Abstract
Introduction
Existing stroke literature demonstrates that rapid recanalization of vessels improves long-term prognosis after acute ischemic stroke. However, further optimization of the speed of the thrombectomy procedure, used to recanalize a blocked vessel, is limited by our minimal knowledge of the clot dimensions pre-procedure. Knowing the clot dimensions would allow planning of the thrombectomy procedure with the appropriate size and length of stent retriever, and determination of the correct site of the stent deployment ensuring total coverage of the clot by the stent retriever.
Methods
We performed a feasibility study to assess if multiphase computed tomography angiography (mCTA) can be used to estimate clot length by comparing CTA imaging data with imaging data obtained from conventional digital subtraction angiography (DSA). A retrospective chart review was performed of patients with clots in the proximal middle cerebral artery and adequate collateral circulation, who underwent both mCTA and DSA.
Results
Clot length was not significantly different on 3D mCTA versus mCTA MIPs, nor was it significantly different on MIP mCTA versus DSA. Pathological evidence also supported our ability to measure clot length on mCTA.
Conclusions
We suggest that mCTA is a reliable and valid measure of clot length in acute ischemic stroke patients.
Keywords: Thrombectomy, stroke, clot length, CTA, MIP, angiography, AIS
Introduction
Acute ischemic stroke (AIS) is associated with high morbidity and mortality in the general population.1 It has been amply demonstrated that hyperacute recanalization is paramount to regain effective neurological function.2,3 However, recent literature has supported the need for further investigation on assessment of clot dimensions and collateral circulation with new imaging techniques.4,5 We performed a feasibility study to assess if multiphase computed tomography angiography (mCTA) is valuable to assess clot length in the hyperacute phase of AIS.
mCTA has recently become a promising imaging technique to better visualize AIS compared to single-phase CTA. Using time-resolved images, mCTA provides pictures of collateral vessel filling, demonstrating not only the location and size of the clot but also the gravity of the filling defect. Recently there has been increasing use of mCTA for AIS detection and characterization owing to its advantages over single-phase CTA. The interrater reliability of using mCTA for AIS detection is high, and its use for prediction of clinical outcome is superior to single-phase CTA.6 Furthermore, mCTA allows improved detection of anterior circulation AIS when compared to single-phase CTA.7 Overall, mCTA is considered a favorable tool for clot detection, and we therefore decided to evaluate the feasibility of its use for accurate clot length measurement.
Length of the clot and its location are important because the success of intravenous (IV) tissue plasminogen activator (TPA) in emergency treatment of patients with AIS is inversely proportional to the clot length. Dissolution of an 8 mm clot with IV TPA alone is almost impossible.8–10 Furthermore, knowing the clot length will allow the interventionist to immediately choose the appropriate size and length of stent retriever device to be used for thrombectomy in order to avoid delays in the procedure and to ensure complete coverage of the clot by the stent, thus enabling correct deployment and avoiding clot fragmentation. Finally, clot length is also a predictor of outcome, infarct size, and hemorrhagic transformation; therefore, the estimation of the length of the clot can be used as a prognostic factor.8
In our retrospective study we compared the length of the clot obtained on mCTA with the one obtained with conventional digital subtraction angiography (DSA) in patients referred to our institution between June 2010 and August 2015 for an acute ischemic event. The working hypothesis of our study was that mCTA is a valid and reliable method of clot length measurement when compared to DSA.
Materials and methods
Participants
We reviewed all cases of AIS processed by the Department of Neuroradiology at the Montreal Neurological Hospital who had undergone both mCTA and conventional DSA imaging, including cases from June 2012 to August 2015. A minority of our AIS patients undergo conventional DSA, therefore this included a total of 37 AIS patients, all of whom had blockages of either the middle cerebral artery (MCA) or the internal carotid artery (ICA). Pertinent patient data were recorded for each case, including epidemiological data, clinical presentation, risk factors, lab results, imaging results, details of the thrombectomy procedure and outcomes with follow-up imaging.
This retrospective study was conducted after receiving the institutional review board approval of our Institution.
Exclusion criteria
Upon initial review of these patients’ images, it was determined that those who had blockages in the ICA were not amenable to clot length measurement on mCTA or DSA, since the lack of blood flow through the ICA abolishes the normal pressure gradient of the vessel causing its collapse on imaging. The collapse of the vessel gives the appearance of a pseudo-clot extending the length of the ICA. This eliminated six of the 37 patients from the study. Furthermore, patients with clots distal to the M1 segment of the MCA did not have adequate collateral flow in order to measure clot length; these nine patients were also not amenable to measurement. Although they had proximal MCA clots, two cases completely lacked collateral circulation on mCTA, and were therefore also eliminated. This left us with 20 qualifying cases of patients with proximal MCA occlusions and adequate collateral circulation. Of those, three patients were eliminated for poor image quality. After completing the file review, 17 patients were eligible for our study.
Clot length measurements
Thrombus length was measured on two types of mCTA: thick maximum intensity projections (MIPs) and three-dimensional (3D) renderings, and on DSA. Measurements on 3D mCTA renderings and DSA imaging were made on IntelePACS® software. Measurements of MIPs were made on Vital Images VITREA® software. To measure clot length on mCTA, all 12 volumes of two-dimensional (2D) MIPs were loaded, and a volume was selected mid-way between peak arterial and peak venous phases in order to obtain maximal visualization of the most distal portion of the occluded MCA, as well as the most retrograde filling of the appropriate collateral vessel.
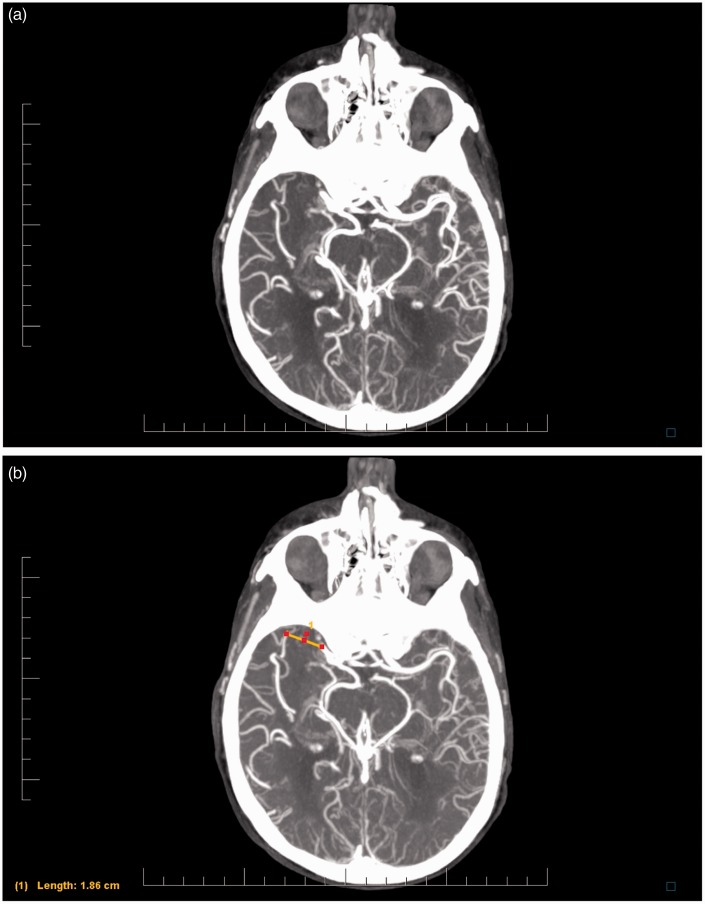
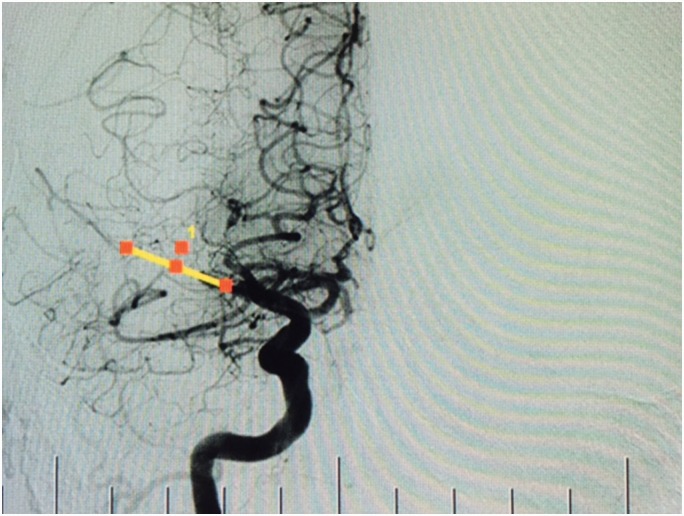
A linear measurement was then made between the most distal filling of the blocked MCA and the most proximal filling of the closest collateral vessel. This distance was used as an estimation of clot length. Figure 1(a) and (b) show MIPs with a blocked MCA before and after measurement, respectively. The same technique was used for the DSA images (Figure 2).
Figure 1.
(a) Maximum intensity projection from multiphase computed tomography angiography, demonstrating an occlusion of the right middle cerebral artery. (b) Demonstration of measurement technique.
Figure 2.
Patient with occlusion of the middle cerebral artery on digital subtraction angiography, with demonstration of measurement technique.
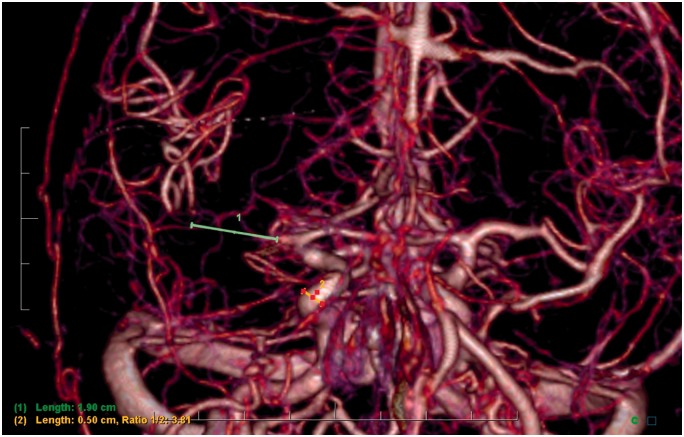
In order to measure clot length on 3D renderings of mCTA, we calibrated our measurement with the 2D MIPs by taking the width of a patent and nearby vessel, input this value on our 3D renderings, and took a linear measurement on our 3D model (Figure 3).
Figure 3.
Patient with occlusion of the middle cerebral artery on three-dimensional multiphase computed tomography angiography rendering, with demonstration of measurement technique.
Statistical analysis
Statistical analysis was performed using SPSS software. First we used a simple unpaired t test to compare the two groups of mCTA, 3D renderings, and MIPs, in order to determine the potential of mCTA for clot length measurement. Then, we used Bland-Altman plots, in which the absolute difference of the datasets and the mean measurements of the datasets are plotted on the Y and X axis, respectively. For both Bland-Altman plots, the mean of the difference is plotted as the middle Y reference line, and the mean of the difference plus or minus two standard deviations (SDs) are plotted as the top and bottom Y reference lines, respectively. We used a linear regression analysis to predict the absolute difference of the datasets based on the mean measurements of the datasets from the Bland-Altman plot: If there is no proportional bias between the two methods of measurement based on clot length (there is no tendency for one measurement technique to overestimate or underestimate the clot length), there should be a nonsignificant regression analysis, i.e. the difference between the measurement techniques should not depend on the value of the mean measurement of clot length. A p value less than 0.05 was considered statistically significant.
Results
Of the 17 eligible cases, we were able to measure clot length on 17 images from mCTA MIPs, nine from 3D mCTA, and nine from DSA (Table 1).
Table 1.
Clot length measurements in our cohort.
| Case no. | Clot length (mm) DSA | Clot length (mm) 3D (mCTA) | Clot length (mm) MIP (mCTA) |
|---|---|---|---|
| 1 | 19.3 | 19 | 18.6 |
| 2 | NA | NA | 6.4 |
| 3 | 5.4 | 5.6 | 5.8 |
| 4 | 17.4 | 13.3 | 13.4 |
| 5 | 15.1 | NA | 20.7 |
| 6 | NA | 18.9 | 10.3 |
| 7 | 15.3 | 15.2 | 13.1 |
| 8 | NA | NA | 26.5 |
| 9 | NA | NA | 9.3 |
| 10 | 12.2 | 8.4 | 9.5 |
| 11 | 16.8 | 8 | 7.1 |
| 12 | NA | 8.6 | 7.6 |
| 13 | 19 | NA | 15.2 |
| 14 | NA | NA | 11.2 |
| 15 | 22.3 | NA | 17.8 |
| 16 | NA | NA | 15.8 |
| 17 | NA | 15.1 | 20.3 |
MIP: maximum intensity projection; DSA: digital subtraction angiography; 3D: three-dimensional; mCTA: multiphase computed tomography angiography; NA: not assessable.
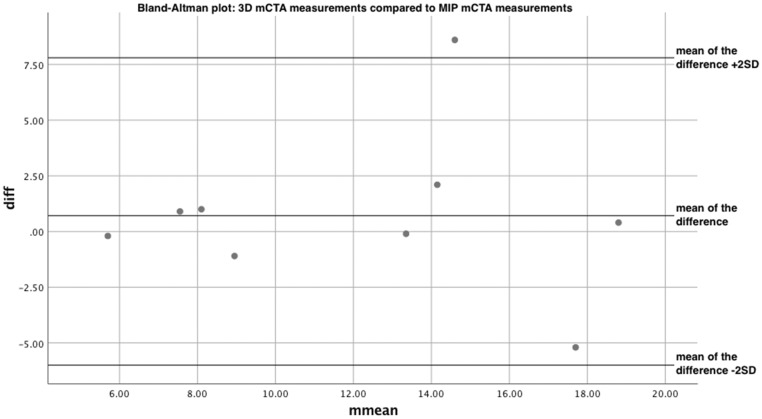
We first compared the two groups of mCTA, 3D renderings and MIPs, in order to evaluate the reliability of mCTA for clot length measurement. A simple t test showed no statistical difference between the two datasets, with t(8) = 0.591, p = 0.571. The Bland-Altman is shown in Figure 4. The mean of the difference is equal to 0.711 and the SD of the difference is equal to 3.61. The plot showed good agreement between the two methods of measurement. A linear regression between the difference of the measurement values and the mean of the measurement values showed no proportional bias between the datasets. The nonsignificant regression equation was found to be (F(1,7)=0.00600, p = 0.938), with an R squared of –0.142.
Figure 4.
Bland-Altman plot: three-dimensional multiphase computed tomography angiography (mCTA) measurements compared to maximum intensity projection mCTA measurements.
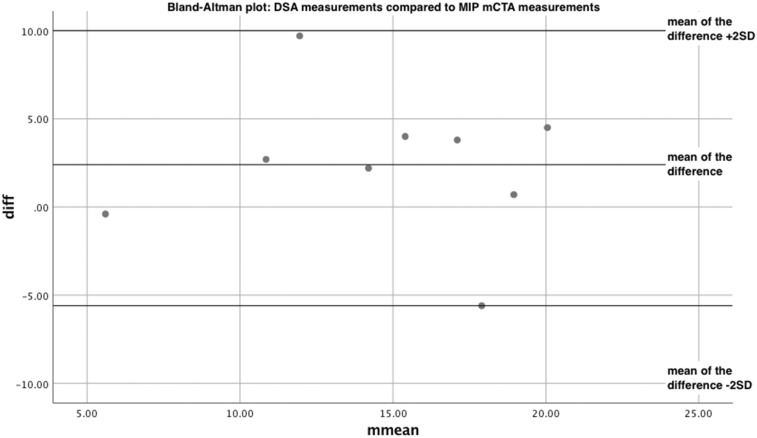
We then compared the MIP mCTA values with our gold standard, DSA, allowing us to evaluate the validity of mCTA. A simple t test showed no statistical difference between the two datasets, with t(8)=1.74, p = 0.120. The Bland-Altman is shown in Figure 5. The mean of the difference is equal to 2.40 and the SD of the difference is equal to 4.14. Again, the plot showed good agreement between the two methods of measurement. A linear regression between the difference of the measurement values and the mean of the measurement values showed no proportional bias between the datasets. The nonsignificant regression equation was found to be (F(1,7) = 0.0500, p = 0.830), with an R squared of 0.00700.
Figure 5.
Bland-Altman plot: digital subtraction angiography measurements compared to maximum intensity projection multiphase computed tomography angiography measurements.
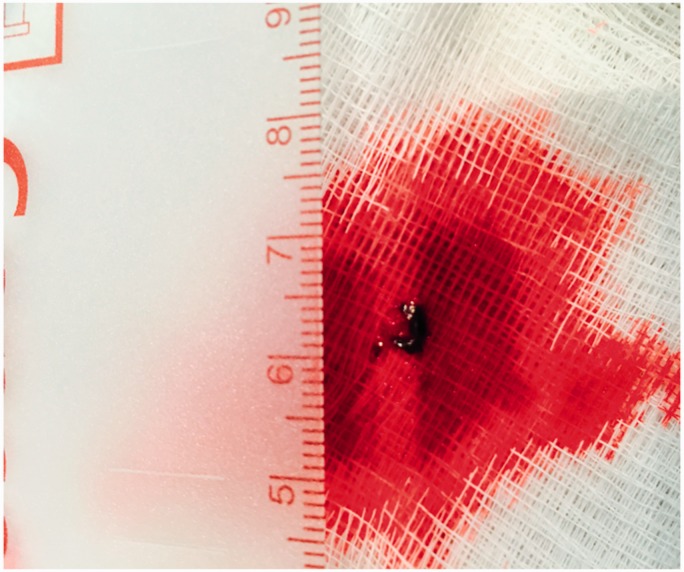
Finally, we used one pathological case post-thrombectomy for practical comparison (Figure 6). The real clot measurement was 6 mm, while it measured 7.1 mm on MIP, 8.0 mm on 3D mCTA, and 16.8 mm on DSA (Table 1, Case no. 11).
Figure 6.
Clot removed from the middle cerebral artery during thrombectomy procedure. Pathological measurement of the clot post-thrombectomy was used for comparison to measurements taken on imaging.
Discussion
The purpose of our study was to evaluate the feasibility of using mCTA to measure clot length in AIS patients. Based on our inclusion and exclusion criteria, a select population of patients became eligible for our study, which represents the actual patient population who would benefit from this technique: patients with proximal MCA clots and adequate collateral circulation.
According to our statistical analysis, there was good agreement between both sets of data.11 This suggests that mCTA appears to be comparable to DSA in terms of validity of clot length measurement, and also appears to be a reliable measure since values made on 3D renderings and MIPs from mCTA are comparable. However, the number of patients who could be evaluated for this study was limited, which restricted the power of our study. Moreover, it has been shown that the measurement of thrombus clot length is dependent on collateral flow.12 In our study, the measurement of clot length both on mCTA and DSA is limited to patients with sufficient collateral flow; as such a number of our patients had missing values due to insufficient collaterals to make a measurement. However, Christoforidis et al. showed that approximately 50% of acute stroke patients have good collateral flow on imaging, and therefore, while not all AIS patients will benefit from this measurement of clot length preoperatively, half of them might.13
Another limitation is the extensive exclusion criteria: Clots in the ICA or distal to the M1 segment of the MCA were not amenable to measurement due to over-estimation of clot length and inability to measure collaterals, respectively. Our ability to measure clot length is limited to AIS in the MCA. Regardless of this limitation, the MCA is the most commonly affected vessel in AIS.14 Image quality was also a limiting factor, as images that were taken without sufficient contrast or images that were blurred because of patient movement could not be evaluated. Finally, taking a linear measurement on a 2D image of a blood vessel is an approximation, making allowance for the potential curvature and the depth of the vessel.
Despite these limitations, measuring clot length on mCTA MIPs was feasible for every patient who met the exclusion criteria, whereas measuring on 3D reconstructions of mCTA was more difficult. Compared to the gold standard, measuring clot length on mCTA MIPs was subjectively easier in most cases than conventional DSA, and impossible for some cases on DSA.
Finally, pathological evidence seems to support the accuracy of mCTA MIPs for measuring clot length (Figure 6). This is further evidence that MIPs can be used to estimate clot length accurately, and it also suggests that it may be better than DSA in certain cases. Given these observations, our hypothesis that mCTA can be used to plan stent placement preoperatively appears feasible.
Missing data points from DSA measurements were due to poor image quality from patient movement, or an inability to reliably visualize the collateral circulation. Similarly, patient movement interfered with 3D mCTA reconstructions. Moreover, 3D mCTA reconstructions were highly sensitive to the adequacy and size of collateral circulation compared to mCTA, since the quality of the reconstruction depends on the quantity of contrast in the vessels and anatomically small collateral vessels had a very tiny amount of contrast within them. These factors accounted for missing data points on 3D mCTA measurements. The above reasoning also accounts for some discrepancy between measurement values using different techniques. For instance, when comparing the 3D mCTA measurements versus MIP measurements: Since tiny collaterals may not appear in the 3D reconstructions, 3D mCTA tends to overestimate the clot length more often than mCTA MIPs.
Recent stroke research has focused on using non-contrast CT (NCCT) to measure clot length, as demonstrated by Riedel et al.15,16 However, the authors note that the hyperdense middle cerebral artery sign (HMCAS) used to measure clot length on NCCT can also appear due to vessel calcification or high hematocrit levels. The sign is also dependent on the timing of the scan since the hemolytic process of the clot determines its hyperdense appearance. We suggest that the HMCAS is not a sensitive enough measure to use in pre-operative thrombectomy planning, as supported by the literature.17,18 While the HMCAS may be a sensitive enough predictor of the efficacy of IV TPA treatment, it is not necessarily sensitive enough to indicate the full length of the clot in the vessel as needed for reliable stent placement. Since our clot length measurement uses collaterals to evaluate the full extent of the blockage in the vessel, it is a more sensitive predictor of clot length to a more exact measure. Case 1 provided in Table 1 supports this notion; only 15 mm of the clot was visible on NCCT, while it measured more than 18 mm on mCTA and more than 19 mm on DSA. This subtle difference is crucial when planning precise stent placement.
In summary, we believe that mCTA may be a useful tool for the interventionist to estimate clot length before thrombectomy. Measuring clot length on mCTA was possible in every eligible patient, and our statistical analysis supports this idea. The benefits of knowing the clot length are numerous;18 however, a significant benefit is to improve the planning and execution of the thrombectomy procedure. Firstly, knowing the clot length can allow the treating physician to predict the potential effectiveness of IV TPA therapy. Secondly, for those patients who undergo thrombectomy, stent size can be selected based on the clot length measurements obtained from mCTA. This will allow the appropriate personnel to equip the angiography suite with a correctly sized stent retriever before the procedure begins. Finally, once the thrombectomy procedure is underway, stent deployment can be based on the clot length measurement, increasing the odds that the entire clot is retrieved and removed by the stent retriever.
From a practical standpoint, the inclusion of mCTA clot length measurement in stroke protocol will not compromise the rapid care that is vital for salvaging brain tissue. Patients being screened for stroke are already set up in the CT machine for baseline plain CT, therefore multiphase CTA images can be rapidly obtained at this time. Thereafter, the length of the clot can be evaluated while the patient is being prepared for thrombectomy, since post-acquisition processing and evaluation of the images does not take longer than five minutes.
For these reasons, we believe that measuring clot length via mCTA in all eligible acute stroke patients will improve the efficiency and accuracy of the thrombectomy procedure, ideally improving patient outcomes.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: A scientific statement from the American Heart Association. Stroke 2009; 40: 3646–3678. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Kaschka IN, Kloska SP, Struffert T, et al. Clinical and radiological outcome after mechanical thrombectomy in acute ischemic stroke: What matters? Neuroradiol J 2016; 29: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke 2017; 12: 606–614. [DOI] [PubMed] [Google Scholar]
- 5.Lin MP, Tsivgoulis G, Alexandrov AV, et al. Factors affecting clinical outcome in large-vessel occlusive ischemic strokes. Int J Stroke 2015; 10: 479–484. [DOI] [PubMed] [Google Scholar]
- 6.Menon BK, d’Esterre CD, Qazi EM, et al. Multiphase CT angiography: A new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015; 275: 510–520. [DOI] [PubMed] [Google Scholar]
- 7.Yu AY, Zerna C, Assis Z, et al. Multiphase CT angiography increases detection of anterior circulation intracranial occlusion. Neurology 2016; 87: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int J Stroke 2008; 3: 230–236. [DOI] [PubMed] [Google Scholar]
- 9.Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011; 42: 1775–1777. [DOI] [PubMed] [Google Scholar]
- 10.Meyne JK, Zimmermann PR, Rohr A, et al. Thrombectomy vs. systemic thrombolysis in acute embolic stroke with high clot burden: A retrospective analysis. Rofo 2015; 187: 555–560. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 12.Qazi EM, Sohn SI, Mishra S, et al. Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci 2015; 42: 381–388. [DOI] [PubMed] [Google Scholar]
- 13.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. Am J Neuroradiol 2005; 26: 1789–1797. [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen TS, Skriver EB, Herning M. Cause of cerebral infarction in the carotid territory. Its relation to the size and the location of the infarct and to the underlying vascular lesion. Stroke 1985; 16: 459–466. [DOI] [PubMed] [Google Scholar]
- 15.Riedel CH, Jensen U, Rohr A, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced computed tomography reconstructions. Stroke 2010; 41: 1659–1664. [DOI] [PubMed] [Google Scholar]
- 16.Miller TS, Brook AL, Riedel CH, et al. Expanding the role of NCCT in acute stroke imaging: Thrombus length measurement and its potential impact on current practice. J Neurointerv Surg 2014; 6: 5–6. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Park JE, Nahrendorf M, et al. Direct thrombus imaging in stroke. J Stroke 2016; 18: 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparian GG, Sanossian N, Shiroishi MS, et al. Imaging of occlusive thrombi in acute ischemic stroke. Int J Stroke 2015; 10: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]