Abstract
Background and purpose
The facial nerve is unique among cranial nerves in demonstrating normal enhancement of particular segments. The effect of varying T1 relaxivities of gadolinium-based contrast agents on facial nerve enhancement is unclear. In this study, we assess differences in normal facial nerve enhancement with two different gadolinium-based contrast agents, gadobutrol and gadopentetate dimeglumine. In addition, we evaluate differences in facial nerve enhancement with spin-echo (SE) T1 versus 3D inversion recovery prepared fast spoiled gradient-echo (FSPGR) post-contrast sequences.
Methods
A total of 140 facial nerves in 70 individuals were evaluated (70 with gadobutrol and 70 with gadopentetate dimeglumine) by two blinded reviewers. Differences in enhancement of facial nerve segments between the two agents were analyzed. Differences in enhancement between SE T1 and FSPGR imaging were also evaluated.
Results
There was no significant difference in facial nerve enhancement between gadobutrol and gadopentetate dimeglumine. Enhancement was commonly observed in the geniculate, tympanic and mastoid segments (98%–100%) with either contrast agent; enhancement was less common in the labyrinthine segments (9%–14%) and lateral canalicular segment (2%–5%). There was a smaller enhancing proportion of labyrinthine and tympanic segments with FSPGR as compared to SE T1 images with gadobutrol.
Conclusion
There is no significant difference in overall enhancement of the facial nerve between gadobutrol and gadopentetate dimeglumine. Mild enhancement of the lateral canalicular portion of the facial nerve may be a normal finding. With FSPGR sequence, there is lesser perceived enhancement of the labyrinthine and tympanic segments of the facial nerve with gadobutrol.
Keywords: Cranial nerve, contrast enhancement, gadolinium, relaxivity
Introduction
Identifying the normal pattern of enhancement of the facial nerve is important, as enhancement in certain segments can be normal, while enhancement of other segments could signify disease.1–3 With different gadolinium based contrast agents with varying magnetic properties, it becomes much more critical to identify any differences that there may be in the pattern of facial nerve enhancement. One of the major determinants for the increase in signal intensity on T1-weighted images following administration of gadolinium-based contrast agents is the shortening of the T1 relaxation. This shortening of the T1 relaxation caused by a magnetic resonance (MR) contrast agent changes with concentration of the contrast agent and is called the relaxivity. The T1 relaxivity depends also on temperature, field strength, and substance in which the contrast agent is dissolved.4,5 The differences in T1 relaxivities between different contrast agents could therefore affect the signal intensity on post-contrast T1-weighted imaging.4,5
In this study we assess whether the differences in magnetic properties between two commonly used gadolinium-based contrast agents, gadobutrol (Gadavist®, Bayer HealthCare Pharmaceuticals Inc) and gadopentetate dimeglumine (Magnevist®, Bayer HealthCare Pharmaceuticals Inc), with different T1 relaxivities4,5 (Table 1), cause a difference in perceived enhancement of the facial nerve on 3T MR imaging (MRI) under similar physiologic environments.
Table 1.
T1 and T2 relaxivities of commercially available gadolinium contrast agents used for evaluating the central nervous system.
| Brand name | T1 relaxivity (l/mmol-s) | T2 relaxivity (l/mmol-s) |
|---|---|---|
| Magnevist® | 4.1 | 4.6 |
| MultiHance® | 6.3 | 8.7 |
| Omniscan™ | 4.3 | 5.2 |
| OptiMARK™ | 4.7 | 5.2 |
| Dotarem® | 3.6 | 4.3 |
| ProHance® | 4.1 | 5.0 |
| Gadavist® | 5.2 | 6.1 |
T1 and T2 relaxivities for commercially available gadolinium-based contrast agents obtained in plasma at 37℃ at 1.5T (data from Rohrer et al.).4
Dehkharghani and colleagues recently demonstrated intrinsically greater T1 signal in the facial nerve on fast spoiled gradient-echo (FSPGR) compared to spin-echo (SE) T1 imaging.6 Our secondary goal, therefore, was to assess whether differences in normal enhancement pattern of the facial nerve would change with FSPGR versus SE T1-weighted MR images with either gadolinium contrast agent.
Methods
Participant selection
After institutional review board approval, we (RR and HM) retrospectively identified 123 consecutive patients (56 with gadopentetate dimeglumine and 67 with gadobutrol) who underwent clinical MRI of the internal auditory canal (IAC) with contrast at 3T, for evaluation of auditory symptoms from July 2012 to January 2013. This time period was chosen as there was overlapping use of gadopentetate dimeglumine and gadobutrol contrast agents. Forty-three patients were excluded because of prior mastoid and IAC surgery (most frequently for vestibular schwannoma), to reduce confounding by potential dural enhancement that could occur following any intracranial surgery. One patient each was excluded because of history of facial pain, trigeminal schwannoma and history of temporal bone trauma. After reviewing the images, we further excluded seven patients with incomplete or motion-degraded MR imaging. This resulted in 35 individuals with gadopentetate dimeglumine-enhanced MR images and 35 individuals with gadobutrol-enhanced MRI included in the study. Medical charts were reviewed for clinical and demographic information to find any additional correlations of interest attributable to facial nerve enhancement.
MR scanning parameters
All participants were imaged on a single 3 Tesla MR (GE SignaDx; Waukesha, WI) scanner with the use of a head coil and had dedicated IAC/temporal bone MR imaging protocol. All individuals had pre-contrast SE T1 imaging to recognize any intrinsic T1 hyperintensity of the facial nerve, as well as fast imaging employing steady-state acquisition (FIESTA, repetition time (TR) 7–9 ms, echo time (TE) 2–3 ms) of the posterior fossa to exclude facial nerve or IAC mass lesion. The post-contrast sequences included thin-section SE T1 (TR 475–600 ms, TE 20 ms, slice thickness 3 mm, slice spacing 0, number of excitations (NEX) 2) in the axial and/or coronal planes, and coronal post-contrast FSPGR (TR 11–12 ms, TE 4–5 ms, TI 450 ms, slice thickness 1.6 mm, NEX 1). All participants had FSPGR and T1-weighted SE images; FSPGR images were performed as the first post-contrast sequence in 19 (of 35) cases with gadobutrol, compared to 11 (of 35) cases with gadopentetate dimeglumine. There were no differences in the parameters for the clinical MRI over the period the data were collected.
Contrast agents
The contrast doses of gadobutrol (0.1 ml/kg, 0.1 mmol/kg) and gadopentetate dimeglumine (0.2 ml/kg, 0.1 mmol/kg) were used as per manufacturer guidelines. The study was performed at a time period when there was shift in practice to the use of gadobutrol from gadopentetate dimeglumine. Therefore, pretest selection that would affect choice of contrast agent between the two populations was reduced.
Image review
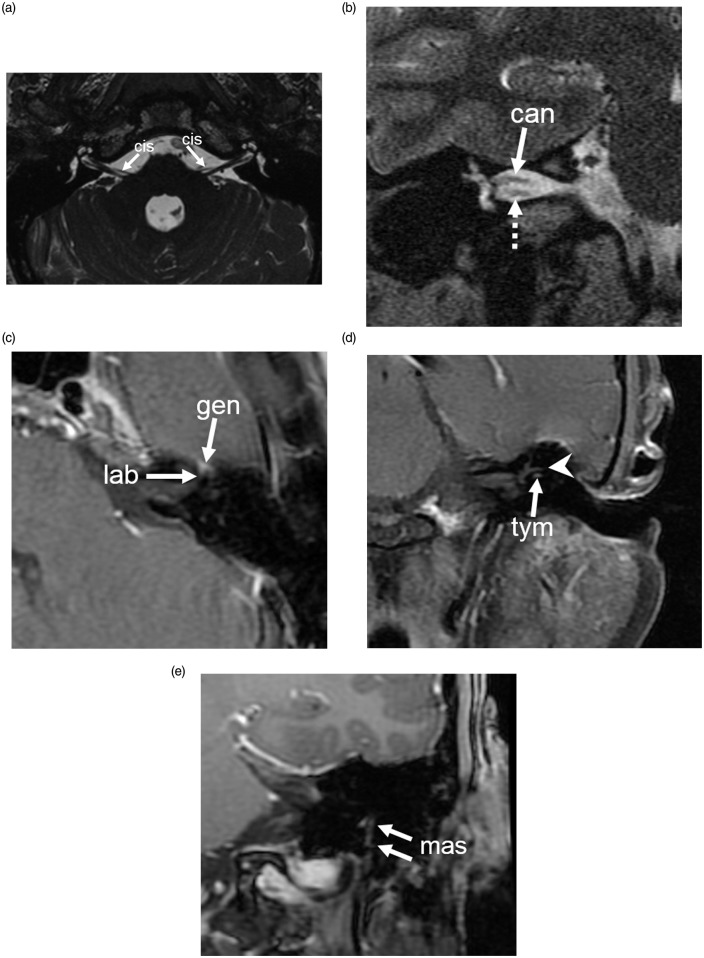
The images were reviewed by two neuroradiologists (HM and RR) blinded to the contrast agent used. Conflicts were resolved by consensus. If consensus could not be reached, the opinion of the senior neuroradiologist (HM) was considered. The intracranial portion of the facial nerve was divided into six segments for evaluation: (1) cisternal, (2) canalicular, (3) labyrinthine, (4) geniculate, (5) tympanic, and (6) mastoid (Figure 1). Enhancement of different portions of the facial nerve had three possible grades: (1) no enhancement, (2) mild enhancement that is less than adjacent vascular enhancement, and (3) intense enhancement that is equal to adjacent vascular structures.3 Enhancement in either SE-T1, FSPGR or both sequences was considered enhancement of the facial nerve segment. Pre-contrast FSPGR images were reviewed in all cases to exclude intrinsic T1 hyperintensity in the facial nerve.
Figure 1.
Segments of facial nerve. (a) Axial FIESTA sequence shows the cisternal segment (cis) of the seventh cranial nerve emerging from the lateral brainstem between the pons and medulla at the level of the facial colliculus. (b) Coronal T2-weighted image shows the canalicular segment of the facial nerve (can) in the superior aspect of the internal auditory canal, with the cochlear nerve (dotted arrow) inferiorly located. (c) Axial gadobutrol contrast-enhanced SE T1 shows the labyrinthine (lab) and geniculate (gen) segments of the facial nerve. (d) Coronal gadobutrol contrast-enhanced SE T1 shows the tympanic segment (tym) of the facial nerve inferior to the lateral semicircular canal (arrowhead). (e) Coronal gadobutrol contrast-enhanced T1 (FSPGR) image shows the vertical mastoid segment (mas) of facial nerve extending from the posterior genu to the stylomastoid foramen. FIESTA: fast imaging employing steady-state acquisition; SE: spin-echo; FSPGR: fast spoiled gradient-echo.
Statistical analysis
For statistical analysis we dichotomized the observed enhancement in each segment of the facial nerve as present (mild or intense enhancement) or absent. We evaluated the differences in proportion of enhancing facial nerves in each segment for either contrast agent. We also analyzed differences in presence of enhancement in different segments of the facial nerve with SE T1 or three-dimensional (3D) FSPGR with either contrast agent. A p value of < 0.05 was considered statistically significant. Agreement between the two reviewers was computed by kappa statistics of the dichotomous value of enhancement or nonenhancement of the facial nerve in each segment. Statistical analysis was performed using Microsoft Excel (Microsoft Office 2010) and R (https://www.r-project.org), a freely available software for statistical computing.
Results
Seventy adults were included in the study (21 males) with average age of 51.3 years (19–88 years). There were 35 participants each imaged by gadobutrol and gadopentetate dimeglumine, i.e. 70 facial nerves evaluated with gadobutrol and 70 with gadopentetate dimeglumine. There was moderate interobserver agreement of facial nerve enhancement with a Cohen’s kappa of 0.43.
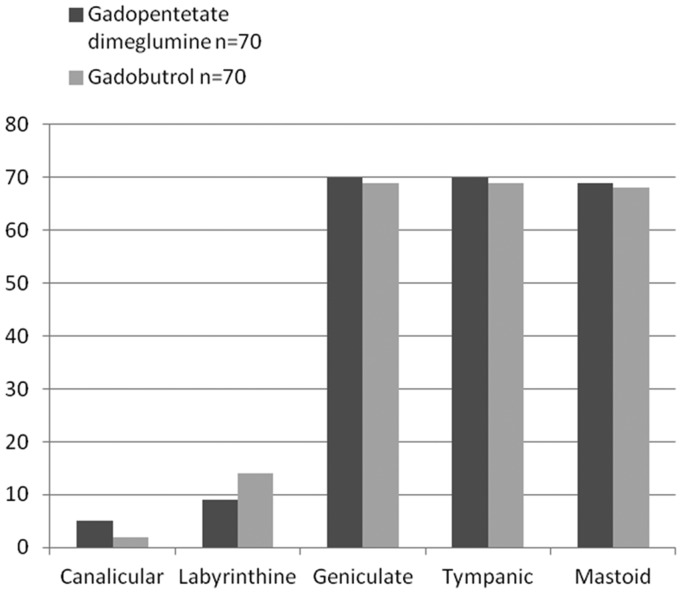
There was no significant difference in the order of post-contrast imaging (T1 SE or FSPGR with either contrast agent). With either contrast agent, enhancement was commonly observed in the labyrinthine, geniculate, tympanic, and mastoid segments of the facial nerves (Figures 2–5, Table 2). No enhancement was identified in the cisternal portion in any participant. There was no significant difference in facial nerve enhancement with use of gadopentetate dimeglumine versus gadobutrol. Most segments of the facial nerve demonstrated mild enhancement, with very few of the geniculate, tympanic and mastoid segments demonstrating intense enhancement, similar to adjacent vascular structures (Figures 6 and 7, Table 3).
Figure 2.
Enhancement of the different segments of the facial nerve with gadobutrol and gadopentetate dimeglumine.
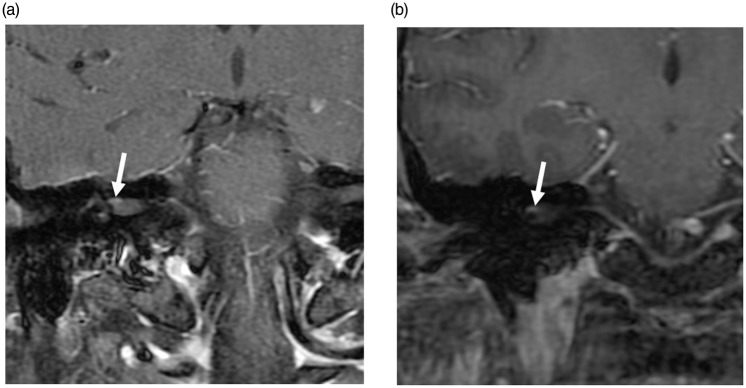
Figure 3.
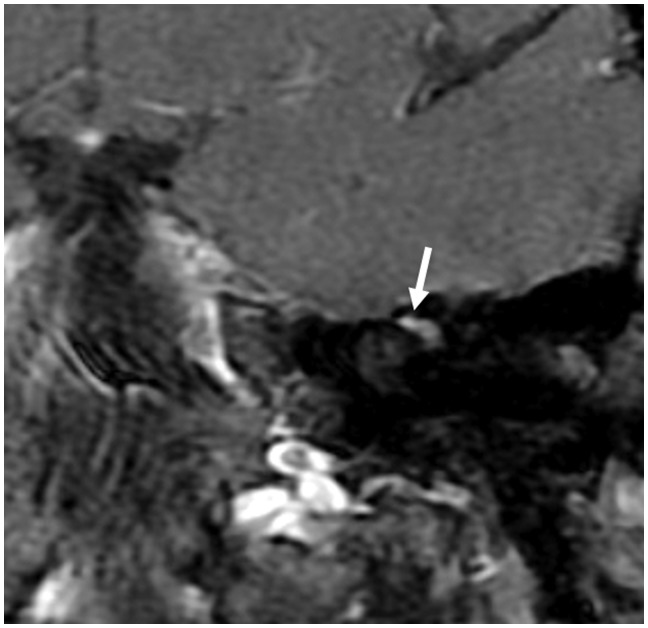
Post-contrast (gadobutrol) SE T1 (a) and FSPGR (b) images demonstrating mild enhancement of the canalicular segment of the right facial nerve (arrows). The left canalicular segment did not enhance (not shown). SE: spin-echo; FSPGR: fast spoiled gradient-echo.
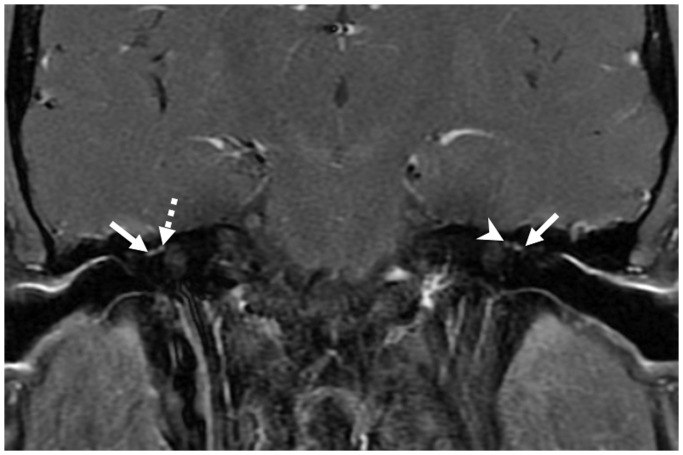
Figure 4.
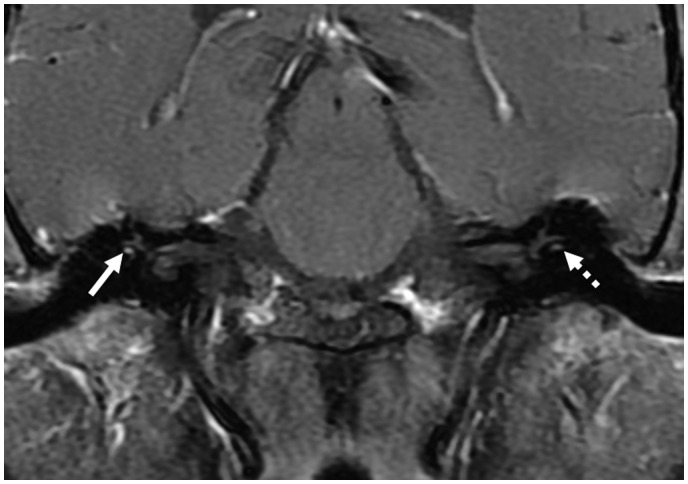
Post-contrast (gadopentetate dimeglumine) coronal spin-echo (SE) T1 image demonstrating mild enhancement of the tympanic segments of the facial nerve bilaterally (solid arrows). There is mild enhancement of the right labyrinthine segment (dotted arrow), but enhancement is not identified in the left labyrinthine segment (arrowhead).
Figure 5.
Post-contrast (gadobutrol) coronal spin-echo (SE) T1 images demonstrating intense left geniculate ganglion (arrowhead) enhancement.
Table 2.
Any enhancement of different facial nerve segments.
| Segments of facial nerve | Gadopentetate dimeglumine n = 70 | Gadobutrol n = 70 | Fisher’s exact p value |
|---|---|---|---|
| Canalicular | 5 (7%) | 2 (3%) | 0.44 |
| Labyrinthine | 9 (13%) | 14 (20%) | 0.36 |
| Geniculate | 70 (100%) | 69 (99%) | 1 |
| Tympanic | 70 (100%) | 69 (99%) | 1 |
| Mastoid | 69 (99%) | 68 (98%) | 1 |
Figure 6.
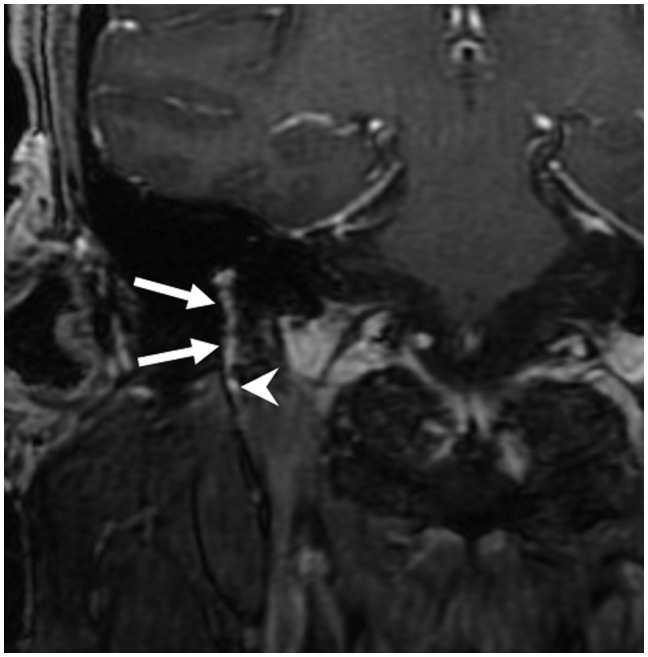
Post-contrast (gadopentetate dimeglumine) coronal spin-echo (SE) T1 image demonstrating intense enhancement of the right tympanic segment (solid arrow) of the facial nerve. The left tympanic segment of the facial nerve showed no definite enhancement (dotted narrow).
Figure 7.
Post-contrast (gadobutrol) coronal FSPGR images demonstrating intense enhancement of the right mastoid segment of the facial artery, similar in intensity to adjacent blood vessel (arrow). The extra-cranial portion of the facial nerve did not enhance. FSPGR: fast spoiled gradient-echo.
Table 3.
Facial nerve segments with intense enhancement.
| Segments of facial nerve with intense enhancement | Gadopentetate dimeglumine n = 70 | Gadobutrol n = 70 |
|---|---|---|
| Canalicular | 0 | 0 |
| Labyrinthine | 0 | 0 |
| Geniculate | 7 | 2 |
| Tympanic | 4 | 0 |
| Mastoid | 4 | 1 |
Enhancement of the canalicular segment was identified in two (3%) facial nerves using gadobutrol and five (7%) facial nerves using gadopentetate dimeglumine, always in the lateral half of the internal auditory canal (distal portion). This enhancement was mild in all cases. The FSPGR sequence and post-contrast SE T1-weighted images each identified canalicular segment enhancement in four nerves. One individual in each contrast group had bilateral canalicular segment enhancement.
When comparing SE T1 versus FSPGR images (Table 4), there was lesser proportion of enhancement in the labyrinthine portion (p = 0.008) and tympanic portion (0.03) of the facial nerve on FSPGR with gadobutrol. There was no significant difference between SE T1 and FSPGR enhancement of the facial nerve in other segments with either contrast agent.
Table 4.
Enhancement of facial nerve with different MR sequences.
| Segments of facial nerve | Gadopentetate dimeglumine (n = 70) |
Gadobutrol (n = 70) |
||||
|---|---|---|---|---|---|---|
| SE T1 | FSPGR | Fischer exact p value | SE T1 | FSPGR | Fischer exact p value | |
| Canalicular | 3 | 3 | 1 | 2 | 2 | 1 |
| Labyrinthine | 9 | 4 | 0.24 | 13 | 3 | 0.008a |
| Geniculate | 70 | 70 | 1 | 68 | 63 | 0.09 |
| Tympanic | 70 | 70 | 1 | 69 | 63 | 0.03a |
| Mastoid | 69 | 69 | 1 | 68 | 63 | 0.09 |
Statistically significant.
MR: magnetic resonance; SE: spin-echo; FSPGR: fast spoiled gradient-echo.
Discussion
The pattern of contrast enhancement that we identified with two commonly used gadolinium agents is similar to previous studies, with enhancement in the labyrinthine, geniculate, tympanic and mastoid segments.1–3,6–9 We also identified enhancement of the distal intracanalicular segment of the facial nerve in a small proportion of our patients with either contrast agent. Although previous studies have attributed enhancement of the intracanalicular segment of the facial nerve to pathology such as Bell’s palsy,1,10 this finding was also identified in normal facial nerves by Hong et al. on 3T MRI using gadobutrol.3 Based on the results or our study, we agree that mild enhancement of the distal intracanalicular facial nerve is a normal finding, and can be seen in a small proportion of the healthy adult population. In our opinion, intense enhancement of the canalicular segment of the facial nerve is more specific for Bell’s palsy, as suggested by other authors.9,10 None of our participants showed enhancement of the cisternal portion of the facial nerve with either contrast agent. This is in keeping with existing knowledge that the cisternal segment does not normally enhance. The cause of facial nerve enhancement has been attributed to the abundant arteriovenous plexus inside the epineurium and perineurium in the tympanic and mastoid segments. Because of this abundant vascularity, contrast material pools within the capillary and venous structures resulting in the visualized enhancement.11 In the narrow labyrinthine canal, the facial nerve has few small endoneural capillaries resulting in enhancement in few individuals.
Interestingly, although FSPGR images can demonstrate a higher intrinsic signal intensity in the facial nerve,6 we identified a fewer number of visible enhancing labyrinthine and tympanic segments on FSPGR as compared to SE T1 images with gadobutrol. The cause of this difference in our findings is unclear. It is possible that timing of post-contrast imaging might play a role. Although there was no significant difference in timing of FSPGR versus SE T1 images with either contrast agent, the FSPGR sequence was frequently the first post contrast sequence with gadobutrol. The shorter lag time to obtain FSPGR images with gadobutrol might have resulted in poor perfusion of the endoneural plexus in the labyrinthine segment of the facial nerve and therefore lesser enhancement with this sequence. It should be noted that enhancement in the labyrinthine segment was not common and that enhancement was mild whenever present (i.e. less than adjacent vascular enhancement).
Although no previous studies have so far evaluated differences in cranial nerve enhancement between different gadolinium-based contrast agents, such comparisons have been made in the normal brain parenchyma or with brain parenchymal lesions.12–16 Griffiths et al. observed no difference in white matter and thalamic enhancement between gadopentetate dimeglumine and gadobutrol (the same gadolinium contrast agents as used in our study) when using the same amount of gadolinium. However, there were differences in enhancement when the same volume of manufacturer’s concentration was used.17 Most of the approved gadolinium contrast agents are available in a concentration of 0.5 mol/l, except for gadobutrol, available in a concentration of 1 mol/l. The approved total administered dose of gadolinium for central nervous system imaging is 0.1 mmol/kg body weight. Thus, a smaller volume of gadobutrol is used when compared to gadopentetate dimeglumine for clinical imaging. This smaller injected volume of gadobutrol may be important for imaging sequences that are time sensitive, such as contrast-enhanced angiography, but is likely of less importance when imaging is delayed by a few minutes when tissue perfusion becomes more important, as in our study, for cranial nerve imaging.
Another study showed differences in enhancement of both normal gray and white matter as well as higher intensity of intracranial tumors with gadobutrol as compared to gadopentetate dimeglumine.15 Since we did not observe any significant difference in enhancement of the facial nerve between the two contrast agents, it is possible that our study is underpowered to demonstrate any true differential enhancement.
The retrospective nature of the study precludes an accurate evaluation of all subject clinical factors. We did not identify facial nerve-related symptoms in these patients on medical chart review, but it is possible that some of the perceived enhancement may be due a subclinical condition such as persistent enhancement from a clinically unapparent or undiagnosed viral, autoimmune or demyelinating condition. However, in the absence of any other imaging abnormality, and lack of facial nerve enlargement on thin-section T2 (FIESTA) images, we believe that the possibility of pathologic enhancement is negligible. Other gadolinium-based contrast agents appear to have much greater T1 relaxivities (e.g. MultiHance®) as compared to gadobutrol and gadopentetate dimeglumine, and these agents, in theory, might show a greater difference in the degree and extent of enhancement. Nevertheless, we have demonstrated the normal pattern of facial nerve enhancement with two commonly used gadolinium-based contrast agents at 3T MRI.
Conclusion
There is no significant difference in overall enhancement of different segments of the facial nerve between gadobutrol and gadopentetate dimeglumine. In the absence of symptoms, mild enhancement of the lateral canalicular portion of the facial nerve may be a normal finding. With gadobutrol, there may be lesser perceived normal enhancement of the labyrinthine and tympanic segments of the facial nerve on FSPGR sequences.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Martin-Duverneuil N, Sola-Martínez MT, Miaux Y, et al. Contrast enhancement of the facial nerve on MRI: Normal or pathological? Neuroradiology 1997; 39: 207–212. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Mends F, Hagiwara M, et al. Imaging the facial nerve: A contemporary review. Radiol Res Pract 2013; 2013: 248039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong HS, Yi BH, Cha JG, et al. Enhancement pattern of the normal facial nerve at 3.0 T temporal MRI. Br J Radiol 2010; 83: 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005; 40: 715–724. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Goerner FL, Snyder C, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol 2015; 50: 330–338. [DOI] [PubMed] [Google Scholar]
- 6.Dehkharghani S, Lubarsky M, Aiken AH, et al. Redefining normal facial nerve enhancement: Healthy subject comparison of typical enhancement patterns—unenhanced and contrast-enhanced spin-echo versus 3D inversion recovery-prepared fast spoiled gradient-echo imaging. AJR Am J Roentgenol 2014; 202: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Mafee MF, Mason T. Value of imaging in disorders of the facial nerve. Top Magn Reson Imaging 2000; 11: 38–51. [DOI] [PubMed] [Google Scholar]
- 8.Gebarski SS, Telian SA, Niparko JK. Enhancement along the normal facial nerve in the facial canal: MR imaging and anatomic correlation. Radiology 1992; 183: 391–394. [DOI] [PubMed] [Google Scholar]
- 9.Kress BP, Griesbeck F, Efinger K, et al. Bell’s palsy: What is the prognostic value of measurements of signal intensity increases with contrast enhancement on MRI? Neuroradiology 2002; 44: 428–433. [DOI] [PubMed] [Google Scholar]
- 10.Tien R, Dillon WP, Jackler RK. Contrast-enhanced MR imaging of the facial nerve in 11 patients with Bell’s palsy. AJR Am J Roentgenol 1990; 155: 573–579. [DOI] [PubMed] [Google Scholar]
- 11.Saremi F, Helmy M, Farzin S, et al. MRI of cranial nerve enhancement. AJR Am J Roentgenol 2005; 185: 1487–1497. [DOI] [PubMed] [Google Scholar]
- 12.Greco A, Parker JR, Ratcliffe CG, et al. Phase III, randomized, double-blind, cross-over comparison of gadoteridol and gadopentetate dimeglumine in magnetic resonance imaging of patients with intracranial lesions. Australas Radiol 2001; 45: 457–463. [DOI] [PubMed] [Google Scholar]
- 13.Anzalone N, Gerevini S, Scotti R, et al. Detection of cerebral metastases on magnetic resonance imaging: Intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 2009; 50: 933–940. [DOI] [PubMed] [Google Scholar]
- 14.Koenig M, Schulte-Altedorneburg G, Piontek M, et al. Intra-individual, randomised comparison of the MRI contrast agents gadobutrol versus gadoteridol in patients with primary and secondary brain tumours, evaluated in a blinded read. Eur Radiol 2013; 23: 3287–3295. [DOI] [PubMed] [Google Scholar]
- 15.Giesel FL, Mehndiratta A, Risse F, et al. Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol 2009; 50: 521–530. [DOI] [PubMed] [Google Scholar]
- 16.Rowley HA, Scialfa G, Gao PY, et al. Contrast-enhanced MR imaging of brain lesions: A large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol 2008; 29: 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths PD, Wilkinson ID, Wels T, et al. Brain MR perfusion imaging in humans. Acta Radiol 2001; 42: 555–559. [PubMed] [Google Scholar]