Abstract
Objective
The aim of this study was to investigate the incidence, risk factors, and treatment of elevated intraocular pressure (IOP) 1 year after vitrectomy in eyes without a history of glaucoma or ocular hypertension.
Patients and methods
This retrospective study comprised 256 eyes from 256 consecutive patients without a history of glaucoma or ocular hypertension who underwent vitrectomy and were followed up for 1 year. The incidence of elevated IOP at 1 year after vitrectomy was calculated. We compared the characteristics of patients with or without elevated IOP to identify possible risk factors for elevated IOP. The treatments used to control IOP were recorded and analyzed.
Results
A total of 50 patients (19.5%) had elevated IOP after vitrectomy at the 1-year follow-up. Tamponade was a significant risk factor for elevated IOP (P<0.05). The cumulative rates of elevated IOP in eyes with air, balanced salt solution, sulfur hexafluoride, perfluoropropane (C3F8), and silicone oil as the tamponade were 0, 10.8%, 5.9%, 19.8%, and 28.4%, respectively (P<0.05). About 68% of cases of elevated IOP occurred within 1 month after vitrectomy. At 1 year after vitrectomy, 29 patients (58.0%) had stopped their IOP-lowering drugs and 21 (42.0%) patients were continuing these drugs. About 65% of ocular hypertension patients who received silicone oil tamponade had not stopped IOP-lowering drugs; this rate was significantly greater than that of ocular hypertension patients who received C3F8 tamponade (18.2%, P<0.05).
Conclusion
Elevated IOP is a common complication after vitrectomy. Silicone oil tamponade was associated with greater risk of elevated IOP and had long-term effects on IOP. Drugs and surgery were used to control IOP, and some patients required long-term IOP-lowering therapy.
Keywords: ocular hypertension, vitrectomy, silicone oil, glaucoma
Introduction
Pars plana vitrectomy (PPV) is the most frequently used surgical procedure to treat a variety of retinal disorders. Ocular hypertension is a common complication after PPV.1–7 Risk factors for ocular hypertension after PPV include a history of glaucoma,8,9 history of diabetes mellitus,8 scleral buckling procedures,10–12 lensectomy,13–15 the use of silicone oil12,16,17 or expanding gas,12,17 and others.6,18 The etiology of ocular hypertension following PPV is complicated, and open-angle and closed-angle mechanisms may be causative factors.
Several studies have investigated that the incidence of ocular hypertension in vitrectomized eyes (including all tamponade methods) varies from 18% to 28%,1–4,6 and the incidence of elevated intraocular pressure (IOP) after vitrectomy with silicone oil ranges from 20% to 56%.5,7 Owing to the development of newer equipment and techniques for PPV and lensectomy, as well as changes to the indications for PPV, it is important to examine the incidence of ocular hypertension after PPV and identify possible risk factors. In addition, little is known about the long-term prognosis of elevated IOP following PPV. Therefore, the objectives of this study were to analyze the incidence of and risk factors for ocular hypertension after PPV in eyes without a previous history of glaucoma or ocular hypertension and to assess the efficacy of pharmacological and surgical management of ocular hypertension following PPV.
Patients and methods
This retrospective study comprised 272 consecutive eyes from 272 patients who underwent PPV surgery at the Department of Ophthalmology and Visual Science, Eye and ENT Hospital of Fudan University, in November 2011 and were followed up for 1 year. This study was approved by the institutional review board/ethics committee of the Eye and ENT Hospital of Fudan University and adhered to the ethical standards of the Declaration of Helsinki. This study was explained to each patient, and each patient provided written informed consent for this study.
PPV was performed using a standard 20 G three-port system in all patients. Filtered air, balanced salt solution (BSS), 20% sulfur hexafluoride (SF6), 14% perfluoropropane (C3F8), or silicone oil were used as tamponades during vitrectomy. PPV was performed together with lensectomy and scleral bucking in some patients. The patients’ demographic, preoperative, intraoperative, and postoperative data were recorded. The preoperative and postoperative data included the best-corrected visual acuity, cup-to-disk ratio, IOP, fundus examination, and ocular medications. The data were recorded at the preoperative visit and postoperatively after PPV at 1 day, 1 week, 1 month after PPV, and monthly thereafter for 1 year. Intraoperative data included the indications for PPV, the tamponade fill during PPV, and the combination of lens surgery or scleral buckling. Ocular hypertension was defined as IOP ≥30 mmHg within 24 h after surgery,12,19 or IOP ≥25 mmHg any time between postoperative day 2 and 6 weeks after surgery, or IOP ≥22 mmHg more than 6 weeks after surgery.20 Pharmacotherapy was the primary mode of managing ocular hypertension. Surgery was considered if IOP could not be controlled with maximum tolerable pharmacotherapy. Surgical interventions included Ahmed glaucoma valve implantation, silicone oil removal, and lensectomy.
Data analysis was conducted using SPSS Software (version 18.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics including mean and SD were calculated for patient characteristics. Pearson’s χ2 test was used to compare categorical variables, and Student’s t-test was used to compare continuous variables. Kaplan–Meier survival analysis was used to determine the cumulative rates of ocular hypertension in each tamponade group, and the rates were compared using the Mantel–Cox log-rank test. P-values of <0.05 were regarded as statistically significant.
Results
A total of 272 eyes from 272 consecutive patients who underwent PPV were followed up for 1 year. Of these, 16 (5.9%) patients who had a history of glaucoma or ocular hypertension were excluded from the analyses. Therefore, 256 eyes from 256 patients were included in the analyses. A total of 50 patients (19.5%) were diagnosed with ocular hypertension on at least one visit. Patients were divided into two groups based on the presence or absence of ocular hypertension.
The demographic characteristics and preoperative data of both groups are summarized in Table 1. Of 50 patients with ocular hypertension, 31 were male (62.0%) and 19 were female (38.0%). Diabetes mellitus was present in 10 patients (20.0%), and systemic hypertension was present in six patients (12.0%). There were 206 patients without ocular hypertension, of whom 125 were male (60.7%) and 81 were female (39.3%). Diabetes mellitus was present in 39 patients (18.9%), and systemic hypertension was present in 43 patients (20.9%). The mean age was 46.3±13.3 years and 50.8±16.6 years in patients with elevated IOP and normal IOP, respectively. The refractive error was −3.6±5.0 D and −3.0±5.6 D in patients with elevated IOP and normal IOP, respectively. The patient demographic characteristics, including age, gender, history of systemic diseases, and refractive degree were not significantly different between the two groups of patients. The mean preoperative IOP was 12.9±4.2 and 12.6±4.1 mmHg in patients with elevated IOP and normal IOP, respectively. The preoperative IOP was not significantly different between the two groups (P=0.980, independent t-test).
Table 1.
Demographics of the study population
| Patients without elevated IOP
|
Patients with elevated IOP
|
P-value | |||
|---|---|---|---|---|---|
| N=206 | % | N=50 | % | ||
| Age (years) | 0.192* | ||||
| Mean ± SD | 50.8±16.6 | 46.3±13.3 | |||
| Range | 5–80 | 9–67 | |||
| Preoperative IOP (mm Hg) | 0.980* | ||||
| Mean ± SD | 12.6±4.1 | 12.9±4.2 | |||
| Range | 5–20 | 3–21 | |||
| Diopter (D) | 0.971* | ||||
| Mean ± SD | −3.0±5.6 | −3.6±5.0 | |||
| Range | −27 to 2 | −14 to 2 | |||
| Gender | 0.864† | ||||
| Male | 125 | 60.68% | 31 | 62.00% | |
| Female | 81 | 39.32% | 19 | 38.00% | |
| Medical history | |||||
| DM | 39 | 18.93% | 10 | 20.00% | 0.863† |
| HP | 43 | 20.87% | 6 | 12.00% | 0.153† |
Notes:
P-value by independent t-test.
P-value by Pearson’s χ2 test.
Abbreviations: DM, diabetes mellitus; HP, hypertension; IOP, intraocular pressure.
The intraoperative characteristics of patients are summarized in Table 2. In patients with elevated IOP, the underlying ocular diseases necessitating PPV included macular diseases (2.0%), rhegmatogenous retinal detachment (48.0%), proliferative diabetic retinopathy (16.0%), trauma-related vitreous retinal diseases (22.0%), vitreous hemorrhage of nondiabetic etiology (4.0%), and other diseases (8.0%). The types of ocular disease were not significantly different between the two groups (P=0.181, Pearson’s χ2 test). However, the incidence of elevated IOP was 3.7% (one of 27 patients) in patients with macular diseases versus 21.4% (49 of 229 patients) in patients with non-macular diseases (including other diseases), and this difference was statistically significant (P=0.028, Pearson’s χ2 test). Tamponade was provided by BSS, 20% SF6, 14% C3F8, and silicone oil in 8.0%, 2.0%, 44.0%, and 46.0% of patients with elevated IOP, respectively. Tamponade was provided by filtered air, BSS, 20% SF6, 14% C3F8, and silicone oil in 4.9%, 16.0%, 7.8%, 43.2%, and 28.2% of patients with normal IOP, respectively. The type of tamponade was significantly different between the two groups (P=0.036, Pearson’s χ2 test).
Table 2.
Intraoperative characteristics of patients with and without elevated IOP
| Patients without elevated IOP
|
Patients with elevated IOP
|
P-value | |||
|---|---|---|---|---|---|
| N=206 | % | N=50 | % | ||
| Diagnosis | 0.181* | ||||
| Macular diseases | 26 | 12.62 | 1 | 2.00 | |
| Vitreous hemorrhage | 19 | 9.22 | 2 | 4.00 | |
| Retinal detachment | 91 | 44.17 | 24 | 48.00 | |
| Trauma related | 40 | 19.42 | 11 | 22.00 | |
| PDR | 24 | 11.65 | 8 | 16.00 | |
| Others | 6 | 2.91 | 4 | 8.00 | |
| Tamponade | 0.036* | ||||
| Air | 10 | 4.85 | 0 | 0.00 | |
| BSS | 33 | 16.02 | 4 | 8.00 | |
| SF6 | 16 | 7.77 | 1 | 2.00 | |
| C3F8 | 89 | 43.20 | 22 | 44.00 | |
| Silicone oil | 58 | 28.16 | 23 | 46.00 | |
| Combined lens surgery | 0.336* | ||||
| Without lens surgery | 133 | 64.56 | 35 | 70.00 | |
| Lensectomy | 43 | 20.87 | 12 | 24.00 | |
| Lensectomy + IOL | 27 | 13.11 | 2 | 4.00 | |
| IOL removal | 3 | 1.46 | 1 | 2.00 | |
| Combined scleral buckling | 0.541* | ||||
| Without SB | 191 | 92.72 | 48 | 96.00 | |
| With SB | 15 | 7.28 | 2 | 4.00 | |
Note:
P-value by Pearson’s χ2 test.
Abbreviations: SF6, sulfur hexafluoride; C3F8, perfluoropropane; BSS, balanced salt solution; IOL, intraocular lens; IOP, intraocular pressure; PDR, proliferative diabetic retinitis; SB, scleral buckling.
PPV was performed together with lens surgery in some patients. Among 50 patients with elevated IOP, 35 (70.0%) underwent PPV without lens surgery, 12 (24.0%) underwent PPV with lensectomy, two (4.0%) underwent PPV with lensectomy and intraocular lens (IOL) implantation, and one (2.0%) underwent PPV with IOL removal. Among 206 patients with normal IOP, 133 (64.6%) underwent PPV without lens surgery, 43 (20.9%) underwent PPV with lensectomy, 27 (13.1%) underwent PPV with lensectomy and IOL implantation, and three (1.5%) underwent PPV with IOL removal. The distribution of surgical procedures was not significantly different between the two groups (P=0.336, Pearson’s χ2 test).
PPV was performed in combination with scleral buckling in 2/50 (4.0%) patients with elevated IOP compared with 15/206 (7.3%) patients with normal IOP. The proportion of patients who underwent PPV together with scleral buckling was not significantly different between the two groups (P=0.541, Pearson’s χ2 test).
After analyzing the risk factors for elevated IOP based on the baseline and surgical procedures as listed in Tables 1 and 2, only PPV tamponade type was a significant risk factor for elevated IOP. We compared the total proportion of eyes with elevated IOP in the different tamponade groups as shown in Figure 1. The number of patients in the air group, the BSS group, and the SF6 group was small; therefore, we combined these three groups with the others group in this comparison. As shown in Figure 1, the total proportion of eyes with elevated IOP in the silicone oil group, the C3F8 group, and the others group was 28.4%, 19.8%, and 7.8%, respectively. The difference between the silicone oil group and the others group was significant (P=0.002, Pearson’s χ2 test). There was also a significant difference between the C3F8 group and the others group (P=0.034, Pearson’s χ2 test). There was no significant difference between the C3F8 group and the silicone oil group (P=0.166, Pearson’s χ2 test). To analyze the association between tamponade and elevated IOP over time, the onset and duration of elevated IOP according to the type of tamponade was examined, and the results are presented in Table 3 and Figure 2. As summarized in Table 3, the elevated IOP was detected at 1 week after surgery in 10 (20.0%) patients, at 1 month in 34 (68.0%) patients, at 3 months in 41 (82.0%) patients, at 4–6 months in two (4.0%) patients, at 7–9 months in one (2.0%) patient, and at 10–12 months in six (12.0%) patients. Therefore, most of the patients experienced elevated IOP within 3 months after PPV. Fewer new cases of elevated IOP occurred at 4–9 months during the first year after PPV, and more new cases occurred at 10–12 months. In patients whose tamponade was provided by BSS or SF6, there were no new cases of elevated IOP beyond 3 months after surgery. By contrast, some patients whose tamponade was provided by C3F8 or silicone oil experienced elevated IOP more than 3 months after surgery. We used Kaplan–Meier analysis to compare the cumulative rates of elevated IOP according to the type of tamponade. As shown in Figure 2, the cumulative rates of elevated IOP at 12 months after PPV were 28.4%, 19.8%, 10.8%, 5.9%, and 0% for silicone oil, C3F8, BSS, SF6, and air, respectively. The cumulative rate of elevated IOP was significantly different between these types of tamponade (P=0.008, Mantel–Cox log-rank test) indicating that the silicone oil tamponade was associated with the greatest risk of elevated IOP, followed by C3F8, SF6, BSS, and air. Therefore, silicone oil and C3F8 tamponades have a greater risk for elevated IOP compared with SF6, BSS, and air tamponades.
Figure 1.
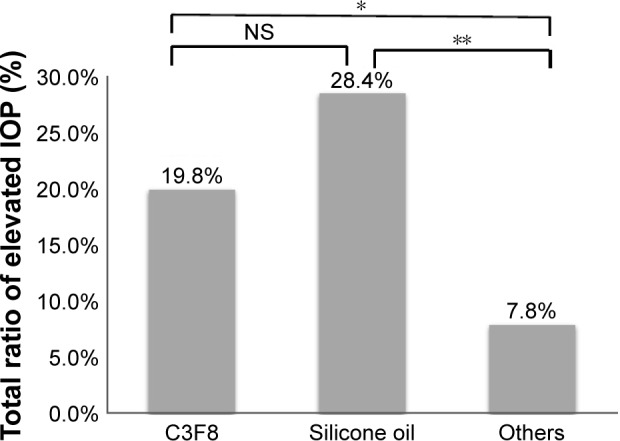
Comparison of the total proportion of eyes with elevated IOP among the different tamponade groups.
Notes: The proportion of eyes with elevated IOP was significantly higher in the silicone oil group than in the others group (combined air, BSS, and SF6 groups; P=0.002, Pearson’s χ2 test). The proportion of eyes with elevated IOP was significantly higher in the C3F8 group than in the others group (P=0.034, Pearson’s χ2 test). There was no significant difference in the proportion of eyes with elevated IOP between the silicone oil group and the C3F8 group (P=0.166, Pearson’s χ2 test). *Significant; **highly significant.
Abbreviations: SF6, sulfur hexafluoride; C3F8, perfluoropropane; BSS, balanced salt solution; IOP, intraocular pressure; NS, not significant.
Table 3.
Number of eyes with elevated IOP according to tampon-ade type and time of onset
| BSS | Air | SF6 | C3F8 | Oil | Total | Cumulative% | |
|---|---|---|---|---|---|---|---|
| 0–1 w | 1 | 0 | 1 | 4 | 4 | 10 | 20.00% |
| 1 w–1 m | 2 | 0 | 0 | 12 | 10 | 24 | 68.00% |
| 1 m–3 m | 1 | 0 | 0 | 3 | 3 | 7 | 82.00% |
| 4 m–6 m | 0 | 0 | 0 | 0 | 2 | 2 | 86.00% |
| 7 m–9 m | 0 | 0 | 0 | 0 | 1 | 1 | 88.00% |
| 10 m–12 m | 0 | 0 | 0 | 3 | 3 | 6 | 100.00% |
| Total | 4 | 0 | 1 | 22 | 23 | 50 |
Notes: Air, filtered air; oil, silicone oil.
Abbreviations: SF6, sulfur hexafluoride; C3F8, perfluoropropane; BSS, balanced salt solution; IOP, intraocular pressure; m, month; w, week.
Figure 2.
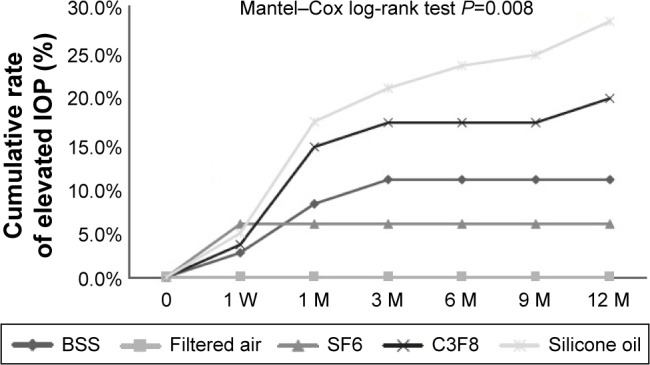
Survival analysis of elevated IOP according to the type of tamponade used in PPV.
Note: The cumulative rate of elevated IOP was significantly different among the five types of tamponade (P=0.008, log-rank test) and was greatest for silicone oil tamponade.
Abbreviations: SF6, sulfur hexafluoride; C3F8, perfluoropropane; BSS, balanced salt solution; IOP, intraocular pressure; M, month; PPV, pars plana vitrectomy; W, week.
Pharmacotherapy was the primary mode of treating elevated IOP. As indicated in Table 4, only topical and systemic IOP-lowering drugs were prescribed in 31/50 (62.0%) patients. These drugs were prescribed for temporary use in 21/50 (42.0%) patients, and for persistent use in 10/50 (20.0%) patients. Surgical interventions to control IOP, including Ahmed glaucoma valve implantation, lensectomy, and silicone oil removal, were used in 15/50 (30.0%) patients. These procedures were successful (ie, achieved a normal IOP without IOP-lowering drugs) in five eyes (10.0%) and were associated with qualified success (ie, achieved normal IOP with IOP-lowering drugs) in 10 eyes (20.0%). In 4/50 (8.0%) eyes with elevated IOP, the IOP-lowering drugs were stopped because of near-total loss of vision; three of these eyes received C3F8 tamponade and one received silicone oil tamponade.
Table 4.
Treatment outcomes of IOP elevation according to tamponade type
| Drugs alone
|
Surgery
|
Drop treatment | |||
|---|---|---|---|---|---|
| Temporary | Persistent | Complete success | Qualified success | ||
| BSS | 2 (50.00%) | 2 (50.00%) | 0 | 0 | 0 |
| SF6 | 1 (100%) | 0 | 0 | 0 | 0 |
| C3F8 | 15 (68.18%) | 3 (13.63%) | 1 (4.55%) | 0 | 3 (13.63%) |
| Oil | 3 (13.04%) | 5 (21.74%) | 4 (17.39%) | 10 (43.48%) | 1 (4.35%) |
| Total | 21 (42.00%) | 10 (20.00%) | 5 (10.00%) | 10 (20.00%) | 4 (8.00%) |
Notes: Results are presented as N (%). Oil, silicone oil.
Abbreviations: SF6, sulfur hexafluoride; C3F8, perfluoropropane; BSS, balanced salt solution; IOP, intraocular pressure.
The outcomes of treating elevated IOP according to the type of tamponade are presented in Table 4. IOP-lowering drugs were prescribed for temporary use in 100.0%, 50.0%, 68.2%, and 13.0% of patients who received SF6, BSS, C3F8, and silicone oil tamponades, respectively. The proportion of patients on long-term IOP-lowering drug use (including patients with persistent use of IOP-lowering drugs and patients with qualified success) was significantly different between the types of tamponade (P=0.004, Pearson’s χ2 test). These results suggest that the duration of IOP-lowering drug use differs between different types of tamponade. Silicone oil typically causes persistent damage to the aqueous outflow pathways and most patients who developed ocular hypertension after silicone oil tamponade required long-term administration of IOP-lowering drugs. By contrast, although C3F8 is associated with damage to the aqueous outflow pathways, these pathways recover over time necessitating short-term administration of IOP-lowering drugs.
Three patients (6.0%) with elevated IOP underwent Ahmed valve implantation, which kept IOP within the normal range. These three patients received silicone oil tamponade. One patient with C3F8 tamponade underwent lensectomy, which was successful. Among 23 patients with elevated IOP after silicone oil tamponade, 12 underwent surgery to remove silicone oil and reduce IOP within 1 year after PPV. As summarized in Table 5, after silicone oil removal, one patient achieved normal IOP without IOP-lowering drugs, 10 patients achieved normal IOP with IOP-lowering drugs, and one patient could not achieve normal IOP with IOP-lowering drugs. This patient underwent Ahmed valve implantation, which resulted in normal IOP.
Table 5.
Outcomes of surgical procedures for the control of IOP
| Complete success
|
Qualified success
|
Failure
|
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Valve implantation | 3 | 100 | 0 | 0 | 0 | 0 |
| Silicone oil removal | 1 | 8.33 | 10 | 83.33 | 1 | 8.33 |
| Lensectomy | 1 | 100 | 0 | 0 | 0 | 0 |
Abbreviation: IOP, intraocular pressure.
Discussion
IOP elevation is one of the most common and severe complications of PPV. Elevated IOP was reported to affect 20%–60% of eyes after PPV.1–5 Various definitions of elevated IOP have been used in previous studies. In our study, ocular hypertension was defined as IOP ≥30 mmHg at 24 h after surgery,12,19 IOP ≥25 mmHg between postoperative day 2 and 6 weeks after surgery, or IOP ≥22 mmHg beyond 6 weeks after surgery and required treatment.20
Many risk factors for elevated IOP after PPV were reported in previous studies. In particular, preexisting glaucoma is a very important risk factor for elevated IOP after PPV.8,9 Phelps and Burton19 reported that among 817 patients, glaucoma preceded retinal detachment historically or based on clinical evidence in nearly 7% of patients. In our original cohort of 272 patients who underwent PPV, 5.9% had a history of glaucoma or ocular hypertension, which is similar to the value in their report. In fact, some patients with preexisting glaucoma were misdiagnosed before PPV. Therefore, the actual prevalence of glaucoma among patients undergoing PPV might exceed 5.9%. To detect risk factors for elevated IOP, other than the history of glaucoma or ocular hypertension, we excluded patients with a history of glaucoma or ocular hypertension from our study.
In our study, the cumulative rate of ocular hypertension after PPV in patients without a history of glaucoma or ocular hypertension was 19.5%, which is similar to the values reported elsewhere.17,21 Ocular hypertension was detected within 1 month after PPV in 68.0% of patients with elevated IOP, consistent with another study.22
Postoperative hemorrhage, inflammation, and pupillary block might contribute to early-onset ocular hypertension. Late-onset ocular hypertension may be caused by anterior synechiae, rubeosis iridis, migration of silicone oil into the anterior chamber, and long-term steroid use.23–25 In previous studies, the risk factors for ocular hypertension after PPV included the history of glaucoma,8 history of diabetes mellitus,8,12 the use of silicone oil and expanding gas tamponade,12,16,17 PPV combined with lensectomy,13–15 and scleral buckling.10–12 In our study, we excluded patients with a history of glaucoma to detect other risk factors and found that only tamponade was a significant risk factor for elevated IOP after PPV. The cumulative rates of ocular hypertension at 1 year after PPV were 28.4%, 19.8%, 10.8%, 5.9%, and 0% for silicone oil, C3F8, BSS, SF6, and air, respectively. In patients who received silicone oil tamponade, the incidence of ocular hypertension continued to increase during the 1-year follow-up. By contrast, in patients who received SF6 or BSS tamponade, there were no new cases of ocular hypertension more than 3 months after PPV. The mechanism of IOP elevation varied among the different tamponade types. Preexisting glaucoma, steroid-induced ocular hypertension, and postoperative inflammation were common mechanisms in the C3F8 and silicone oil groups. Furthermore, the migration of emulsified silicone oil into the anterior chamber might block or infiltrate the trabecular meshwork to cause angle-closure glaucoma or open-angle glaucoma with trabeculitis.24,26
The duration of ocular hypertension also differed among the tamponade types. Of note, most cases of ocular hypertension were persistent in patients who received silicone oil tamponade and most of these patients continued to use IOP-lowering drugs until the last visit at 1 year after surgery, whereas most cases of ocular hypertension were temporary in patients who received C3F8 tamponade. These results indicate that the type of tamponade is a significant determinant of the incidence and duration of ocular hypertension after PPV.
There are conflicting reports on whether lensectomy is a risk factor for ocular hypertension.13,14,27–29 In one long-term study of patients with late-onset open-angle hypertension or glaucoma after PPV, the incidence of late-onset open-angle hypertension was 15% in nonphakic eyes versus 1.4% in phakic eyes (P<0.05).13 In another study, the incidence of late-onset open-angle glaucoma was 2% in phakic eyes versus 13% in pseudophakic eyes (P<0.05).14 The results of these studies suggest that the presence of a crystalline lens reduces the risk of open-angle glaucoma, and that lensectomy is a risk factor for late-onset open-angle glaucoma. However, in another study of late-onset open-angle glaucoma or ocular hypertension after simple PPV with or without lens surgery, lens surgery was not a risk factor for late-onset open-angle glaucoma.29 Moreover, another study that examined the incidence of elevated IOP at 48 h after simple PPV combined with or without lensectomy and IOL implantation suggested that lensectomy is a risk factor for postoperative short-term ocular hypertension.15 However, in the current study, the incidence of elevated IOP was not significantly different between patients who did or did not undergo lens surgery. We speculate that, although lensectomy in combination with PPV might increase postoperative inflammation and the risk of open-angle ocular hypertension, it could decrease the risk of pupil block, which leads to closed-angle ocular hypertension especially when expanding gas or silicone oil is used as the tamponade in PPV. This might explain why lensectomy was not a significant risk factor for ocular hypertension after PPV in our study.
Once ocular hypertension was detected, the patient was treated pharmacologically or surgically to lower IOP. Surgical procedures to lower IOP in this study included silicone oil removal, Ahmed valve implantation, and lensectomy. Overall, 14/23 eyes with elevated IOP after silicone oil tamponade underwent IOP-lowering surgery compared with 1/22 eyes that received C3F8 tamponade. About 65% of eyes with elevated IOP after silicone oil tamponade required persistent administration of IOP-lowering drugs compared with 14% of eyes after C3F8 tamponade. These results highlight the different clinical outcomes of various tamponades used in PPV.
In the study by Budenz et al,30 silicone oil removal alone was associated with an overall success rate of 62.5% (including complete success and qualified success) for reducing silicone oil-related ocular hypertension. In a study by Nguyen et al,21 silicone oil removal alone had a success rate of 57.1%. In the current study, silicone oil removal surgery was performed in 12 patients to control IOP. This procedure was defined as complete success in one (8.3%) patient, as qualified success in 10 (83.3%) patients, and was unsuccessful in one (8.3%) patient. This patient underwent Ahmed glaucoma valve implantation, which controlled IOP. Therefore, the overall success rate (including complete success and qualified success) of silicone oil removal to control IOP was higher in our study than in earlier studies.30 The higher success rate of silicone oil removal to control IOP in our study could be related to the introduction of newer IOP-lowering drugs, particularly prostaglandin analogs, in recent years.
Conclusion
In this study, tamponade, especially with expanding gas or silicone oil, was the only significant risk factor for ocular hypertension after PPV in patients without a history of glaucoma. Lensectomy and scleral buckling were not significant risk factors for ocular hypertension after PPV in our study. The cumulative rate of ocular hypertension was higher in patients who received silicone oil tamponade than in patients who received other types of tamponade. Patients with ocular hypertension after PPV with silicone oil tamponade required long-term treatment with IOP-lowering drugs or surgery. Thus, patients who undergo PPV with silicone oil tamponade require long-term monitoring of IOP to detect ocular hypertension. The shortcoming of this study is the lack of angle description of ocular hypertension. Our study should help clinicians to estimate the risk and management of ocular hypertension after PPV. The prospective investigations with longer follow-up may strengthen our results in the future.
Acknowledgments
This study was supported by the Chinese National Natural Science Foundation (NSFC81100667) and Chinese International Science and Technology Cooperation Program (No 2015DFA31340). Dr Yuan Fang and Dr Qingqing Long are co-first authors for this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Faulbom J, Conway BP, Machemer R. Surgical complications of pars plana vitreous surgery. Ophthalmology. 1978;85(2):116–125. doi: 10.1016/s0161-6420(78)35684-0. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg RS, Peyman GA, Huamonte FU. Elevation of intraocular pressure after pars plana vitrectomy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;200(2):157–161. doi: 10.1007/BF00414365. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM, VanHorn DL. Late complications of pars plana vitrectomy surgery. Ophthalmology. 1978;85(2):126–140. doi: 10.1016/s0161-6420(78)35683-9. [DOI] [PubMed] [Google Scholar]
- 4.Ghartey KN, Tolentino FE, Freeman HM, McMeel JW, Schepens CL, Aiello LM. Closed vitreous surgery. XVII. Results and complications of pars plana vitrectomy. Arch Ophthalmol. 1980;98:1248–1252. doi: 10.1001/archopht.1980.01020040100014. [DOI] [PubMed] [Google Scholar]
- 5.Antoun J, Azar G, Jabbour E, et al. Vitreoretinal surgery with silicone oil tamponade in primary uncomplicated rhegmatogenous retinal detachment. Retina. 2016;36(10):1906–1912. doi: 10.1097/IAE.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 6.Parke DW, 3rd, Sisk RA, Houston SK, Murray TG. Ocular hypertension after intravitreal triamcinolone with vitrectomy and phacoemulsification. Clin Ophthalmol. 2012;6:925–931. doi: 10.2147/OPTH.S32934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas JB, Knorr HL, Rank RM, Budde WM. Intraocular pressure and silicone oil endotamponade. J Glaucoma. 2001;10(2):102–108. doi: 10.1097/00061198-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Henderer JD, Budenz DL, Flynn HW, Schiffman JC, Feuer WJ, Murray TG. Elevated intraocular pressure and hypotony following silicone oil retinal tamponade for complex retinal detachment. Arch Ophthalmol. 1999;117(2):189–195. doi: 10.1001/archopht.117.2.189. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Berrocal MH, Rodriguez FJ, et al. Intraocular pressure elevation after uncomplicated pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2014;34(10):1985–1989. doi: 10.1097/IAE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 10.Hartley RE, Marsh RJ. Anterior chamber depth changes after retinal detachment. Br J Ophthalmol. 1973;57(8):546–550. doi: 10.1136/bjo.57.8.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreiger AE, Hodgkinson BJ, Frederick AR, Jr, Smith TR. The results of retinal detachment surgery. Analysis of 268 operations with a broad scleral buckle. Arch Ophthalmol. 1971;86(4):385–394. doi: 10.1001/archopht.1971.01000010387005. [DOI] [PubMed] [Google Scholar]
- 12.Muether PS, Hoerster R, Kirchhof B, Fauser S. Course of intraocular pressure after vitreoretinal surgery: is early postoperative intraocular pressure elevation predictable? Retina. 2011;31(8):1545–1552. doi: 10.1097/IAE.0b013e31820f4b05. [DOI] [PubMed] [Google Scholar]
- 13.Koreen L, Yoshida N, Escariao P, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012;32(1):160–167. doi: 10.1097/IAE.0b013e318217fffb. [DOI] [PubMed] [Google Scholar]
- 14.Luk FO, Kwok AK, Lai TY, Lam DS. Presence of crystalline lens as a protective factor for the late development of open angle glaucoma after vitrectomy. Retina. 2009;29(2):218–224. doi: 10.1097/IAE.0b013e31818ba9ca. [DOI] [PubMed] [Google Scholar]
- 15.Yang HK, Woo SJ, Park KH, Park KH. Intraocular pressure changes after vitrectomy with and without combined phacoemulsification and intraocular lens implantation. Korean J Ophthalmol. 2010;24(6):341–346. doi: 10.3341/kjo.2010.24.6.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mi CW, Thompson JT. Long-term follow-up of intraocular pressure after vitrectomy in eyes without preexisting glaucoma. Retina. 2015;35(12):2543–2551. doi: 10.1097/IAE.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 17.Framme C, Klotz S, Wolf-Schnurrbusch UE, Wiedemann P, Wolf S. Intraocular pressure changes following 20G pars-plana vitrectomy. Acta Ophthalmol. 2012;90(8):744–749. doi: 10.1111/j.1755-3768.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeng KW, Fine HF, Wheatley HM, Roth D, Connors DB, Prenner JL. Incidence of steroid-induced ocular hypertension after vitreoretinal surgery with difluprednate versus prednisolone acetate. Retina. 2014;34(10):1990–1996. doi: 10.1097/IAE.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 19.Desai UR, Alhalel AA, Schiffman RM, Campen TJ, Sundar G, Muhich A. Intraocular pressure elevation after simple pars plana vitrectomy. Ophthalmology. 1997;104(5):781–786. doi: 10.1016/s0161-6420(97)30233-4. [DOI] [PubMed] [Google Scholar]
- 20.Al-Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil injection. J Glaucoma. 2005;14(1):40–46. doi: 10.1097/01.ijg.0000145811.62095.fa. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen QH, Lloyd MA, Heuer DK, et al. Incidence and management of glaucoma after intravitreal silicone oil injection for complicated retinal detachments. Ophthalmology. 1992;99(10):1520–1526. doi: 10.1016/s0161-6420(92)31771-3. [DOI] [PubMed] [Google Scholar]
- 22.Phelps CD, Burton TC. Glaucoma and retinal detachment. Arch Ophthalmol. 1977;95(3):418–422. doi: 10.1001/archopht.1977.04450030060003. [DOI] [PubMed] [Google Scholar]
- 23.Gedde SJ. Management of glaucoma after retinal detachment surgery. Curr Opin Ophthalmol. 2002;13(2):103–109. doi: 10.1097/00055735-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Honavar SG, Goyal M, Majji AB, Sen PK, Naduvilath T, Dandona L. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology. 1999;106(1):169–176. doi: 10.1016/S0161-6420(99)90017-9. discussion 177. [DOI] [PubMed] [Google Scholar]
- 25.Van Aken E, Lemij H, Vander Haeghen Y, de Waard P. Baerveldt glaucoma implants in the management of refractory glaucoma after vitreous surgery. Acta Ophthalmol. 2010;88(1):75–79. doi: 10.1111/j.1755-3768.2008.01428.x. [DOI] [PubMed] [Google Scholar]
- 26.Mangouritsas G, Mourtzoukos S, Portaliou DM, Georgopoulos VI, Dimopoulou A, Feretis E. Glaucoma associated with the management of rhegmatogenous retinal detachment. Clin Ophthalmol. 2013;7:727–734. doi: 10.2147/OPTH.S42792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U. Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension – a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1407–1414. doi: 10.1007/s00417-010-1409-7. [DOI] [PubMed] [Google Scholar]
- 28.Ki-I Y, Yamashita T, Uemura A, Sakamoto T. Long-term intraocular pressure changes after combined phacoemulsification, intraocular lens implantation, and vitrectomy. Jpn J Ophthalmol. 2013;57(1):57–62. doi: 10.1007/s10384-012-0207-7. [DOI] [PubMed] [Google Scholar]
- 29.Lalezary M, Kim SJ, Jiramongkolchai K, Recchia FM, Agarwal A, Sternberg P., Jr Long-term trends in intraocular pressure after pars plana vitrectomy. Retina. 2011;31(4):679–685. doi: 10.1097/IAE.0b013e3181ff0d5a. [DOI] [PubMed] [Google Scholar]
- 30.Budenz DL, Taba KE, Feuer WJ, et al. Surgical management of secondary glaucoma after pars plana vitrectomy and silicone oil injection for complex retinal detachment. Ophthalmology. 2001;108(9):1628–1632. doi: 10.1016/s0161-6420(01)00658-3. [DOI] [PubMed] [Google Scholar]


