Abstract
Objective
We aimed to characterise the magnetic resonance imaging (MRI) features of a case series of primary gliosarcoma, with the inclusion of diffusion-weighted imaging and perfusion imaging with dynamic susceptibility contrast MRI.
Materials and methods
We conducted a retrospective study of cases of primary gliosarcoma from the Pathology Department database from January 2006 to December 2014. Clinical and demographic data were obtained. Two neuroradiologists, blinded to diagnosis, assessed tumour location, signal intensity in T1 and T2-weighted images, pattern of enhancement, diffusion-weighted imaging and dynamic susceptibility contrast MRI studies on preoperative MRI.
Results
Seventeen patients with primary gliosarcomas had preoperative MRI study: seven men and 10 women, with a mean age of 59 years (range 27–74). All lesions were well demarcated, supratentorial and solitary (frontal n = 5, temporal n = 4, parietal n = 3); 13 tumours abutted the dural surface (8/13 with dural enhancement); T1 and T2-weighted imaging patterns were heterogeneous and the majority of lesions (12/17) showed a rim-like enhancement pattern with focal nodularities/irregular thickness. Restricted diffusion (mean apparent diffusion coefficient values 0.64 × 10–3 mm2/s) in the more solid/thick components was present in eight out of 11 patients with diffusion-weighted imaging study. Dynamic susceptibility contrast MRI study (n = 8) consistently showed hyperperfusion in non-necrotic/cystic components on relative cerebral volume maps.
Conclusions
The main distinguishing features of primary gliosarcoma are supratentorial and peripheral location, well-defined boundaries and a rim-like pattern of enhancement with an irregular thick wall. Diffusion-weighted imaging and relative cerebral volume map analysis paralleled primary gliosarcoma with high-grade gliomas, thus proving helpful in differential diagnosis.
Keywords: Gliosarcoma, magnetic resonance imaging, diffusion magnetic resonance imaging, perfusion imaging, brain neoplasms, diagnosis, differential
Introduction
Primary gliosarcoma (PGS) is a rare central nervous system neoplasm, accounting for from 1.8% to 2.6% of all forms of glioblastoma multiforme (GBM).1 According to the 2016 World Health Organization classification2 it is a biphasic tumour, with alternating areas displaying glial and mesenchymal differentiation, it is considered a grade IV tumour and a subtype of GBM IDH-wild type. The mesenchymal metaplastic component may have fibroblastic, osseous, cartilaginous, muscle or adipose cell lineage.3 The occurrence of similar genetic alterations in both glial and mesenchymal components supports the concept of a monoclonal origin of the metaplastic mesenchymal differentiation from the astrocytic component.4
The PGS distinguishing features from GBM may be gross macroscopic features,5–9 genetic alterations and the greater propensity to metastasise extracranially.8,10 It has a slightly worse prognosis than GBM although some cases of long surviving patients have been described.4 Genetically they have a similar profile to primary glioblastoma, except for a much lower frequency of epidermal growth factor receptor amplification (8% in small series versus up to 50% of primary GBMs).11,12 Imaging features of PGS may be identical to GBM and insufficient to distinguish both entities, but some of them occur more frequently in gliosarcomas.13
The current literature consists mostly of case reports and small case series focusing on clinical and histopathological data, imaging features being sparsely described.14–16 Moreover, to the best of our knowledge, data concerning diffusion-weighted imaging (DWI) were described only by Zhang et al.16 and Damodaran et al.17 Zhang et al.16 also referred to magnetic resonance spectroscopy (MRS), but no data on perfusion magnetic resonance were detailed. We intend to characterise the magnetic resonance imaging (MRI) features of a case series of PGS, including diffusion-weighted imaging (DWI) and perfusion imaging with dynamic susceptibility contrast (DSC) MRI.
Materials and methods
We conducted a retrospective observational study. All patients with a histological diagnosis of brain gliosarcoma from January 2006 to December 2014 were selected from the Pathology Department database. In order to include only PGS, patients with a previous intracranial neoplasm diagnosis were excluded.
To characterise our sample the clinical records were reviewed concerning demographic and clinical data, treatment and outcomes (summarised in Table 1). Seventeen patients with PGS constitute our sample: seven men and 10 women, ranging from 27 to 74 years old (mean age of 59 years). The clinical presentation was mostly compatible with an expansive intracranial lesion (more commonly, focal neurological deficit, intracranianal hypertension and seizures). Eleven patients had a Karnofsky performance status (KPS) of 90 or 100 (64.7%), and two patients had a KPS equal to or below 60 (11.8%), remaining the same after surgery. The postoperative MRI (performed in the first 72 hours after surgery) showed a near total resection in 15 patients (88.2%; more than 90% of the tumour removed) and a complete resection in two patients (11.8%; absence of residual enhancement) (Table 1). Following surgery, most of the patients (n = 16) were treated with chemoradiotherapy according to the Stupp protocol.18 At the time of first progression eight patients (44.4%) underwent surgical re-intervention. With regard to chemotherapy, six patients received bevacizumab plus irinotecan, three patients received procarbazine, lomustine and vincristine, and five patients did not have clinical conditions to receive another treatment (Table 1). The median overall survival was 12 months (ranging from 4 to 31 months; two patients are alive, with 45 months and 18 months of follow-up); the overall survival was calculated from the date of diagnosis to the date of death. One patient developed a pulmonary metastasis 5 months after the diagnosis.
Table 1.
Clinical data of the 17 patients with PGS.
| Case | Age | Sex | Clinical presentation | Surgical resection | KPS | Radiotherapy | TMZ | Time to progression (months) | Surgical re-intervention | 2nd Line chemotherapy | Overall survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | Focal deficit | Near total | 100 | External beam | + | 7 | + | PCV | 12 |
| 2 | 61 | M | Focal deficit; ICH | Near total | 100 | External beam | + | 5 | − | − | 8 |
| 3 | 71 | M | Focal deficit | Near total | 90 | External beam | + | 4 | − | − | 5 |
| 4 | 59 | M | Focal deficit | Near total | 100 | External beam | + | 3 | + | − | 11 |
| 5 | 60 | F | ICH | Near total | 90 | External beam | + | 7 | + | PCV | 13 |
| 6 | 63 | M | Focal deficit | Near total | 70 | External beam | + | 5 | + | BCV | 31 |
| 7 | 52 | M | Focal deficit; ICH | Near total | 90 | External beam | + | 6 | − | BCV | 15 |
| 8 | 62 | M | Impaired consciousness | Near total | 30 | − | − | 1 | − | − | 4 |
| 9 | 64 | F | Focal deficit | Near total | 60 | External beam | + | 9 | − | PCV | 12 |
| 10 | 64 | F | Focal deficit | Near total | 90 | External beam | + | 6 | − | BCV | 8 |
| 11 | 59 | F | ICH | Near total | 100 | External beam | + | 3 | + | − | 5 |
| 12 | 74 | F | ICH | Near total | 70 | − | a | 1 | − | − | 11 |
| 13 | 27 | F | Seizures, ICH Focal deficit | Total | 100 | External beam | + | 2 | + | − | 45+(alive) |
| 14 | 63 | F | Focal deficit | Near total | 90 | External beam | + | 12 | + | BCV | 18+(alive) |
| 15 | 66 | F | Focal deficit | Near total | 80 | External beam | + | 5 | − | BCV | 14 |
| 16 | 49 | F | Focal deficit | Near total | 70 | External beam | + | 2 | − | − | 6 |
| 17 | 52 | F | ICH | Total | 90 | External beam | b | 9 | + | BCV | 18 |
ICH: intracranial hypertension; KPS: Karnofsky performance score; TMZ: temozolomide; PCV: procarbazine, lomustine and vincristine; BCV: bevacizumab; a: only temozolomide; b: only 3 cycles of temozolomide.
All preoperative brain MRIs were reviewed by two neuroradiologists, blinded to diagnosis, assessing tumour location, signal intensity in T1-weighted images and T2-weighted images, pattern of contrast enhancement, surrounding oedema and behaviour regarding DWI study, and relative cerebral volume (rCBV) maps obtained from DSC MRI. Patients without available preoperative MRI were excluded. MRI scans were performed using a 1.5 Tesla MR scanner (Magnetom Symphony, Siemens, Germany) or 3 Tesla MR scanner (Magnetom Trio, Siemens, Germany) with a standard head coil. Each MRI examination included at least pre-contrast axial T1-weighted spin echo (SE), T2-weighted fast spin echo (FSE) images and fluid-attenuated inversion recovery (FLAIR) images. DWI was acquired in the transverse plane using an SE echo-planar imaging sequence with encoding gradient in three orthogonal directions (b = 1000 s/mm2). The quantitative apparent diffusion coefficient (ADC) was calculated by the mean of three individual regions of interest (ROI) with 5 pixels/0.1 cm2 placed in the correspondent slice position of T2 FSE (the software for ADC calculation for each ROI is automatic using a monoexponential algorithm). DSC MRI was performed using a fat-suppressed T2*-weighted gradient echo echo-planar sequence (TR = 2000 ms at 3 T and 1790 ms at 1.5 T, TE = 30 ms, flip angle 90°, field of vision 210 cm, matrix 128 × 128, slice thickness 5 mm, gap 1 mm); the tumour was preloaded approximately 3 minutes prior to the acquisition with one fourth of total contrast volume (0.1 mmol/kg of Gd-Dota (Dotarem, Guerbet, France)); images were first acquired at baseline (five datasets) and then during the first pass of a bolus injection of the remaining dose, administrated via an 18 gauge peripheral venous catheter with a power injector at 5 mL/s, followed by a saline flush at the same rate. Post-contrast axial, coronal and sagittal planes T1-weighted images were obtained.
Statistical analysis was performed using IBM SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
The imaging features of patients with preoperative magnetic resonance are described in Table 2. All tumours were supratentorial and solitary lesions, located in the frontal lobe in five patients, in the temporal lobe in four patients, in the parietal lobe in three patients and involving more than one lobe in five patients. Thirteen tumours were located peripherally, abutting the dural surface, and eight of them had concurrent dural enhancement. All tumours presented a clear demarcation from the brain parenchyma and have round, lobulated or irregular shapes with surrounding vasogenic oedema. T1 and T2-weighted imaging patterns were generally heterogeneous, with predominant T1 hypointensity and T2 hyperintensity (further detailed in Table 2). Only one patient had a tumour with a significant haemorrhagic area (Figure 1), although we found a high proportion of patients with small hyperintense foci in T1-weighted imaging (11/17 patients), which suggests small haemorrhagic deposits. The enhancement was marked in all tumours, with the majority of lesions (n = 12) showing a pattern of rim-like enhancement with small focal nodularities or irregular thickness; three patients had a more homogeneous solid enhancement and two patients had a mix pattern with both rim-like and solid components of enhancement; only in one patient was ependymal lining enhancement identified (Figure 2). DWI was performed in 11 of the 17 patients; qualitative analyses of DWI and ADC maps identified areas of restricted diffusion located in the more solid or thick components of the tumour in eight patients (73%) (Figure 3); two lesions had no restricted diffusion; one patient had a compromised appreciation of DWI due to haemorrhage. Mean ADC values were 0.64 × 10−3 mm2/s (minimum 0.47 × 10–3 mm2/s and maximum 0.81 × 10−3 mm2/s) in tumours with components with restricted diffusion and 1.1 × 10–3 mm2/s in the other two subjects. DSC MRI was obtained in eight patients, and the qualitative analysis of the rCBV maps showed that the non-necrotic/cystic components of the lesion had hyperperfusion; two patients had available the mean perfusion curve after placement of a ROI in a hyperperfusion and enhancing tumour area, with a percentage of signal intensity recovery (PSR) over 60% (Figure 4). In the remaining six patients the PSR was impossible to obtain because the studies were older and the acquisition images of DSC MRI were not available.
Table 2.
Neuroradiological features based on magnetic resonance imaging.
| Neuroradiological features | Number of tumours (17) |
|---|---|
| Clear demarcation of tumour edge | 17 |
| Location | |
| Frontal lobe | 5 (3 involving corpus callosum) |
| Temporal lobe | 4 (1 involving the insula) |
| Parietal lobe | 3 |
| Fronto-temporal lobe | 2 |
| Fronto-parietal lobes | 1 |
| Parieto-occipital lobes | 1 |
| Fronto-temporo-parieto-insular lobes | 1 |
| Tumour abutting the dura | 13 |
| Yes, without dural enhancement | 5 |
| Yes, with dural enhancement | 8 |
| No | 4 |
| Peritumoral oedema | 17 |
| Mild | 3 |
| Moderate | 11 |
| Marked | 3 |
| Signal Intensity in T1-weighted images | |
| Hypointense | 3 |
| Mostly hypointense with hyperintense foci | 8 |
| Mostly hypointense with isointense and hyperintense foci | 3 |
| Mostly hypointense with isointense foci | 2 |
| Mostly isointense with an hypointense area | 1 |
| Signal Intensity in T2-weighted images | |
| Mostly hyperintense with hypointense foci | 5 |
| Mostly isointense with an hyperintense area | 2 |
| Mostly hyperintense with isointense and hypointense foci | 7 |
| Mostly hyperintense with isointense foci | 3 |
| Enhancement pattern | |
| Rim enhancement with nodularity/irregular thick wall | 12 |
| Mostly solid enhancement | 3 |
| Rim enhancement with nodularity/irregular thick wall and a solid component | 2 (1 with ependymal enhancement) |
| Diffusion-weighted image | 11 |
| Restricted diffusion in solid/thick component | 8 |
| Without restricted diffusion | 2 |
| Unreadable (haematoma) | 1 |
| Perfusion-weighted image | |
| (concerning solid/thick components of tumour) | 8 |
| rCBV (qualitative analysis) hyperperfusion | 8 |
| Perfusion mean curve with PSR > 60% | 8 |
rCBV: relative cerebral volume; PSR: percentage of signal intensity recovery.
Figure 1.
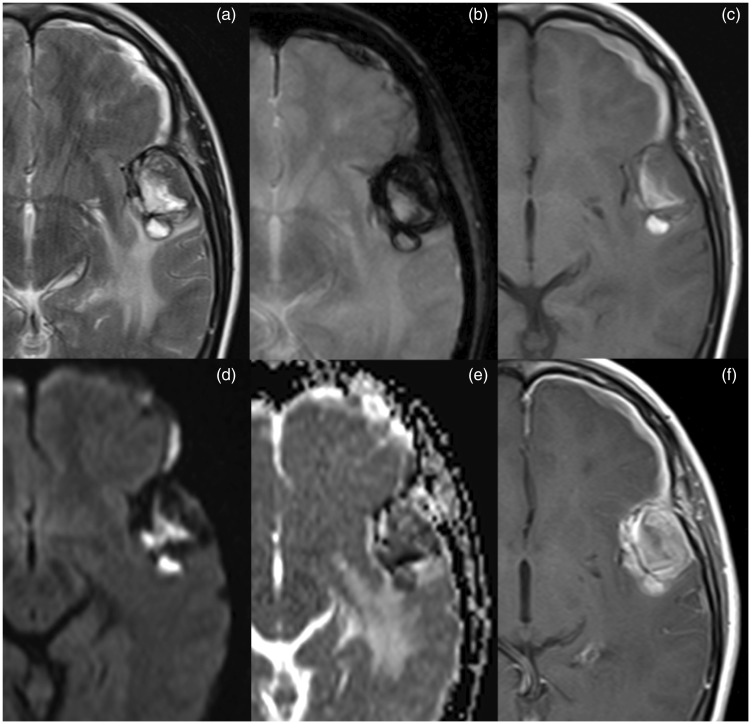
Brain MRI. Lesion in the left temporal with heterogeneous signal in T2-weighted imaging (a) and enhancement (f); lesion has high signal components in T1-weighted imaging (c) compatible with haemorrhagic content and presents a peripheral rim of low signal in T2* (b) suggesting haemosiderin deposition. Diffusion-weighted imaging and apparent diffusion coefficient map (d and e) show heterogeneous signal due to the presence of blood products.
Figure 2.
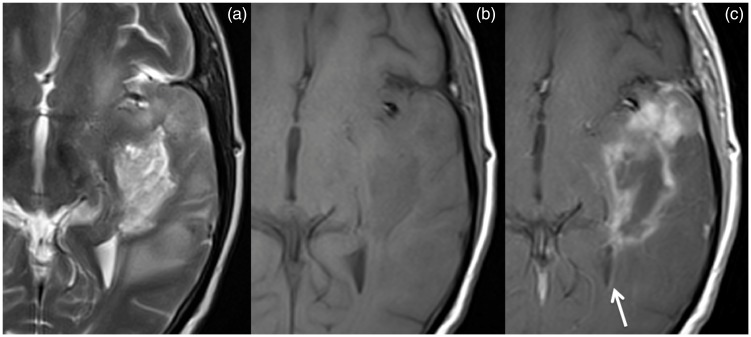
Brain MRI. Primary gliosarcoma extending from the temporal cortical surface to the temporal horn of the lateral ventricle, heterogenous, but with predominant hyperintensity in T2-weighted imaging (a) and hypointensity in T1-weighted imaging (b); the enhancement is more ‘solid’ near the temporal cortical surface and more rim-like in the more central component; it reaches the vicinity of the ventricular system being possible to find ependymal lining enhancement (c, arrow).
Figure 3.
Brain MRI. Post-contrast T1-weighted imaging (b, arrow) depicting the striking pattern of intratumoral strip enhancement; this finding possibly relates to neoangiogenesis, because linear stripes in T2-weighted imaging (a, arrow) resemble vascular structures. The peripheral component of the tumour shows hyperintensity on diffusion-weighted imaging (c, arrowhead) with a correspondent low signal on the apparent diffusion coefficient map (d, arrowhead), thus having restricted diffusion.
Figure 4.
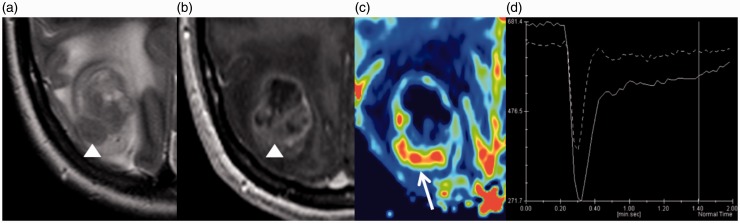
Brain MRI. Tumour that presents a more iso to hypointense T2 signal in the solid component (a, arrowhead), with a conspicuous enhancement (b, arrowhead). In the non-necrotic/cystic tumour components, the relative cerebral volume map shows hyperperfusion (c, arrow) and the mean perfusion curve has a percentage of signal intensity recovery over 60% (d, continuous line).
Discussion
To date, and to the best of our knowledge, the literature contains a limited number of case series reports with more than 10 cases of gliosarcoma.1,6,8, 9,14–17,19–24 Moreover, only four of those reported on detailed imaging features.14–16,25
PGS is a grade IV tumour according to the WHO classification26 that shares demographic, clinical and imaging features and management with GBM – ultimately the differentiation is histopathological.3,27 PGS is classically characterised as a poor prognosis tumour, with a median survival in untreated patients of 4 months8 and from 6.25 to 11.5 months in treated patients.1,8,19–22 The PGS metastasis rate is 11%,10,28–38 the lungs being the most frequent location, followed by the liver and lymph nodes.39,40 The reported metastatic foci are constituted by biphasic elements30–32 or exclusively by sarcomatous component,28,34,35 which points to the sarcomatous component as the potential metastatic source by haematogenous spreading, and corroborates the premise that PGS is a distinct entity from GBM. Due to our reduced sample size we cannot comment further on demographic and clinical features, but they are generally in accordance with previously reported data.
The general imaging pattern of PGS, at a first glance, shares the same imaging features as GBM precluding a safe imaging distinction.
In our series all tumours were supratentorial and more frequently located in the frontal lobe. The temporal lobe1,9,15,16,19,21 and frontal lobe6,14,17,20,22,41 are the more frequent reported locations; this variance may be due to different and usually small sample sizes. Han et al.14 also described involvement of the corpus callosum and Han et al.14 and Zhang et al.16 both described involvement of more than one lobe. Infratentorial, intraventricular and multifocal lesions at presentation are rare;14,16,41 accordingly, in our small sample, we have not found these aspects.
We found a high frequency of peripherally located tumours, which abutted the dural surface (13/17 cases), with concomitant dural enhancement depicted in eight patients. This pattern of predominantly peripheral location has been widely described in previously published studies, and suggests a possible distinguishing feature from GBM.6,14,16 As previously reported, all tumours had relatively smooth and well-demarcated boundaries and peri-lesional vasogenic oedema; however, the possibility of no surrounding oedema has been pointed out.14,16
The signal intensity in T1 and T2-weighted imaging is variable and heterogeneous, although described as generally hypointense in T1-weighted imaging and hyperintense in T2-weighted imaging, compared to white matter. Han et al. and Zhang et al. described in their series a high proportion of cases in which the enhancing components of gliosarcoma were less intense/intermediate signal intensity in T2-weighted imaging, postulating that they were the representation of the sarcomatous component.14,16 We identified only one patient with a tumour that clearly presented a more iso to hypointense T2 signal solid component (while compared to the signal of white matter) that had a particularly conspicuous enhancement (patient number 13, with 45 months of follow-up) (Figure 4). The areas of hyperintensity on T2-weighted imaging were described as the gliomatous component, with associated necrotic and cystic changes.14,16 However, we found that the T2 hyperintensity components (excluding the necrotic-cystic areas, with the T2 signal more close to cerebrospinal fluid) also had intense enhancement after contrast. Therefore, a more accurate definition of ‘intermediate signal intensity in T2’ is probably needed, perhaps in comparison to white matter, in order more reliably to compare this feature with the reported findings. As first reported by Han et al.14 we found a more striking pattern of intratumoral strip enhancement in one patient, possibly related to neoangiogenesis (as in T2-weighted imaging the strips resemble vascular structures) (Figure 3), although an irregular thick wall of rim-like enhancement pattern was a frequent feature (12/17 cases). Damodaran et al.17 described central location with transpendymal infiltration into the ventricles as an opposite pattern to the peripherally situated tumours; we were not able to find other reports concerning ependymal enhancement. We identified one patient with this feature (Figure 2), probably due to contiguous tumoural infiltration of the temporal horn of the lateral ventricle; this patient did not present with ‘a central location pattern’ because the tumour extended from the temporal cortical surface to the temporal horn of the lateral ventricle. Thus, it may be possible to find ependymal lining enhancement once the tumour reaches the vicinity of the ventricular system and becomes able to infiltrate by contiguous spreading.
To the best of our knowledge, only Zhang et al. and Damodaran et al. described DWI features.16,17 Zhang et al. mentioned the heterogeneous signal of DWI, with relatively high signal intensity areas corresponding to cellular components, while hypointense areas were the necrotic-cystic components, suggesting that this could be useful to display further the different composition of the tumour.16 However, no detail was provided concerning the relatively high signal areas, namely if they were really areas of restricted diffusion. Damodaran et al. described restricted diffusion in cellular components in 45% of PGS of the cases and a mixed pattern in the remaining tumours.17 We also found in DWI relatively high signal intensity areas corresponding to cellular components and hypointense areas corresponding to necrotic-cystic components of the tumour in all patients. However, only eight out of 11 patients had a mild restricted diffusion in the more solid components, by ADC maps qualitative evaluation and quantitative analysis (mean ADC of 0.64 × 10–3 mm2/s (minimum 0.47 × 10–3 mm2/s and maximum 0.81 × 10–3 mm2/s). These results are consistent with malignant tumours.42,43
Concerning DSC MRI, similar to GBM, we consistently found hyperperfusion in rCBV maps in the more solid components of the tumour. This feature was not characterised in the so far published case series of PGS, being one additional feature to consider that could be helpful in the differential diagnosis. PSR determination could help further in the differential diagnosis, because the two examples that we have found are similar to those found in GBM (distinct PSR from the metastasis, where the PSR is expected to be low).44
The small sample size and its retrospective nature are the main limitations of our study. The impossibility of determining the PSR derived from the DSC MRI in all patients with perfusion-weighted imaging (PWI) study was a drawback, but we still consider that this report adds further information concerning imaging features of PGS, namely by describing qualitatively and quantitatively the DWI studies, and by detailing the behaviour of these tumours in rCBV maps, although paralleling the DWI and PWI findings with high-grade glioma findings.
In conclusion, this case series illustrates a wide range of potential imagiological appearances of the gliosarcoma, despite all of them apparently being diagnosed with the same WHO criteria.
Overall, we can conclude that when a tumour presents in a supratentorial and peripheral location, with well-defined boundaries, a pattern of enhancement rim-like with an irregular thick wall, gliosarcoma should be included in the list of differential diagnoses. Further studies with a greater number of cases detailing DWI and PWI are needed; however, the presented data may be additional aids to shorten the list of differential diagnoses.
We declare that this study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Due to the retrospective nature of this study, patient consent and approval by our institutional review board was waived. All procedures followed were in accordance with institutional guidelines.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: a clinical study. Radiother Oncol 2001; 61: 57–64. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 3.Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med 2007; 131: 397–406. [DOI] [PubMed] [Google Scholar]
- 4.Linhares P, Martinho O, Carvalho B, et al. Analysis of a synchronous gliosarcoma and meningioma with long survival: a case report and review of the literature. Surg Neurol Int 2013; 4: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervoni L, Celli P. Cerebral gliosarcoma: prognostic factors. Neurosurg Rev 1996; 19: 93–96. [DOI] [PubMed] [Google Scholar]
- 6.Han SJ, Yang I, Ahn BJ, et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer 2010; 116: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri F, Stella L, Benvenuti D, et al. Cerebral gliosarcomas: correlation of computed tomographic findings, surgical aspect, pathological features, and prognosis. Neurosurgery 1990; 26: 261–267. [PubMed] [Google Scholar]
- 8.Morantz RA, Feigin I, Ransohoff J., III Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg 1976; 45: 398–408. [DOI] [PubMed] [Google Scholar]
- 9.Salvati M, Caroli E, Raco A, et al. Gliosarcomas: analysis of 11 cases do two subtypes exist? J Neurooncol 2005; 74: 59–63. [DOI] [PubMed] [Google Scholar]
- 10.Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol 2000; 54: 373–378. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 11.Actor B, Cobbers JM, Buschges R, et al. Comprehensive analysis of genomic alterations in gliosarcoma and its two tissue components. Genes Chromosomes Cancer 2002; 34: 416–427. [DOI] [PubMed] [Google Scholar]
- 12.Reis RM, Konu-Lebleblicioglu D, Lopes JM, et al. Genetic profile of gliosarcomas. Am J Pathol 2000; 156: 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn AG, Jhaveri MD, Salzman KL. Diagnostic imaging. Brain, 3rd edn Philadelphia, PA: Elsevier, 2016. , p. xxi, i–xiv pp. [Google Scholar]
- 14.Han L, Zhang X, Qiu S, et al. Magnetic resonance imaging of primary cerebral gliosarcoma: a report of 15 cases. Acta Radiol 2008; 49: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Rojas AE, Diaz-Perez JA, Ariza-Serrano LM, et al. Primary gliosarcoma of the brain: radiologic and histopathologic features. Neuroradiol J 2013; 26: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang BY, Chen H, Geng DY, et al. Computed tomography and magnetic resonance features of gliosarcoma: a study of 54 cases. J Comput Assist Tomogr 2011; 35: 667–673. [DOI] [PubMed] [Google Scholar]
- 17.Damodaran O, van Heerden J, Nowak AK, et al. Clinical management and survival outcomes of gliosarcomas in the era of multimodality therapy. J Clin Neurosci 2014; 21: 478–481. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 19.Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg 1998; 89: 425–430. [DOI] [PubMed] [Google Scholar]
- 20.Meis J, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma: a clinicopathologic study of 26 Radiation Therapy Oncology Group cases. Cancer 1991, pp. 2342–2349. [DOI] [PubMed] [Google Scholar]
- 21.Parekh HC, O’Donovan DG, Sharma RR, et al. Primary cerebral gliosarcoma: report of 17 cases. Br J Neurosurg 1995; 9: 171–178. [DOI] [PubMed] [Google Scholar]
- 22.Perry JR, Ang LC, Bilbao JM, et al. Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer 1995; 75: 2910–2918. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar C, Sharma MC, Sudha K, et al. A clinico-pathological study of 29 cases of gliosarcoma with special reference to two unique variants. Ind J Med Res 1997; 106: 229–235. [PubMed] [Google Scholar]
- 24.Singh G, Das KK, Sharma P, et al. Cerebral gliosarcoma: analysis of 16 patients and review of literature. Asian J Neurosurg 2015; 10: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwyer KW, Naul LG, Hise JH. Gliosarcoma: MR features. J Comput Assist Tomogr 1996; 20: 719–723. [DOI] [PubMed] [Google Scholar]
- 26.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO Classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda T, Yasumichi K, Suzuki T. Immunohistochemistry of gliosarcoma with liposarcomatous differentiation. Pathol Int 2008; 58: 396–401. [DOI] [PubMed] [Google Scholar]
- 28.Beaumont TL, Kupsky WJ, Barger GR, et al. Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol 2007; 83: 39–46. [DOI] [PubMed] [Google Scholar]
- 29.Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: a case report and review of the literature. Neurosurgery 1985; 17: 413–418. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenreich T, Devlin JF. A complex of glioblastoma and spindle-cell sarcoma with pulmonary metastases. Arch Pathol 1958, pp. 56–549. [PubMed] [Google Scholar]
- 31.Feigin I, Gross SW. Sarcoma arinsing in glioblastoma of the brain. Am J Pathol 1954, pp. 633–653. [PMC free article] [PubMed] [Google Scholar]
- 32.Garret R. Glioblastoma and fibrossarcoma of the brain with extracranial metastases. Cancer 1958, pp. 888–894. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama J, Mori T, Hori S, et al. Gliosarcoma with multiple extracranial metastasess. Case Report. Neurol Med Chir (Tokyo) 1989, pp. 938–943. [DOI] [PubMed] [Google Scholar]
- 34.Ojeda VJ, Sterrett GF. Cerebral gliosarcoma, pulmonary adenoid-cystic carcinoma, and pulmonary metastatic gliosarcoma: report of an untreated case. Pathology 1984; 16: 217–221. [DOI] [PubMed] [Google Scholar]
- 35.Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer 1969; 24: 270–276. [DOI] [PubMed] [Google Scholar]
- 36.Weaver D, Vandenberg S, Park TS, et al. Selective peripancreatic sarcoma metastases from primary gliosarcoma. Case report. J Neurosurg 1984; 61: 599–601. [DOI] [PubMed] [Google Scholar]
- 37.Wharton SB, Whittle IR, Collie DA, et al. Gliosarcoma with areas of primitive neuroepithelial differentiation and extracranial metastasis. Clin Neuropathol 2001; 20: 212–218. [PubMed] [Google Scholar]
- 38.Yokoyama H, Ono H, Mori K, et al. Extracranial metastasis of glioblastoma with sarcomatous component. Surg Neurol 1985; 24: 641–645. [DOI] [PubMed] [Google Scholar]
- 39.Dawar R, Fabiano AJ, Qiu J, et al. Secondary gliosarcoma with extra-cranial metastases: a report and review of the literature. Clin Neurol Neurosurg 2013; 115: 375–380. [DOI] [PubMed] [Google Scholar]
- 40.Han SJ, Yang I, Tihan T, et al. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol 2010; 96: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas A, Kumar N, Kumar P, et al. Primary gliosarcoma – clinical experience from a regional cancer centre in north India. Br J Neurosurg 2011; 25: 723–729. [DOI] [PubMed] [Google Scholar]
- 42.Chiang IC, Kuo YT, Lu CY, et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 2004; 46: 619–627. [DOI] [PubMed] [Google Scholar]
- 43.Usinskiene J, Ulyte A, Bjornerud A, et al. Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 2016; 58: 339–350. [DOI] [PubMed] [Google Scholar]
- 44.Cha S, Lupo JM, Chen MH, et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2007; 28: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]