Abstract
Background
Acute ischemic stroke due to basilar artery occlusion (BAO) is associated with a dismal prognosis and, even though endovascular treatment (EVT) contributed to an improvement in clinical outcomes, patient selection is difficult and frequently results in futile recanalization. We investigated the prognostic value of baseline ADC quantification in patients with BAO undergoing EVT.
Methods
We retrospectively evaluated MRI at admission in 11 patients with BAO undergoing EVT. Ischemic lesions were defined on baseline DWI and minimum ADC (minADC), ADC ratio and total area were quantified. Final infarction area was determined on follow-up T2WI/CT. We assessed the correlation between imaging parameters, recanalization grade and clinical scores (NIHSS at admission, NIHSS and mRS at discharge and mRS at three months) using Spearman rank correlation coefficient and correcting for multiple comparisons with the false discovery rate (FDR).
Results
Lower values of minADC at admission MRI are strongly correlated with higher scores in NIHSS (rs = −0.845, p = 0.001) and mRS at discharge (rs = −0.743, p = 0.009). We also found a negative correlation between minADC and NIHSS at admission (rs = −0.67, p = 0.02), mRS at three months and difference between pre- and post-treatment ischemic area (rs = −0.664, p = 0.026) that lost significance with FDR correction. Ischemic area and TICI grade were not significantly associated with clinical results.
Conclusions
ADC quantification of ischemic lesions at baseline MRI seems to predict clinical outcome in patients with BAO undergoing EVT, more importantly than ischemic area or TICI grade.
Keywords: Stroke, ADC, basilar occlusion
Introduction
Basilar artery occlusion (BAO) is a rare form of acute ischemic stroke, with an estimated incidence of 1/100,000 per year,1 standing out for its dismal prognosis, with a high mortality rate and poor functional outcomes in survivors. More recently, technical improvement and availability of endovascular treatment (EVT) has changed this devastating scenario.1 Mechanical thrombectomy, in particular, has led to higher recanalization rates and better short-term clinical outcomes.2,3 However, the recent Endovascular Stroke Treatment (ENDOSTROKE) study revealed that in BAO the success of recanalization does not necessarily imply good clinical results.4 On the other hand, National Institutes of Health Stroke Scale (NIHSS) score and pre-treatment imaging changes both on magnetic resonance imaging (MRI) and computed tomography (CT) were indicated as independent predictors of clinical outcome in recent studies,4–6 highlighting the importance of applying initial prognostic markers for patient selection, avoiding “futile recanalization.”
Among imaging techniques in stroke, diffusion-weighted imaging (DWI) has been pointed to as a marker of cytotoxic edema in acute ischemic brain tissue with high risk of irreversible infarction. Although most studies have focused on anterior circulation, in BAO pre-treatment DWI also seems to be an outcome predictor tool, relying on lesion load and distribution.5,7–10 Previous studies quantitatively assessed apparent diffusion coefficient (ADC) maps in anterior circulation stroke, suggesting that low ADC values reflect tissue damage severity.11,12
We therefore aimed to explore the possible prognostic value of DWI metrics, including quantification of the infarcted area and ADC in patients with BAO undergoing EVT.
Methods
Patients
We retrospectively identified in the neuroangiography unit stroke database 46 patients with BAO undergoing EVT from January 2009 to March 2015. Eleven patients were selected for this study considering the following inclusion criteria: (1) ischemic posterior circulation stroke with acute cerebellar and/or brainstem symptoms with less than 12 hours; (2) pre-intervention DWI; (3) digital subtraction angiography (DSA) documenting BAO and final reperfusion grade; (4) EVT performed in the first 24 hours; (5) post-treatment imaging study (MRI/CT) documenting final infarct area. Thirty-five patients were excluded for not meeting one or more of the inclusion criteria.
Patients were characterized with respect to demographic data, clinical presentation, times to imaging tests and therapeutics, and symptom severity assessed by stroke specialists according to NIHSS and the modified Rankin Scale (mRS). NIHSS at admission was considered as clinical baseline and NIHSS and mRS at discharge as clinical outcome measures.
Imaging protocols and analysis
A 1.5 T Siemens Magnetom Avanto MR scanning system was used. Pre-intervention imaging protocol included: whole-brain echo planar imaging (EPI) sequence for DWI images (repetition time (TR) = 2700–3200 ms, echo time (TE) = 90 ms, matrix 128 × 128, slice thickness of 5 mm) as well as automatically calculated ADC maps using b0 and b1000 images, fluid-attenuated inversion recovery (FLAIR) sequence (5 mm slice thickness).
Post-processing was performed with Osirix software v.5.7.1. All regions of interest (ROIs), both in initial and follow-up exams, were manually draw in consensus by two neuroradiologists (D.P. with five years’ experience and I.F. with 10 years’ experience) blinded to outcome and clinical data. We defined ROIs corresponding to areas of acute ischemia (areas of abnormal hyperintensity on initial DWI) that we transferred to ADC maps for calculations of minimum ADC (minADC) in each ROI (Figures 1 and 2). We did not include small-scattered isolated foci of less than 1 cm diameter (Figure 2(a)). Although we carefully avoided the inclusion of cerebrospinal fluid (CSF), because of the dispersion of ischemic areas and its location in the posterior fossa (larger tissue-CSF interface), standard deviations were higher than in normal parenchyma and mean ADC was highly influenced by maximum ADC voxels containing CSF. Consequently, we found minADC to be a more precise parameter of ischemic lesion. MinADC was selected for each patient. Total acute ischemic area was calculated from the sum of all ROI areas. We also defined two control circular ROIs (1 cm diameter) on each centrum semiovale, for ADC ratio calculation.
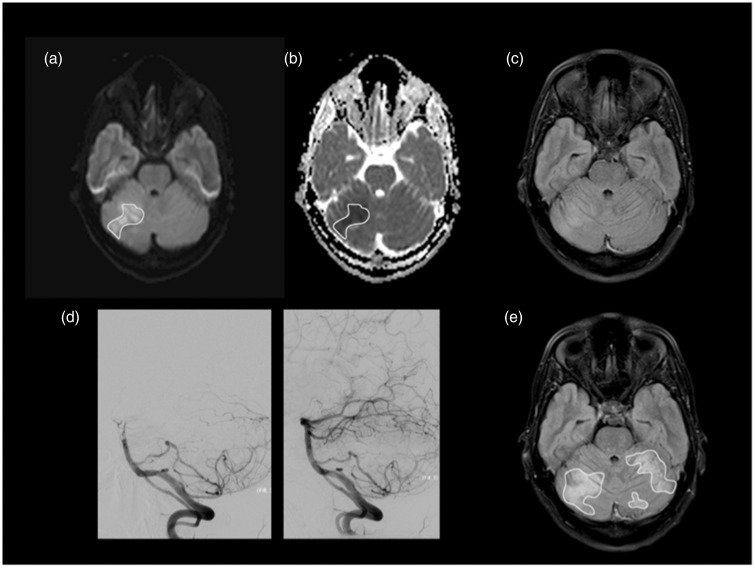
Figure 1.
Patient 5, with occlusion at the distal basilar artery, was admitted with an NIHSS of 5. MRI scan was performed seven hours after symptom onset showing areas of restricted diffusion on right cerebellar hemisphere representing acute ischemia involving the superior cerebellar artery territory. We manually drew several ROIs including areas of hyperintensity at b1000 (a), subsequently imported to ADC map (b). The minADC value in this patient was 0.323 × 10−3mm2/s. A faint high signal is already seen on FLAIR at the corresponding location (c). Successful recanalization (TICI = 3) with mechanical thrombectomy using a stent retriever device was achieved three hours after initial MRI (d). The follow-up MRI was performed on day 4 and showed extension of the ischemic area to both cerebellar hemispheres superiorly (e). The patient had an NIHSS of 1 and mRS of 1 at discharge and no neurological deficits at three-month follow-up (mRS = 0). NIHSS: National Institutes of Health Stroke Scale; MRI: magnetic resonance imaging; ROI: region of interest; FLAIR: fluid-attenuated inversion recovery; ADC: apparent diffusion coefficient; TICI: Thrombolysis in Cerebral Infarction score.
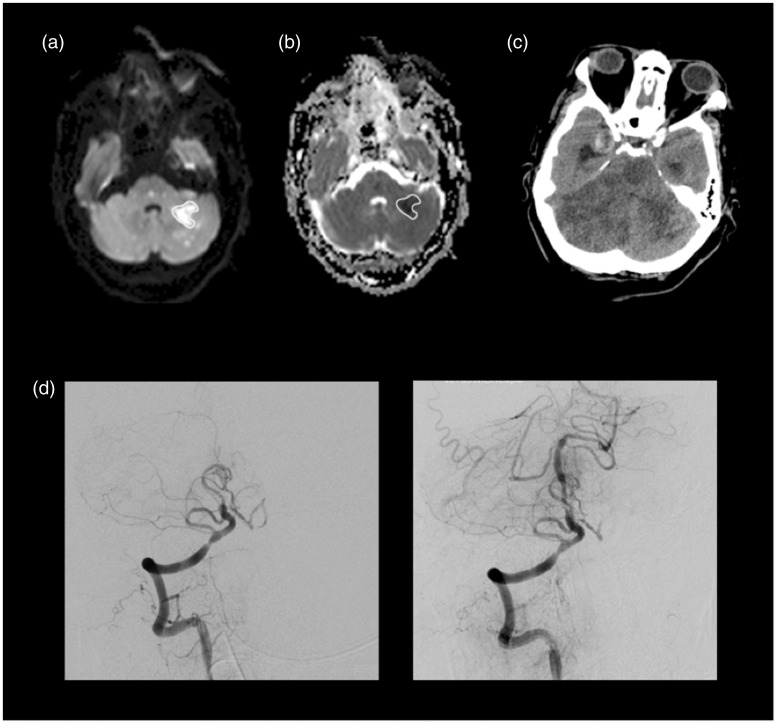
Figure 2.
Patient 11, with occlusion at the proximal basilar artery, was admitted with an NIHSS of 7. MRI scan showed multiple small areas of restricted diffusion representing acute ischemia on midbrain and pons bilaterally and on the left cerebellar hemisphere and temporo-occipital medial region. We did not take into account foci smaller than 1 cm when manually drawing ROIs including areas of hyperintensity at b1000 (a), subsequently imported to ADC map (b). MinADC measured in this patient was 0.219 × 10−3mm2/s. Despite successful recanalization (TICI = 3) with mechanical thrombectomy using a stent retriever device and balloon for dilatation (d), the follow-up CT two days after showed an extensive ischemic area involving both cerebellar hemispheres and midbrain (c), complicated with hemorrhagic transformation and supratentorial hydrocephalus, that culminated on patient death. NIHSS: National Institutes of Health Stroke Scale; MRI: magnetic resonance imaging; ROI: region of interest; FLAIR: fluid-attenuated inversion recovery; ADC: apparent diffusion coefficient; TICI: Thrombolysis in Cerebral Infarction score; CT: computed tomography.
Final infarction area was evaluated on follow-up T2-weighted images or CT in the few cases without follow-up MRI. CT was used as follow-up on patients 1, 4 and 11 for logistical and patient safety issues, since these were clinically unstable patients.
Statistical analysis
Statistical analysis was performed using SPSS software v.21. Because of the small size of our sample, we performed an exploratory analysis with the non-parametric Spearman correlation coefficient to evaluate possible relationships between outcome measures (NIHSS and mRS at discharge) and potential prognostic markers including age, minADC, ADC ratio, pre-treatment ischemic area, difference between pre- and post-treatment ischemic area, NIHSS at admission and recanalization according to Thrombolysis in Cerebral Infarction (TICI) grade. A p value <0.05 was considered significant. To avoid false positives, we used false discovery rate (FDR) correction for multiple comparisons, considering a Q value of 0.1.
Results
A total of 11 patients (eight male, three female) with a mean age of 58.7 years (minimum/maximum (min/max) 23/78) were included. Clinical scores and EVT results are summarized in Table 1. Median NIHSS at admission, 24 hours and discharge was 7 (min/max: 4/32), 5 (min/max: 1/37) and 9 (min/max: 1/37), respectively. The median mRS at discharge was 4 (min/max: 1/6). Three patients died.
Table 1.
Patients characterization according to demographic data, clinical scores, ischemic areas and endovascular therapy.
| Patient/ Sex/Age | NIHSS at admission | NIHSS at discharge | mRS at discharge | mRS at 3 months | Initial ischemic area (cm2) | Follow-up ischemic area (cm2) | Basilar occlusion site | TICI | IA treatment | minADC (×10−6mm2/s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/M/66 | 30 | 30 | 5 | 5 | 127.7 | 241.3 | Proximal | 3 | Trevo | 132 |
| 2/F/78 | 32 | 32 | 6 | 6 | 7.8 | 15.7 | Distal | 3 | tPA | 242 |
| 3/M/54 | 4 | 3 | 1 | 0 | 27.7 | 31.4 | Proximal | 3 | Trevo | 289 |
| 4/M/53 | 17 | 37 | 6 | 6 | 10.5 | 330.8 | Distal | 0 | Trevo | 170 |
| 5/M/23 | 5 | 1 | 1 | 0 | 19.7 | 31.2 | Distal | 3 | Trevo | 323 |
| 6/M/61 | 6 | 3 | 2 | 0 | 14.6 | 6.8 | Mid | 2a | Trevo, stent | 262 |
| 7/M/49 | 11 | 5 | 3 | 1 | 4.1 | 23.8 | Distal | 2c | Trevo | 224 |
| 8/M/61 | 4 | 18 | 4 | a | 3.9 | 12.9 | Distal | 2a | Solitaire | 252 |
| 9/M/64 | 9 | 1 | 1 | 1 | 9.2 | 23.6 | Mid | 3 | Wingspan | 290 |
| 10/F/71 | 4 | 4 | 4 | 2 | 1.1 | 2.2 | Proximal | 3 | Solitaire | 309 |
| 11/F/66 | 7 | 22 | 6 | 6 | 18.0 | 145.2 | Distal | 3 | Trevo, balloon | 219 |
Patient 8 was lost to follow-up at three months.
NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; TICI: Thrombolysis in Cerebral Infarction score; IA: ischemic area; minADC: minimum apparent diffusion coefficient; M: male; F: female; tPA: tissue plasminogen activator.
All patients, except patient 2, were treated with stent retriever devices, with complete recanalization (TICI = 3) in 64% (n = 7). Only in patient 4 no reperfusion was achieved at all (TICI = 0) after seven passages, and death occurred after seven days. This patient had the largest difference between follow-up and baseline ischemic areas (320.4 cm2) and the second lowest minADC value (0.17 × 10−3mm2/s).
Acute ischemic areas on baseline MRIs were highly variable, ranging from a small lacunar infarct with 1.1 cm2 to diffuse brainstem ischemia occupying 127.7 cm2 (mean = 22.2 cm2). Difference from post-treatment area (mean 78.6 cm2; range 2.2–330.8 cm2) was always positive except for patient 6—the only one with a follow-up exam in a chronic phase (20 months), with atrophy probably contributing to this negative differential. All other patients had a follow-up exam on the first 12 days after ictus.
Mean of minADC value in ischemic areas was 0.25 ± 0.058 × 10−3mm2/s. For control ROIs, minADC mean was 0.65 ± 0.063 × 10−3mm2/s.
Association between clinical scores and imaging variables
Table 2 summarizes the correlation analysis between clinical scores and possible predictors.
Table 2.
Spearman rank sum correlations between clinical scores at admission, discharge and three months and potential prognostic predictors.
| Admission |
Discharge |
Three months |
||||||
|---|---|---|---|---|---|---|---|---|
| NIHSS |
NIHSS |
mRS |
mRS |
|||||
| rs | p | rs | p | rs | p | rs | p | |
| Age | 0.22 | 0.52 | 0.34 | 0.30 | 0.50 | 0.12 | 0.556 | 0.095 |
| minADC | −0.67 | 0.02 | −0.85 | 0.001 | −0.74 | 0.009 | −0.642 | 0.045 |
| ADC ratio | −0.53 | 0.09 | −0.52 | 0.10 | −0.441 | 0.18 | −0.443 | 0.2 |
| IAb | 0.15 | 0.67 | −0.09 | 0.8 | −0.162 | 0.63 | −0.231 | 0.522 |
| IAf–b | 0.54 | 0.09 | 0.48 | 0.14 | 0.441 | 0.18 | 0.611 | 0.061 |
| TICI | −0.02 | 0.96 | −0.28 | 0.389 | −0.17 | 0.62 | 0.008 | 0.983 |
Significant results are marked in bold.
NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; minADC: minimum apparent diffusion coefficient; IAb: ischemic area in baseline; IAf–b: ischemic area at follow-up minus baseline; TICI: Thrombolysis in Cerebral Infarction score.
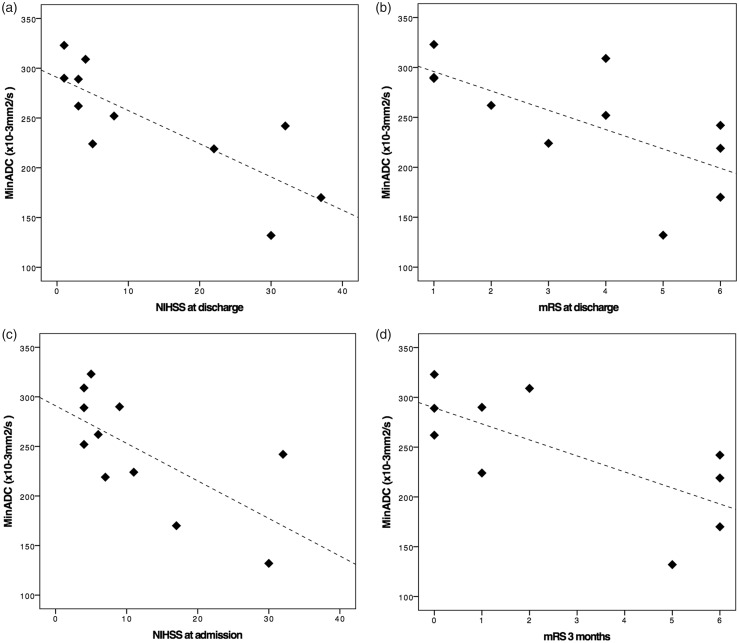
We found a significant negative association between minADC values in acute ischemic areas and the four clinical variables considered on our analysis (Figure 3): baseline NIHSS (rs = −0.67, p = 0.02), NIHSS at discharge (rs = −0.845, p = 0.001), mRS at discharge (rs = −0.743, p = 0.009) and mRS at three months (rs = −0.642, p = 0.045). As expected, clinical scores at discharge (NIHSS and mRS) were strongly correlated with each other (rs = 0.942, p < 0.001), as well as measures at discharge and mRS at 90 days (rs = 0.856, p = 0.002 for NIHSS and rs = 0.933, p < 0.001 for mRS). However, baseline NIHSS was not correlated with clinical scores discharge (rs = 0.59, p = 0.06 for NIHSS and rs = 0.53, p = 0.1 for mRS), but had a moderate correlation with mRS at three months (rs = 0.65, p = 0.042).
Figure 3.
Scatter plots graphically representing relationships between minADC and NIHSS at discharge (a), mRS at discharge (b), NIHSS at admission (c) and mRS at three months (d). minADC: minimum apparent diffusion coefficient; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale.
MinADC values and ADC ratio were also inversely correlated with the difference of pre- and post-treatment ischemic area (respectively rs = −0.664, p = 0.026; rs = −0.682, p = 0.021).
The area of ischemic lesions, reperfusion score and age were not significantly associated with clinical results (Table 2). Also, we did not find any association between age and ADC values (rs = −0.142, p = 0.68 for minADC; rs = 0.018, p = 0.96 for ADC ratio), ischemic areas (rs = −0.21, p = 0.53 for baseline ischemic area; rs = −0.196, p = 0.563 for difference of pre- and post-treatment ischemic area) or TICI grade (rs = 0.45, p = 0.164).
After correction for multiple comparisons, negative correlation between minADC and both clinical scores at discharge remained significant. As expected, positive correlation between NIHSS and mRS at discharge, and between these scores and mRS at three months, also survive to multiple comparisons correction.
Discussion
By quantifying ADC in BAO, our study demonstrated that lower minADC values, probably reflecting more severe tissue damage, are strongly associated with higher scores in NIHSS and mRS at discharge, meaning worse clinical outcome at short term. On the other hand, ischemic areas at baseline and difference between pre- and post-intervention were not significantly correlated with clinical scores, confirming our hypotheses that ADC quantification might be a helpful prognostic tool, even better than lesion size, which does not reflect per se ischemia severity.
We also found an association between lower minADC values and long-term outcome that did not survive to multiple comparisons correction, probably because of the lower variability of scores in such a small sample size, with a dichotomous distribution of mRS at three months (≤2 or ≥5).
Recanalization was successful (TICI = 2b or 3) in all cases except one. Therefore, we did not expect significant associations between this parameter and clinical outcome. In fact, in line with previous literature, our results confirm that even when good reperfusion is achieved patients can have a poor outcome, which might be predicted at baseline MRI by ADC quantification. Ultimately, the prognostic value of minADC values could be useful for selecting patients to EVT, avoiding futile recanalization procedures.
Furthermore, we found that minADC and ADC ratio were negatively associated with ischemic area difference, reflecting larger ischemic progression, although not surviving to FDR correction. This tendency is in agreement with previous reports13,14 showing that severely decreased ADC values represent, with higher probability, irreversible ischemia.
Interestingly, baseline NIHSS was not correlated with NIHSS and mRS at discharge in our sample, reflecting the already established contribution of EVT2 in changing the poor prognosis of BAO in some patients. The fact that NIHSS was not originally designed for posterior circulation stroke might underestimate its clinical impact. However, it has been largely applied and validated in the literature15,16 and, in our patients, the good correlation between mRS and NIHSS as well as between minADC and both clinical scores supports its clinical use.
Major limitations of our exploratory study are the small sample size and its retrospective design. However, to our knowledge this is the first study to quantify ADC in BAO and we believe that the strong correlations reported, achieving significance even in a small group, encourage future prospective studies.
Conclusions
In this small series, we report that ADC values of ischemic lesions in BAO undergoing EVT are correlated with clinical outcome, being a potential imaging prognostic factor, possibly more relevant than lesion size. Our results emphasize the need for further prospective research on MRI parameters in BAO, with potential impact in the selection of patients for EVT.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mattle HP, Arnold M, Lindsberg PJ, et al. Basilar artery occlusion. Lancet Neurol 2011; 10: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 2.Mak CH, Ho JW, Chan KY, et al. Intra-arterial revascularization therapy for basilar artery occlusion—a systematic review and analysis. Neurosurg Rev 2016; 39: 575–580. [DOI] [PubMed] [Google Scholar]
- 3.Mokin M, Sonig A, Sivakanthan S, et al. Clinical and procedural predictors of outcomes from the endovascular treatment of posterior circulation strokes. Stroke 2016; 47: 782–788. [DOI] [PubMed] [Google Scholar]
- 4.Singer OC, Berkefeld J, Nolte CH, et al. Mechanical recanalization in basilar artery occlusion: The ENDOSTROKE study. Ann Neurol 2015; 77: 415–424. [DOI] [PubMed] [Google Scholar]
- 5.Yoon W, Kim SK, Heo TW, et al. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015; 46: 2972–2975. [DOI] [PubMed] [Google Scholar]
- 6.Son S, Kim YW, Oh MK, et al. Initial factors affecting the clinical outcome after successful recanalization via MR-based mechanical thrombectomy in patients with acute ischemic stroke due to basilar artery occlusion. J Neurointerv Surg 2016; 8: 889–893. [DOI] [PubMed] [Google Scholar]
- 7.Cho TH, Nighoghossian N, Tahon F, et al. Brain stem diffusion-weighted imaging lesion score: A potential marker of outcome in acute basilar artery occlusion. Am J Neuroradiol 2009; 30: 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourand I, Machi P, Nogué E, et al. Diffusion-weighted imaging-lesion score of the brain stem as a predictor of functional outcome in acute basilar artery occlusion treated by mechanical thrombectomy with a Solitaire stent. Cerebrovasc Dis 2014; 35: 13. [Google Scholar]
- 9.Renard D, Landragin N, Robinson A, et al. MRI-based score for acute basilar artery thrombosis. Cerebrovasc Dis 2008; 25: 511–516. [DOI] [PubMed] [Google Scholar]
- 10.Karameshev A, Arnold M, Schroth G, et al. Diffusion-weighted MRI helps predict outcome in basilar artery occlusion patients treated with intra-arterial thrombolysis. Cerebrovasc Dis 2011; 32: 393–400. [DOI] [PubMed] [Google Scholar]
- 11.Shen JM, Xia XW, Kang WG, et al. The use of MRI apparent diffusion coefficient (ADC) in monitoring the development of brain infarction. BMC Med Imaging 2011; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Na DG, Thijs VN, Albers GW, et al. Diffusion-weighted MR imaging in acute ischemia: Value of apparent diffusion coefficient and signal intensity thresholds in predicting tissue at risk and final infarct size. Am J Neuroradiol 2004; 25: 1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer PW, Hassankhani A, Putman C, et al. Characterization and evolution of diffusion MR imaging abnormalities in stroke patients undergoing intra-arterial thrombolysis. AJNR Am J Neuroradiol 2004; 25: 951–957. [PMC free article] [PubMed] [Google Scholar]
- 14.Fiehler J, Knudsen K, Kucinski T, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke 2004; 35: 514–519. [DOI] [PubMed] [Google Scholar]
- 15.Lee WJ, Jung KH, Ryu YJ, et al. Acute symptomatic basilar artery stenosis: MR imaging predictors of early neurologic deterioration and long-term outcomes. Radiol 2016; 280: 193–201. [DOI] [PubMed] [Google Scholar]
- 16.Muir KW, Weir CJ, Murray GD, et al. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 1996; 27: 1817–1820. [DOI] [PubMed] [Google Scholar]