipital region without diffusion restriction (Figure 1). Post-surgical changes were seen in the left occipital lobe. Brain MRI on three-month follow-up showed complete resolution of enhancement (Figure 2).
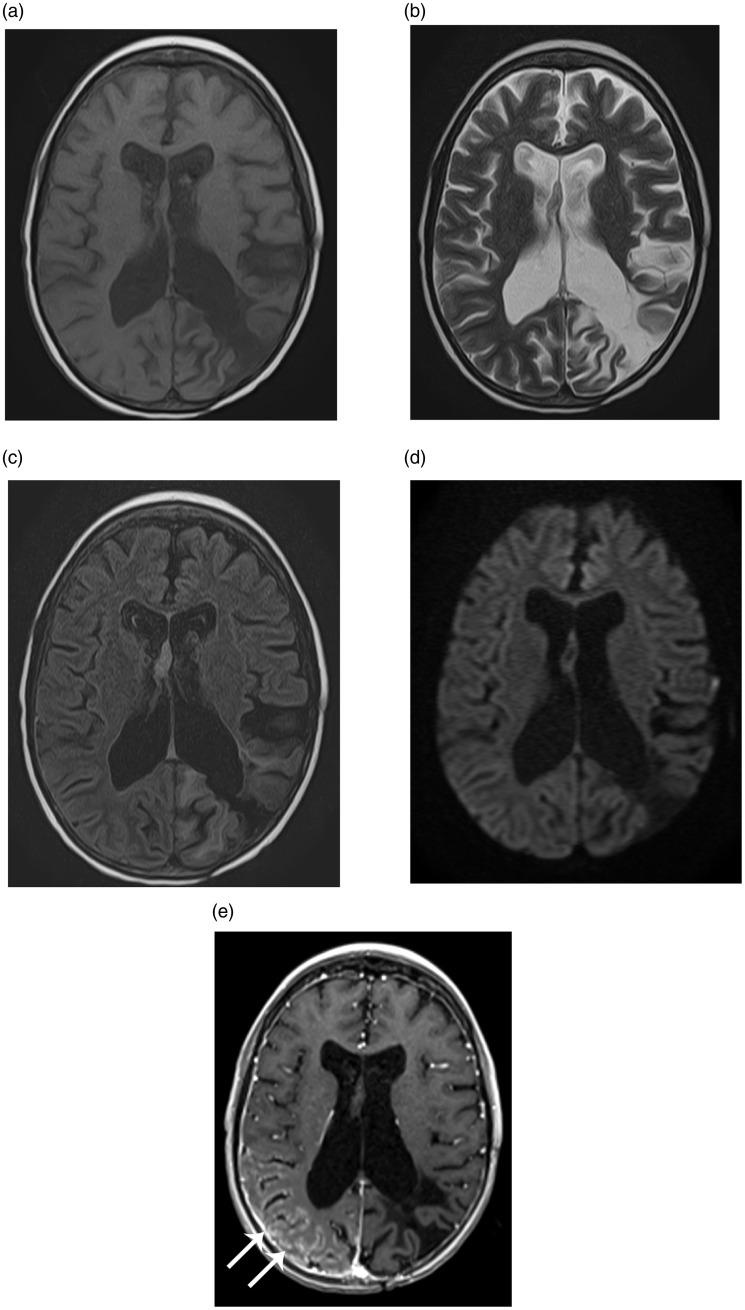
Figure 1.
Magnetic resonance (MR) images of the brain at the time of admission demonstrate gyriform enhancement (arrows in e) in the right parieto-occipital region without diffusion restriction. Note post-surgical encephalomalacia in the left parietooccipital region. (a) Axial T1-weighted image. (b) Axial T2-weighted image. (c) Axial fluid attenuation inversion recovery (FLAIR). (d) Axial diffusion-weighted image. (e) Axial T1-weighted post contrast image.
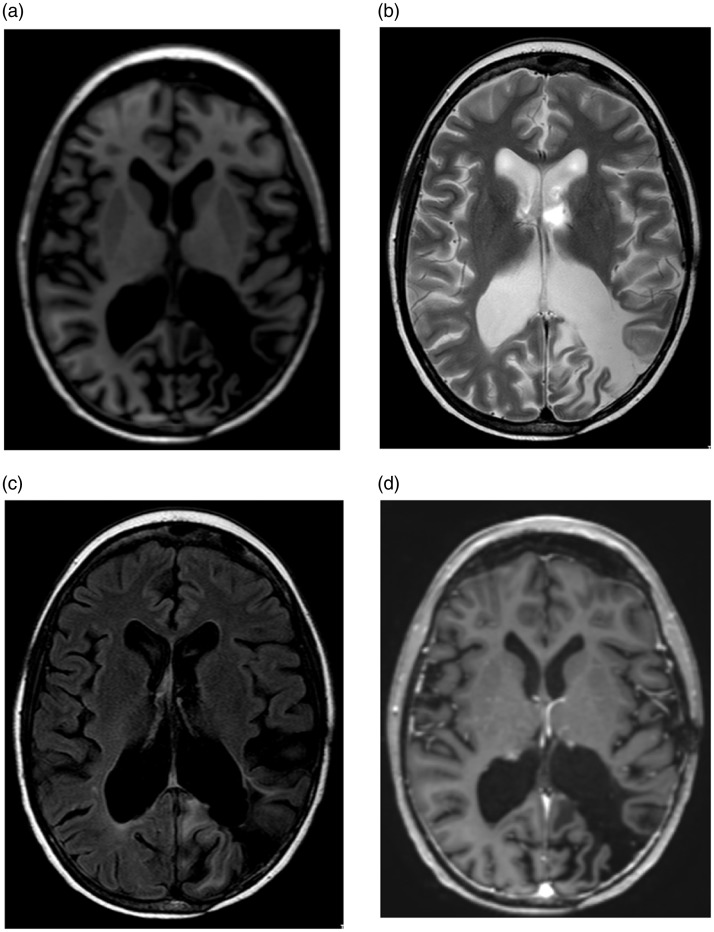
Figure 2.
Follow-up magnetic resonance (MR) images of the brain after three months demonstrate resolution of enhancement in the right parieto-occipital region. (a) Axial T1-weighted image. (b) Axial T2-weighted image. (c) Axial FLAIR. (d) Axial T1-weighted post contrast image.
Discussion
SMART syndrome is a late complication of brain radiation characterized by recurrent neurologic symptoms including migraine type of headaches, hemispheric neurological impairment, and seizures.1,2
This syndrome can be seen in both adult and pediatric age groups, typically in young adults. It is usually associated with radiation dosage of more than 50 Gy; however, cases of SMART syndrome with less than 50 Gy have also been reported.3,4
The time of onset of symptoms after radiation treatment is variable and ranges from 1–37 years.5 There is no particular association between specific tumor type and SMART syndrome; however, most of the tumors primarily originate in the central nervous system with a small proportion of metastatic lesions.6 The most common symptom is a headache followed by seizures and stroke-like symptoms.6 The symptoms are typically subacute in onset and include stroke-like features such as homonymous hemianopsia, hemiparesis, aphasia, sensory defects, seizures, and migraine type of headaches. Duration of symptoms is variable and ranges from hours to weeks.
The first case series of patient with symptoms similar to SMART syndrome was described in four children with “complicated migraine-like episodes after radiation therapy” by Shuper et al. in 1995.7 They described four children with a history of prior radiation for posterior fossa tumors (ependymoma and primitive neuroectodermal tumor (PNET)) and pineal PNET. These patients presented with migraine-like headaches associated with neurological impairment such as vision loss, numbness, weakness, and aphasia. None of them had seizures. According to the authors, no structural abnormality was noted on the MRI. Bartleson et al.3 reported two adult patients in 2003 with a history of radiation therapy for lymphoma and hemangioblastoma with transient, intense enhancement of the posterior cerebral gyri on MRI. In 2006, Black et al.1 proposed revisions to Bartleson et al.’s diagnostic criteria of SMART syndrome (Table 1).3
Table 1.
Diagnostic criteria for stroke-like migraine attacks after radiation therapy (SMART) syndromeas.
| Criteria |
|---|
| A. Remote history of external beam cranial irradiation without evidence of residual or recurrent neoplasm. |
| B. Prolonged, reversible signs and symptoms referable to a unilateral cortical region beginning years after irradiation. Manifestations may include: Visuospatial deficits Confusion Hemisensory deficits Hemiparesis Aphasia Seizures Headaches with the attacks An antecedent migraine with or without aura starting after irradiation. |
| C. Transient, diffuse, unilateral cortical gadolinium enhancement of the cerebral gyri sparing the white matter within a previous radiation field. |
| D. Not attributable to other disorder. |
Table taken from Black et al.1, copyright 2006, Sage Publishing.
Imaging findings
Brain MRI findings are characteristic and include unilateral hyperintense cortical signal on T2-weighted and FLAIR sequences with gyriform enhancement. The most commonly affected areas are posterior temporal, parietal, and occipital lobes. The enhancement typically resolves in 2–5 weeks but may persist up to 12 weeks.2 Abnormal findings on brain MRI are not always present8,9 but appear to be present in most of the cases.5 The pattern of gyriform enhancement is also not universal, and seen in about 72.2% of cases.6 Interestingly, enhancement on MRI was absent in about 54% of patients in one series.9 Diffusion restriction is usually absent in the region of cortical abnormalities.2 Three out of 11 patients showed diffusion restriction in a series reported by Black et al. and were thought to be due to superimposed infarcts.2 Gomez-Cibeira et al.10 described magnetic resonance (MR) spectroscopy findings in a patient with SMART syndrome with decreased N-acetyl-aspartate (NAA), increased creatine, and mild elevation of choline peaks. A lactate peak was not noted suggesting that mitochondrial dysfunction, vasospasm, or ischemic mechanisms were not involved. Khanipour et al.11 observed that susceptibility weighted imaging (SWI) may help in discriminating SMART syndrome from other differential diagnoses based on the identification of multiple parenchymal hypointensities, which are presumed to be radiation-induced cavernous malformations. However, these findings suggest prior radiation exposure and are not specific for SMART syndrome. There is limited literature on the role of perfusion studies in SMART syndrome, and a few case reports suggest that there is increased perfusion in the region of enhancement.12,13
Pathophysiology
The pathophysiology of SMART syndrome is not well understood due to the rarity of the disease and lack of histopathological findings in all of the reported cases. However, radiation is a likely cause as it was seen in all reported cases of the SMART syndrome. Radiation is known to cause delayed long-term neurotoxicity and can be seen from three months to a few years after treatment.14 These manifestations in the central nervous system include radiation necrosis, leukoencephalopathy, and myelopathy.15 Histopathological effects of delayed radiation neurotoxicity are blood-brain-barrier disruption, endothelial cell damage, vascular endothelial growth factor (VEGF) up-regulation, perivascular inflammation, thrombus formation, smooth muscle proliferation, and fibrinoid necrosis of the vessel wall subsequently leading to vessel narrowing and occlusion.16–19 However, it remains unclear if one or all of these factors are responsible for SMART syndrome. In a case series reported by Shuper et al.,7 the autopsy done on one patient (who died 1.5 years after the onset of a migraine-like headache episodes) did not reveal any vascular damage. They concluded that transient vascular instability secondary to radiation therapy was the cause of a complicated migraine-like headache in these patients. Farid et al.4 did not support the radiation-induced cerebrovascular reserve dysfunction as the underlying mechanism of SMART syndrome. They proposed that neuronal dysfunction may be a factor in the pathophysiology of SMART syndrome. This neuronal dysfunction may be related to cortical spreading depression (slowly propagating wave of neuronal and glial depolarization) which is seen in patients with migraines. The radiation effects responsible for SMART syndrome are likely multifactorial including cerebral hyperexcitability with impaired autoregulatory parameters, endothelial damage with impairment of the trigeminovascular system and lowering of the threshold for cortical spreading depression resulting in migraine-like headaches and increased risk for seizures.1,4,20 The trigeminovascular system consists of axons which provide the nociceptive innervation to the intracranial vasculature and the meninges, and activation of this system plays an important role in the pathophysiology of migraines.21 Maloney et al.5 reported a case of surgically induced SMART syndrome after the resection of a dural-based right middle cranial fossa metastatic lesion. They noted that the medial temporal lobe lesion was in close proximity to the trigeminal ganglion during surgery and speculated that the surgical manipulation could have disrupted the trigeminovascular system and set off the SMART syndrome. However, it is to be noted that the patient also received radiation in the form of Gamma Knife radiosurgery and whole-brain radiation therapy, five and two years before the onset of symptoms respectively, and prior radiation may have been a contributing factor for SMART syndrome in this case report with possible precipitation by the surgery.
There are no specific histopathological findings of SMART syndrome. In a case series reported by Black et al., brain biopsy (done on 4/11) patients) demonstrated nonspecific gliosis without specific pathology related to SMART syndrome.2
Prognosis
SMART syndrome was thought to be reversible; however, Black et al.2 reported that 45% of patients (total 11) in their series had an incomplete recovery with permanent imaging sequelae (cortical laminar necrosis) seen in 27% of patients. Tomek et al.22 reported a case of a patient with SMART syndrome with prolonged unresponsiveness with very slow recovery. Another case report by Singh et al.23 reported permanent neurological deficit with atrophy on MRI in a patient with SMART syndrome. Overall, it appears that the natural history of SMART syndrome is favorable with complete recovery in 83% of cases.6
Treatment
There is no specific treatment for SMART syndrome and treatment is usually targeted towards controlling the symptoms, such as anticonvulsive therapy.20,24
Differential diagnoses
Posterior reversible encephalopathy syndrome (PRES) shares some similarities with SMART syndrome with vasculopathy and endothelial injury being common factors in both disorders.25,26 However, differentiation from SMART syndrome should be possible in most cases based on clinical history (hypertension, organ transplantation, autoimmune disorders) and imaging findings that include bilateral symmetric vasogenic edema involving the cortex and white matter.26,27 Enhancement is not common and seen in 21–38% of patients.28,29 The most common location is the parieto-occipital lobe followed by the frontal lobe.27
A hemiplegic migraine can be familial or sporadic.30 Familial hemiplegic migraine (FHM) is characterized by a migraine with aura and reversible hemiparesis with at least one first- or second-degree relative having the same symptoms.31 Sporadic hemiplegic migraine is characterized by the same symptoms without a family history. The literature on MRI findings in these conditions is sparse and ranges from normal findings to increased T2 cortical signal with or without enhancement.31–34
Transient MR signal changes after a seizure is another potential differential diagnosis which is typically seen in patients with status epilepticus. MRI findings include increased T2 signal in the cortical-subcortical distribution with diffusion restriction and gyral enhancement.35 Findings can be seen in other areas away from the seizure focus such as the thalamus (especially pulvinar nucleus) and cerebellum.36,37
Other differential diagnoses include meningitis and carcinomatosis; however, these conditions can be ruled out with the help of clinical history and laboratory analysis.
Conclusion
In conclusion, the diagnosis of SMART syndrome should be considered whenever gyriform enhancement is noted in the parieto-occipital region in a patient with migraine-type headaches, seizures, and stroke-like symptoms with a remote history of brain radiation. Appropriate diagnosis of SMART syndrome is essential to avoid unnecessary invasive brain biopsy and other expensive tests.
Funding
No funding was received for this project.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Black DF, Bartleson JD, Bell ML, et al. SMART: Stroke-like migraine attacks after radiation therapy. Cephalalgia 2006; 26: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 2.Black DF, Morris JM, Lindell EP, et al. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: A case series. AJNR Am J Neuroradiol 2013; 34: 2298–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartleson JD, Krecke KN, O'Neill BP, et al. Reversible, strokelike migraine attacks in patients with previous radiation therapy. Neuro Oncol 2003; 5: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farid K, Meissner WG, Samier-Foubert A, et al. Normal cerebrovascular reactivity in stroke-like migraine attacks after radiation therapy syndrome. Clin Nucl Med 2010; 35: 583–585. [DOI] [PubMed] [Google Scholar]
- 5.Maloney PR, Rabinstein AA, Daniels DJ, et al. Surgically induced SMART syndrome: Case report and review of the literature. World Neurosurg 2014; 82: e7–e240. [DOI] [PubMed] [Google Scholar]
- 6.Rigamonti A, Lauria G, Mantero V, et al. SMART (stroke-like migraine attack after radiation therapy) syndrome: A case report with review of the literature. Neurol Sci 2016; 37: 157–1561. [DOI] [PubMed] [Google Scholar]
- 7.Shuper A, Packer RJ, Vezina LG, et al. ‘Complicated migraine-like episodes' in children following cranial irradiation and chemotherapy. Neurology 1995; 45: 1837–1840. [DOI] [PubMed] [Google Scholar]
- 8.Cordato DJ, Brimage P, Masters LT, et al. Post-cranial irradiation syndrome with migraine-like headaches, prolonged and reversible neurological deficits and seizures. J Clin Neurosci 2006; 13: 586–590. [DOI] [PubMed] [Google Scholar]
- 9.Partap S, Walker M, Longstreth WT, Jr, et al. Prolonged but reversible migraine-like episodes long after cranial irradiation. Neurology 2006; 66: 1105–1107. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Cibeira E, Calleja-Castano P, Gonzalez de la Aleja J, et al. Brain magnetic resonance spectroscopy findings in the stroke-like migraine attacks after radiation therapy (SMART) syndrome. J Neuroimaging 2015; 25: 1056–1058. [DOI] [PubMed] [Google Scholar]
- 11.Khanipour Roshan S, Salmela MB, McKinney AM. Susceptibility-weighted imaging in stroke-like migraine attacks after radiation therapy syndrome. Neuroradiology 2015; 57: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 12.Ardicli D, Gocmen R, Oguz KK, et al. Cerebral hyperperfusion in a child with stroke-like migraine attacks after radiation therapy syndrome. Neuropediatrics 2016; 47: 259–262. [DOI] [PubMed] [Google Scholar]
- 13.Nar Senol P, Gocmen R, Karli Oguz K, et al. Perfusion imaging insights into SMART syndrome: A case report. Acta Neurol Belg 2015; 115: 807–810. [DOI] [PubMed] [Google Scholar]
- 14.Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist 2003; 9: 180–188. [DOI] [PubMed] [Google Scholar]
- 15.Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 2013; 20: 485–502. [DOI] [PubMed] [Google Scholar]
- 16.Brown WR, Thore CR, Moody DM, et al. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res 2005; 164: 662–668. [DOI] [PubMed] [Google Scholar]
- 17.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000; 60: 321–327. [PubMed] [Google Scholar]
- 18.Nordal RA, Nagy A, Pintilie M, et al. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: A role for vascular endothelial growth factor. Clin Cancer Res 2004; 10: 3342–3353. [DOI] [PubMed] [Google Scholar]
- 19.Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys 1986; 12: 1965–1969. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Q, Yang L, Tan LM, et al. Stroke-like migraine attacks after radiation therapy syndrome. Chin Med J (Engl) 2015; 128: 2097–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buture A, Gooriah R, Nimeri R, et al. Current understanding on pain mechanism in migraine and cluster headache. Anesth Pain Med 2016; 6: e35190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomek M, Bhavsar SV, Patry D, et al. The syndrome of stroke-like migraine attacks after radiation therapy associated with prolonged unresponsiveness in an adult patient. Neurologist 2015; 19: 49–52. [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Hsu CC. Stroke-like migraine attacks after radiation therapy (SMART) syndrome causing permanent neurological deficit. Ann Indian Acad Neurol 2016; 19: 129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehan J. SMART syndrome: A reversible complication after radiation therapy and surgery for brain tumors. World Neurosurg 2014; 82: e193–e194. [DOI] [PubMed] [Google Scholar]
- 25.Marra A, Vargas M, Striano P, et al. Posterior reversible encephalopathy syndrome: The endothelial hypotheses. Med Hypotheses 2014; 82: 619–622. [DOI] [PubMed] [Google Scholar]
- 26.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008; 29: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao B, Yu BX, Li RS, et al. Cytotoxic edema in posterior reversible encephalopathy syndrome: Correlation of MRI features with serum albumin levels. AJNR Am J Neuroradiol 2015; 36: 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007; 189: 904–912. [DOI] [PubMed] [Google Scholar]
- 29.Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc 2010; 85: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen LL, Olesen J. Sporadic hemiplegic migraine. Cephalalgia 2004; 24: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen LL, Eriksen MK, Roemer SF, et al. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125: 1379–1391. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia H, Babtain F. Sporadic hemiplegic migraine with seizures and transient MRI abnormalities. Case Rep Neurol Med 2011; 2011: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha YH, Millett D, Kane M, et al. Adult-onset hemiplegic migraine with cortical enhancement and oedema. Cephalalgia 2007; 27: 1166–1170. [DOI] [PubMed] [Google Scholar]
- 34.Jacob A, Mahavish K, Bowden A, et al. Imaging abnormalities in sporadic hemiplegic migraine on conventional MRI, diffusion and perfusion MRI and MRS. Cephalalgia 2006; 26: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 35.Kim JA, Chung JI, Yoon PH, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: Periictal diffusion-weighted imaging. AJNR Am J Neuroradiol 2001; 22: 1149–1160. [PMC free article] [PubMed] [Google Scholar]
- 36.Cartagena AM, Young GB, Lee DH, et al. Reversible and irreversible cranial MRI findings associated with status epilepticus. Epilepsy Behav 2014; 33: 24–30.24614522 [Google Scholar]
- 37.Katramados AM, Burdette D, Patel SC, et al. Periictal diffusion abnormalities of the thalamus in partial status epilepticus. Epilepsia 2009; 50: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]