Abstract
Regulation of human immune cell cytokine production in vivo is not well understood due in part to limitations on imposing experimental conditions. We proposed that life-imposed conditions (pregnancy, birth, age, gender), combined with large sample size, repeat sampling, and family-based recruitment would serve to reveal peripheral blood cell-derived cytokine patterns reflective of in vivo regulation regarding Th1/Th2 balance and familial correlation. Mononuclear cells were obtained from 483 trios in the Tucson Infant Immune Study: from mothers pre- and postpartum, infants at birth and at 3 mo, and fathers. Con A/PMA-stimulated supernatants were assayed by ELISA for IFN-γ, IL-4, IL-13, IL-5, and IL-10 and allergen-stimulated supernatants for IFN-γ, IL-4, and IL-13. Mitogen-stimulated prepartum samples were not globally Th2 biased, differing from postpartum only by a modestly reduced IFN-γ:IL-5 ratio. Prepartum samples actually produced less IL-10 and IL-13 although more IL-5 than paternal samples. Newborns were also not globally Th2 biased, with mitogen stimulation producing ~10-fold less IL-4, IL-5, and IFN-γ than adults but only 2- to 3-fold less IL-13 and IL-10. Despite these group differences, all cytokines showed marked positive intraindividual correlations (all p < 0.001). Allergen stimulation gave results consistent with a lack of global Th2 bias. Mitogen stimulation revealed parent-child and parent-parent correlations. Thus, rather than a global Th2 bias, cytokine production in pregnant mothers and newborns appears regulated so as to maintain a relative balance among the cytokines, with the nature of the balance differing in mothers and infants and with production influenced by familial factors that include shared environment.
Very little is known as yet about the in vivo regulatory pathways that control the capacity of adaptive immune cells in humans to produce cytokines. This lack occurs in large part because of individual heterogeneity, obvious limitations to controlling experimental conditions, and challenges in gaining access to the major sites of immune activity in vivo. Nevertheless, hypotheses of immune regulation can be tested by sampling circulating immune cells from individuals in different maturational stages or experiencing different life events or conditions and, if cytokine production patterns associated with the stages or events can be identified, inferences of regulation can be made. Also, sampling family members can allow the assessment of familial patterns such that inferences regarding genetic or environmental influences can be drawn.
An example of this approach has been the testing of the proposal made by Wegmann et al. (1) some years ago that pregnancy is a Th2-polarized condition. With Th2 cells at that time having been newly described as critical to Ab production but not involved in the induction of CTLs, a Th2 status or bias for pregnancy provided a satisfying explanation for the well-known immunologic enigma of the pregnant female immune system permitting the presence and growth of the fetal allograft. This proposal of altered immune status developed in part from animal model studies demonstrating that immune responses to infection or to administration of a Th1 cytokine (IFN-γ or IL-2) increased the risk of abortion of the fetus (2). Further support was provided by studies in humans reporting greater production of Th1 and/or lesser production of Th2 cytokines from PBMCs at the time of spontaneous abortion (3, 4) or during preeclampsia (5) when compared with PBMCs from women with normal pregnancies. Still, these differences may represent immune occurrences during and perhaps limited to extreme circumstances and thus they show the maternal immune status of normal pregnancy only in relation to (and as different from) the pathologic state likely due either to acute infection or a prepregnancy condition. Although a few studies have compared the immune status of normal pregnancy more directly to that of the immune status in nonpregnant or postpartum women (4–7), they have been mainly cross-sectional case control studies, most with relatively small group sizes and unspecified matching criteria, and the results have been conflicting.
Despite the lack of clarity, the proposed concept of Th2 bias in pregnancy has held on and has been extended to the immune system of the newborn child (8), following the logic that the child’s immune system, having been exposed mainly to the supposed Th2 or the Th2-inducing maternal environment, is also likely skewed toward Th2. Support of this proposal, however, comes from only a few murine (9) and human studies (10), whereas other studies are not in agreement. Indeed, Wilson and Lewis (11) and Wilson et al. (12) demonstrated that the capacities to produce both IFN-γ and IL-4 were suppressed to approximately the same degree in newborns compared with adults. Nonetheless, the concept of “Th2ness” for the pregnant mother and newborn permeate much of the current thinking regarding the immune susceptibility status of the mother and the readiness of the infant to encounter a world laden with pathogens. The importance of establishing the immune status at birth is underscored by several studies that suggest that variations in early immune development have long-term sequelae with regard to the prevalence of many diseases (13–15).
Another approach to elucidating in vivo regulatory mechanisms in infants is to determine whether familial patterns can be identified in relation to cytokine production. This approach has been used to a very limited extent and only in relation to maternal-child (and not paternal-child) relations (16), thus leaving unaddressed whether such an influence is environmental or genetic.
The limitations of our understanding of immune regulation in vivo in relation to the immune status of pregnancy and birth suggest a need for investigating these issues within a large population of parents and newborn infants. In the study presented here, we report on cytokine production following stimulation of mononuclear cells in blood samples from mothers during the third trimester of pregnancy and 3 mo postpartum, fathers, and infants at birth and 3 mo after birth. These trios are enrolled in the Tucson Infant Immune Study (IIS). The spectrum of cytokines produced by mitogen stimulation was assessed as an approximate indicator of T cell polarization status in relation to the processes of pregnancy for the mother, and birth for the infant, and compared with samples taken 3 mo after these events. Similarly, allergen stimulation was compared among these groups. In addition, we have sought evidence for whether the cytokine production capacities of the mother and the father relate to the child’s capacity to produce cytokines at birth or 3 mo.
Materials and Methods
Subjects
Pregnant women visiting their obstetrician between 25 and 44 wk of gestation were approached by the study nurse without selection and asked whether they and the child’s father would like to enroll themselves and their newborn in the IIS, a prospective birth cohort study of the development of immune markers for asthma risk in childhood. Between 1996 and 2004, 484 infants and their parents were enrolled as trios in the study. Parents completed a questionnaire at enrollment describing their respiratory health history and their child’s health was followed prospectively during the first year of life by questionnaires. Allergy in the parents was defined as physician diagnosed asthma or allergic rhinitis. Diagnoses were made by physicians independent of the study investigative team. Skin test positivity was defined as a wheal of at least 3 mm (sum of perpendicular diameters) to any of 15 aeroallergens. The University of Arizona Institutional Review Board approved the study and informed consent was obtained for all participants.
Samples
Heparinized blood samples were obtained during the last trimester of pregnancy and processed within 24 h of collection (prepartum sample, n 428; mean age, 29.5 (SD 6.1) years; mean gestational age, 37.2 (SD 1.9) wk) and ~3 mo postpartum (postpartum sample, n 438; postpartum mean, 3.5 (SD 1.7) months). A heparinized blood sample was collected from the fathers at enrollment or at the 3-mo visit (paternal sample, n 365; mean age, 32.3 (SD 6.6) years). Heparinized umbilical cord blood samples were collected at birth (cord sample, n 294) and peripheral blood samples were collected from the infants at 3 mo of age (3-mo infant sample, n 323; mean age, 2.8 (SD 1.2) months). Samples were obtained from one or more members of the 483 trios of the 484 enrolled in IIS.
PBMC and cord blood mononuclear cell (CBMC)3 stimulations and cytokine analyses
PBMCs were isolated by gradient sedimentation with lymphocyte separation medium (LSM; MP Biomedicals) from 7 to 14 ml of heparinized blood. The mononuclear cell layer was removed, washed with HBSS, and resuspended at 2 × 106 cells/ml in RPMI 1640 (RPMI+) containing 5% heat-inactivated FCS, 1 mM L-glutamine, 10 mM HEPES, and 50 U penicillin/50 μg/ml streptomycin. Cord blood mononuclear cells were similarly isolated by gradient sedimentation with LSM with further steps to remove nucleated RBC (17, 18). The plasma and mononuclear cell layers were removed, added to 6% dextran T500 in 0.9% NaCl (ratio of 1:4 dextran to the cell/plasma layer), and incubated for 10 min at 37°C. The CBMCs were reisolated by LSM gradient sedimentation of the dextranplasma/cell mixture, washed with HBSS without Ca2+ or Mg2+, and resuspended in RPMI+ medium. Cytospin slides were prepared and stained, and the percent mononuclear cells was determined. Cell concentration was adjusted to 2 × 106 mononuclear cells.
For mitogen stimulation, 2-ml cultures were incubated without stimulant (control) or with 10 μg/ml Con A and 10 ng/ml PMA (Con A/PMA; Sigma-Aldrich) for (randomly) 18–24 h (19, 20). For Ag stimulation, 2-ml cultures were incubated without stimulant or with 25 μg/ml Alternaria (Alternaria culture filtrate) or 25 μg/ml Bermuda (both from Greer Laboratories) for (randomly) 66–72 h. Supernatants were collected following centrifugation and aliquots were frozen at −70°C until cytokine assays were performed. Concentrations of IFN-γ, IL-4, IL-5, IL-10 (R&D Systems), and IL-13 (Diaclone) in the culture supernatants were measured by ELISA using commercially available kits. The limits of detection thresholds in pg/ml were 15.6, 0.25, 7.8, 3.1, and 7.8 for IFN-γ, IL-4, IL-5, IL-13, and IL-10, respectively. Values below the limit of detection were assigned values of half the lowest standard.
Data management
Data are managed by Epi-Logs, a system developed at the Arizona Respiratory Center. Key features of this software for enrollment and follow up include management of contact information, appointment scheduling, specimen and questionnaire collections, and bar-coded label printing for biorepository items. For the laboratory, Epi-logs guides assay set-up processes, runs quality control/quality assurance procedures, and uploads data into a data warehouse.
Statistical analysis
For each sample type and each individual cytokine measured, the distribution of values was initially assessed for the percentage of values that were undetectable. Distributions with >20% undetectable values (IL-4 in cord and IL-5 in cord and infant 3-mo samples) were analyzed as categorical variables using nonparametric techniques. Cytokine distributions with <20% undetectable values were considered as continuous variables and because of strong skew to the right were log transformed to yield approximately normal distributions, permitting the use of parametric analytic techniques. Cytokine ratios were calculated for those variables without truncation and were also log transformed. Means between groups were compared by Student’s unpaired t test. Random effects models were used to assess differences in samples obtained from the same group at two points (mothers pre- and postpartum and infants at birth and 3 mo). Pearson correlation was used to assess the relation between cytokines within samples or the same cytokine between sample types for all data sets containing <20% undetectable values. For data sets with >20% undetectable values, analysis of percent positive between groups was assessed by χ2. Multivariate regression was used to examine gender-based cytokine differences adjusting for other potential modifiers. Significance level for p values for all analyses was set at 0.01 to control for multiple comparisons. Analyses were performed using SPSS for Windows version 15.0 and STATA version 9.0.
Results
Cytokine production in mothers prepartum, mothers postpartum, and fathers
Production of IFN-γ, IL-4, IL-5, IL-13, and IL-10 in response to Con A/PMA stimulation of prepartum PBMCs is shown in the first box of each panel in Fig. 1. Comparing the cytokines, IFN-γ was produced in the greatest amounts with a geometric mean of 5270 pg/ml (geometric SD 3.5, n = 420) being 21-, 47-, 61- and 512-fold greater than the concentrations of IL-13, IL-10, IL-5, and IL-4, respectively. When prepartum production of each cytokine was compared with production in the postpartum samples (second box in each panel of Fig. 1), no significant differences were noted except that IL-4 production (Fig. 1B) was modestly lower in the prepartum supernatants. Comparison of maternal to paternal cytokine production (third box in each panel of Fig. 1) revealed very similar levels for IFN-γ and for IL-4. Fathers’ cells produced greater amounts of IL-13 (Fig. 1C) and less IL-5 (Fig. 1D) than were produced by the prepartum samples. Fathers’ cells also produced more IL-10 (Fig. 1E) than did the mothers’ cells either pre- or postpartum.
FIGURE 1.
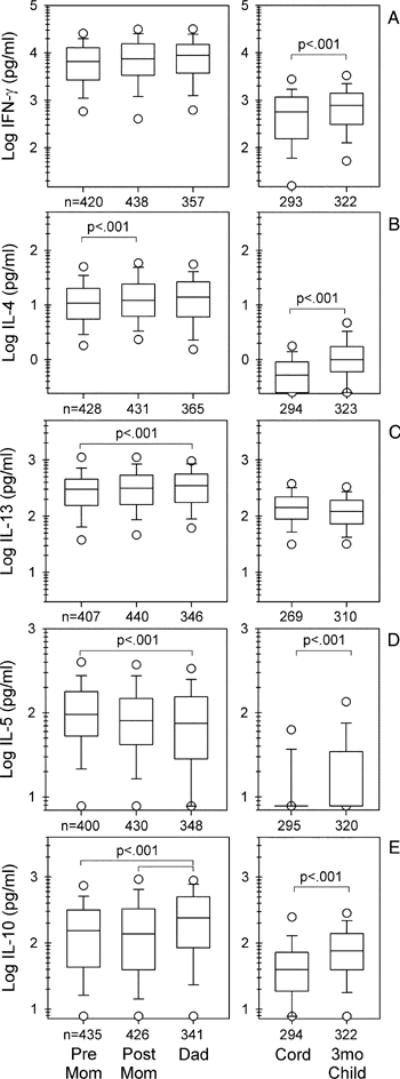
Cytokine production from mitogen-stimulated blood mononuclear cells of parents and infants. Although most data sets displayed are continuous and approximate log normal distributions, to maintain consistency throughout, data are shown as box plots, with boxes indicating 25–75% of values, lines within boxes indicating medians, whiskers 5 and 95% of values, and values beyond the whiskers as open symbols. Statistical comparisons shown for the two samples from mothers (pre mom and post mom) for all cytokines and the two samples from the infants (cord and 3-mo child) for IFN-γ, IL-13, and IL-10 were analyzed by random effects (by time). Pre-mom-to-dad and post-mom-to-dad comparisons were analyzed by Student’s t test. Wide brackets refer to pre-mom-to-dad comparisons. Value of p in E applies to both brackets. Cord-to-3-mo-child IL-4 and IL-5 comparisons were assessed (as percent detectable) by χ2. Pre Mom, Maternal prepartum samples; Post Mom, maternal postpartum samples; Dad, paternal samples; Cord, venous umbilical cord sample; 3mo Child, infant samples obtained at 3 mo of age.
Cytokine production in the parents was also compared by ratios of Th1:Th2 cytokines (Table I) and reflected what could be predicted from the individual cytokines for IFN-γ, IL-5, and IL-10. The mean IFN-γ:IL-5 ratio was significantly lower in prepartum samples compared with postpartum and paternal samples. The mean IFN-γ:IL-10 ratios did not differ for the two sample points for the women but these ratios were higher than the mean ratio for the fathers, suggestive of a gender-based increase in IL-10 in the fathers. In contrast, the more modest postpartum increase in IL-4 and the paternal increase in IL-13 production were not reflected in Th1:Th2 cytokine ratio differences.
Table I.
Cytokine ratios of mitogen-induced cytokine production from PBMCs of pregnant mothers, mothers at 3 mo postpartum, and fathers
| Cytokine Ratios | Mothers Pregnant | Mothers 3 mo postpartum | Fathers |
|---|---|---|---|
| IFN-γ:IL-4 | |||
| Log mean | 2.7102 | 2.7105 | 2.7241 |
| Log SD | 0.3749 | 0.4270 | 0.4775 |
| n | 412 | 424 | 354 |
| IFN-γ:IL-13 | |||
| Log mean | 1.3176 | 1.3246 | 1.3336 |
| Log SD | 0.3690 | 0.4170 | 0.4518 |
| n | 403 | 436 | 337 |
| IFN-γ:IL-5 | |||
| Log mean | 1.7853a,b | 1.8976 | 2.0108 |
| Log SD | 5313 | 0.6155 | 0.6263 |
| n | 390 | 427 | 341 |
| IFN-γ:IL-10 | |||
| Log mean | 1.6687b | 1.7133c | 1.5080 |
| Log SD | 0.5230 | 0.5970 | 0.5137 |
| n | 414 | 422 | 332 |
p < 0.01 for comparison of pregnant to postpartum mothers.
p < 0.001 for comparison of pregnant mothers to fathers.
p < 0.001 for comparison of postpartum mothers to fathers.
Among the IIS trios, 24% consisted of at least one Hispanic parent, 58% had two non-Hispanic white parents, and the remaining 18% were inclusive of many ethnicities. Allergy (asthma and/or rhinitis) prevalence did not differ in Hispanic and non-Hispanic white mothers (p = 0.27), but Hispanic fathers had a lower prevalence than non-Hispanic white fathers (15% vs 35%, p = 0.001). Upon using multivariate analysis, gender differences remained evident for IL-13 and IL-10 production and became significant for IL-5. Thus, with adjustment for smoking and ethnicity, prepartum and postpartum maternal IL-5 production was significantly greater than paternal IL-5 production (p <.001 and p = 0.008, respectively; see supplemental Tables 1 and 24) and these differences were independent of similar IL-5-raising influences of allergy (asthma or allergic rhinitis (p < 0.001) and skin test reactivity (p < 0.001). For IL-10 production, gender differences were strong (p < 0.001) but with fathers having greater production compared with mothers pre- and postpartum (p < 0.001 for both). Prepartum but not postpartum IL-13 production was lower than that of fathers (p = 0.002). In contrast to IL-5, production of neither IL-10 nor IL-13 was affected significantly by allergy and skin test reactivity. Postpartum maternal IL-5 was higher and IL-10 was lower than paternal IL-10 production. The only ethnic difference in cytokine production that was significant was a lower production of IL-13 in Hispanics.
Relation of mitogen-induced cytokine production within parental samples
Despite little difference in mean values of cytokine production between prepartum and postpartum samples, it was still possible that mothers might show an inverse relation of Th2 to Th1 cytokine production that might be more exaggerated in pregnancy. We thus assessed the degree and direction of correlation among the cytokines, pairwise, within subjects. These comparisons are shown separately for mothers prepartum, mothers postpartum, and fathers in Table III. Even though, as noted above, the mean concentrations of the secreted cytokines differed from one another over several hundredfold, the level of production of each of the Th2 cytokines was found to be closely and directly correlated not only to the level of production of the other Th2 cytokines but also to the level of production of the Th1 cytokine IFN-γ. This strong direct correlation pattern was evident in both prepartum and postpartum samples from the mothers and also in the samples from the fathers, with correlation coefficients ranging from 0.378 to 0.841 (all p < 0.001; Table III). Furthermore, correlation between IL-10 and each of the Th1 and Th2 cytokines was also direct and impressively strong. In accord, correlation coefficients among the ratios of IFN-γ:Il-4, IL-13, IL-5, and IL-10 were as high or higher as those for the individual cytokines (data not shown).
Table III.
Correlation of cytokines from mitogen-stimulated PBMCs from pregnant mothers, postpartum mothers, and fathers
| Samples from | Pearson Correlation Coefficients (n)
|
|||
|---|---|---|---|---|
| IFN-γ | IL-4 | IL-13 | IL-5 | |
| Pregnant mothers | ||||
| IL-4 | 0.696a (412) | |||
| IL-13 | 0.739a (403) | 0.789a (402) | ||
| IL-5 | 0.463a (390) | 0.655a (392) | 0.692a (384) | |
| IL-10 | 0.565a (414) | 0.705a (419) | 0.661a (402) | 0.593a (395) |
| Mothers postpartum | ||||
| IL-4 | 0.668a (424) | |||
| IL-13 | 0.738a (436) | 0.808a (427) | ||
| IL-5 | 0.378a (427) | 0.677a (416) | 0.550a (428) | |
| IL-10 | 0.537a (422) | 0.667a (413) | 0.600a (423) | 0.542a (412) |
| Fathers | ||||
| IL-4 | 0.704a (354) | |||
| IL-13 | 0.634a (337) | 0.841a (343) | ||
| IL-5 | 0.504a (341) | 0.745a (344) | 0.775a (332) | |
| IL-10 | 0.691a (332) | 0.800a (334) | 0.693a (322) | 0.605a (321) |
Significant at the 0.001 level (two tailed).
Cytokine production in infants at birth and 3 mo of age
Cytokine production in the neonates is shown in Fig. 1 (the two right-most boxes in each panel). The rank order of levels of cytokine production in CBMCs was the same as for the adult PBMCs, with IFN-γ production the greatest, although only 3- and 11-fold greater than IL-13 and IL-10, respectively. Production of IL-4 and IL-5 was very low (undetectable for 46 and 77% of the newborns, respectively). IFN-γ and IL-13, however, were detectable in >95% of cord samples and IL-10 in >80%. By 3 mo of age, each of the cytokines except IL-13 increased in production and only IL-5 had >20% undetectable values (47%) at this age point (Fig. 1). IFN-γ:IL-13 ratios decreased significantly from birth to 3 mo (Table II). No gender, gestational age, or ethnicity differences were evident for production of any of the cytokines at either age (data not shown). Neither infant nor maternal cytokine production was significantly affected by maternal colds during pregnancy (data not shown).
Table II.
Cytokine ratios from mitogen-induced cytokine production from CBMCs of infants at birth and PBMCs at 3 mo
| Cytokine Ratios | Infants at Birth | Infants at 3 mo |
|---|---|---|
| IFN-γ:IL-4 | ||
| Log mean | NCa | 2.7812 |
| Log SD | NC | 0.5044 |
| n | 293 | 322 |
| IFN-γ:IL-13 | ||
| Log mean | 0.4819b,c | 0.7386c |
| Log SD | 0.4837 | 0.4601 |
| n | 268 | 310 |
| IFN-γ:IL-10 | ||
| Log mean | 1.0401c | 0.9444c |
| Log SD | 0.6460 | 0.5002 |
| n | 293 | 322 |
NC, Not computed because of large proportion of undetectable IL-4 values.
p < 0.001 comparing infants at birth to infants at 3 mo.
p < 0.001 comparing infants to mothers postpartum (from Table I).
Relation of mitogen-induced cytokine production within infant samples
The relation among the different cytokines secreted by stimulated CBMCs within individual cord samples and, similarly, the relation for cytokines produced by PBMCs at 3 mo is shown in a correlation matrix in Table IV, except for those cytokines with a large proportion (>20%) of undetectable values (IL-4 at birth and IL-5 at both ages). The pattern is similar to that of the parents in that both Th1 and Th2 cytokines as well as IL-10 show strong direct correlations with one another.
Table IV.
Correlation of cytokines from mitogen-stimulated CBMCs at birth and PBMCs at 3 mo of age
| Pearson Correlation Coefficients (n)
|
|||
|---|---|---|---|
| Samples from | IFN-γ | IL-4 | IL-13 |
| Infants at birth | |||
| IL-4 | NCa | ||
| IL-13 | 0.589b (268) | NC | |
| IL-10 | 0.281b (293) | NC | 0.273b (269) |
| Infants at 3 mo | |||
| IL-4 | 0.473b (322) | ||
| IL-13 | 0.560b (310) | 0.574b (310) | |
| IL-10 | 0.492b (322) | 0.558b (322) | 0.440b (310) |
NC, Not computed because of large proportion of undetectable IL-4 values.
Pearson correlation coefficient is significant at the 0.001 level (two tailed).
Quantitative differences in cytokine production by parents and infants
Despite the general correlation pattern being similar to that of the adults, some differences are noted when comparing the mean cytokine production from the immune cells of the newborns to that of the adults. The mean amount of IFN-γ produced was on the order of 10-fold less than that of the adults (Fig. 1). The large proportion of undetectable values for IL-4 and IL-5 preclude a comparison of means but a comparison of medians shows IL-4 and IL-5 are also produced in amounts on the order of 10-fold lower than the amounts produced from adult cells. This is not the case, however, for either IL-13 or IL-10 production, each of which is reduced by only 2- to 3-fold from adult levels. Although by 3 mo of age, significant increases have occurred for all cytokines except IL-13, mean and/or median values remain substantially below those of the parents.
Cytokine Th1:Th2 ratio assessment (supplemental Table II) revealed that the IFN-γ:IL-4 ratio at 3 mo of age was similar to that of mothers at 3 mo postpartum (Table I), further demonstrating that, within individuals, production of both cytokines is similarly decreased compared with adults. In contrast, IFN-γ:IL-13 and IFN-γ:IL-10 ratios at 3 mo and at birth were substantially lower than parent ratios (Table II vs I). Thus, production of Th2 cytokines did not show an across-the-board elevation, but IL-13 production was increased in early life.
Relation of parent-parent and parent-infant mitogen-stimulated cytokine production
Cytokine production (we reasoned) might be correlated among family members, either through genetic mechanisms or mechanisms involving shared environment or possibly both. Such relations, however, might be masked or mimicked by technical variation such as a small variation among subjects in time of culture during stimulation. To assess whether such factors might have influenced our data, we first compared the cytokine production of the two maternal samples (Table V). These samples showed a significant direct correlation, demonstrating sufficient biologic similarity to outweigh any technical contributions to variation. Going on to compare mother with father samples to assess possible shared environment influences, we found a significant correlation was also evident for cytokine production between postpartum mothers and fathers for IFN-γ, IL-4, IL-13, and IL-10. In comparisons of parents to children, there was no evidence of correlation of cytokine production by either parent with that of the child at birth (data not shown). However, production of IFN-γ and IL-13 from mothers’ PBMCs postpartum correlated significantly with production of these cytokines from infants at 3 mo (Table V). Also, IFN-γ production from the fathers’ PBMCs correlated with that from PBMCs of the infant at 3 mo. No correlation was evident between the two samples obtained from the children (data not shown). Very similar correlation coefficients were obtained upon limiting the analyses to the Hispanic population (although significance is decreased with the reduction in group size; data not shown).
Table V.
Correlation of individual cytokines by PBMCs from prepartum vs postpartum mothers, mothers vs fathers, mothers vs 3-mo infants, and fathers vs 3-mo infants
|
Samples from |
Pearson Correlation Coefficients (n)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Samples from mothers prepartum
| ||||||||
| IFN-γ | IL-4 | IL-13 | IL-5 | IL-10 | IFN-γ:IL-4 | IFN-γ:IL-13 | IFN-γ:IL-10 | |
| Mothers postpartum | 0.197a (379) | 0.365a (381) | 0.272a (369) | 0.325a (357) | 0.345a (384) | 0.450a (360) | 0.425a (361) | 0.446a (361) |
| Fathers | 0.027 (313) | 0.093 (330) | 0.095 (296) | 0.141 (294) | 0.210a (315) | 0.016 (306) | 0.055 (284) | −0.059 (290) |
| Infants at 3 mo | 0.081 (281) | 0.091 (290) | 0.080 (264) | NCb | −0.120 (292) | 0.098 (277) | 0.155 (261) | 0.001 (277) |
|
| ||||||||
| Samples from | Samples from mothers postpartum
|
|||||||
| IFN-γ | IL-4 | IL-13 | IL-5 | IL-10 | IFN-γ:IL-4 | IFN-γ:IL-13 | IFN-γ:IL-10 | |
|
| ||||||||
| Fathers | 0.150c (343) | 0.179a (349) | 0.183a (335) | 0.035 (328) | 0.256a (323) | 0.043 (334) | 0.062d (323) | −0.030 (312) |
| Infants at 3 mo | 0.167a (302) | 0.137 (298) | 0.207a (294) | NC | 0.101 (299) | 0.138 (292) | 0.239a (290) | 0.033 (0.571) |
|
| ||||||||
| Samples from | Samples from fathers
|
|||||||
| IFN-γ | IL-4 | IL-13 | IL-5 | IL-10 | IFN-γ:IL-4 | IFN-γ:IL-13 | IFN-γ:IL-10 | |
|
| ||||||||
| Infants at 3 mo | 0.163c (244) | 0.043 (252) | 0.161 (228) | NC | 0.106 (235) | 0.152 (242) | 0.140 (219) | 0.120 (227) |
Significant at the 0.001 level (two tailed).
NC, Not computed because of the large proportion of undetectable IL-5 values.
Significant at the 0.01 level (two tailed).
Not significant by Pearson but Spearman ρ = 0.165, p = 0.003.
Relation of allergen-stimulated cytokine production in parents and infants
To determine whether cytokine production by specific allergens might differ in relation to Th2 bias during pregnancy and birth, we compared cytokine production following stimulation with two allergens common in the southwest environment, Alternaria and Bermuda. The data, given as percent with detectable cytokine production in Table VI, demonstrate that the rank order of frequency of detectable cytokine production upon Alternaria stimulation was greater for IL-13 than IL-4 or IFN-γ in mothers and fathers and that mothers prepartum did not differ from postpartum mothers or from fathers in the frequency of these responses. When stimulated by Bermuda, the frequency rank order differed with IL-4 produced most frequently and was increased in prepartum vs postpartum samples, although the prepartum responses were equivalent to those of the fathers. Thus, pregnancy showed an enhanced frequency of IL-4 (but not IL-13) responses to the grass allergen Bermuda. Stratifying by allergy revealed only that IL-13 production to Alternaria was reduced in pregnancy compared with fathers among allergics (supplemental Table 3). Stratifying by immediate skin test reactivity in the parents indicated that IL-4 responses were increased in frequency for mothers prepartum for both allergens but only among the nonatopics (supplemental Table 4). Ethnicity did not affect sensitization to these allergens.
Table VI.
Allergen-stimulated cytokine production comparing PBMCs of mothers prepartum, mothers postpartum, and fathers
| Allergen | Cytokine | Mothers Prepartum | Mothers 3 mo Postpartum | Fathers |
|---|---|---|---|---|
| Alternaria | IFN-γ | |||
| % detectable | 20.0 | 29.7 | 29.5 | |
| n | 250 | 269 | 244 | |
| IL-4 | ||||
| % detectable | 32.8 | 24.2 | 31.1 | |
| n | 250 | 277 | 244 | |
| IL-13 | ||||
| % detectable | 46.8 | 52.0 | 56.8 | |
| n | 250 | 277 | 243 | |
| Bermuda | IFN-γ | |||
| % detectable | 5.4 | 7.7 | 7.0 | |
| n | 205 | 235 | 201 | |
| IL-4 | ||||
| % detectable | 36.9a | 22.1b | 32.2 | |
| n | 206 | 244 | 202 | |
| IL-13 | ||||
| % detectable | 13.1 | 21.1 | 19.3 | |
| n | 206 | 242 | 202 |
p < 0.01 for comparison of prepartum to postpartum mothers.
p < 0.001 for comparison of postpartum mothers to fathers.
In infants at birth (Table VII), IFN-γ and IL-13 were produced at frequencies (for Alternaria) similar to or (for Bermuda) even greater than those in the parent samples. In contrast, IL-4 production was extremely infrequent at birth. From birth to 3 mo, IFN-γ and IL-4 showed little change, whereas IL-13 production decreased. These data, along with those from mitogen stimulation, suggest that IL-13 and IL-4 production have quite distinct patterns of production in early life.
Table VII.
Allergen-induced cytokine production comparing CBMCs and 3-mo PBMCs of infants
| Allergen | Cytokine | Child at Birth | Child at 3 mo |
|---|---|---|---|
| Alternaria | IFN-γ | ||
| % detectable | 34.8 | 36.5 | |
| n | 210 | 104 | |
| IL-4 | |||
| % detectable | 5.2 | 3.8 | |
| n | 211 | 104 | |
| IL-13 | |||
| % detectable | 52.2a | 34.6 | |
| n | 209 | 104 | |
| Bermuda | IFN-γ | ||
| % detectable | 16.4 | 12.3 | |
| n | 195 | 73 | |
| IL-4 | |||
| % detectable | 2.0 | 2.7 | |
| n | 197 | 73 | |
| Il-13 | |||
| % detectable | 27.4a | 9.6 | |
| n | 197 | 73 |
p < 0.01 for comparison of child at birth vs at 3 mo.
Discussion
Findings from our study demonstrate fundamental characteristics of mitogen-stimulated cytokine production in a large population of triads: very similar cytokine production during and after pregnancy (except for small but significantly lower means for IL-4 and the IFN-γ:IL-5 ratio); very strong direct correlations of Th1 to Th2 cytokines and to IL-10; discernable characteristic patterns of production by age (with a relative enhancement in IL-13 and IL-10 production for infants compared with adults) and by gender in adults (with higher IL-13 and IL-10 and lower IL-5 in males); and familial patterns that include both parent-parent and parent-child correlations. These findings allow us to address several issues regarding the in vivo regulation of the capacity of human immune cells to produce and secrete adaptive immune cytokines. From our findings, we suggest (and expand on below) that 1) a general Th2 bias is not evident in pregnancy or at birth; 2) regulation of cytokine production results in strong intrasample correlations that are evident in both males and females and are maintained within groups at major life stages (infancy vs adulthood), and while undergoing major events (like pregnancy and birth) and despite relative between-group shifts in production of certain cytokines; and 3) shared environment appears to participate in the familial correlations in cytokine production.
The differentiation of Th1 and Th2 cells identified first in mice and subsequently (although less cleanly) in humans led to the proposal that at least some human conditions might be associated with immunity that is preponderantly Th1 or Th2, with one such proposed Th2 condition being pregnancy. We examined PBMCs from 400 women obtained during their third trimester of pregnancy and found, first, that upon stimulation with Con A/PMA, the Th1 cytokine IFN-γ was produced in many times greater amounts than the Th2 cytokines. Also, the mean production levels of IFN-γ, IL-13, and IL-5 did not differ in the same women assessed 3 mo postpartum, whereas the mean level of IL-4 produced was actually lower during pregnancy compared with postpartum. These findings provide no support for the oft-cited concept that pregnancy is a Th2-biased condition.
Our findings differ from those of Marzi et al. (4) who demonstrated lower IFN-γ and higher IL-4 production from PHA A–stimulated PBMCs obtained during pregnancy when compared with PBMCs from control nonpregnant women. Also, Reinhard et al. (5) and Saito et al. (6) found increased IL-4-positive and decreased IFN-γ-positive cells following ionomycin/PMA stimulation during pregnancy compared with nonpregnant and postpartum controls. However, Keski-Nisula et al. (7), upon stimulation with Con A/PMA, found both IL-4 and IFN-γ production to be lower during pregnancy than was the case for samples obtained 3 mo later. Rastogi et al. (8), in a study limited to women with asthma, were unable to detect differences in intracellular IFN-γ and IL-4 following stimulation with ionomycin/PMA in samples obtained during pregnancy compared with those postpartum. Although it is possible that different stimuli may be responsible for some of these disparities, small group size for most of these studies and (as discussed further below) environmental differences between pregnant and control groups might also be involved.
We pursued further the possibility of a Th2 bias in pregnancy in at least some women (especially those producing high levels of Th2 cytokines) by determining whether inverse correlations or even a shift toward the null might be evident between Th1 and Th2 cytokines within individuals. Our data, however, demonstrated strong direct correlations among all of the cytokines produced, with IL-4, IL-5, and IL-13 correlations to IFN-γ that were comparable to or greater than those obtained postpartum. These correlations (that arise because PBMC samples producing low levels of one cytokine produce low levels of the other cytokines and similarly for high production) suggest that the presence of a regulatory mechanism functioning to maintain a relative balance of the cytokines produced within individuals that appears more important than actual amounts. The consistency of this balance (seen not only within mothers, but also within fathers and within infants) suggests functional significance. Given that the chromosomal locations are different for the genes for IFN-γ (chromosome 12), IL-4, IL-5, and IL-13 (chromosome 5), and IL-10 (chromosome 1) and that production of these cytokines involves more than one cell subtype (at least CD4+ and CD8+ cells and possibly NK cells) and cell state (naive vs effector status), (21, 22) it seems unlikely that a single common transcriptional regulatory mechanism operating simultaneously for each of the cytokine genes is responsible. Instead, we suggest that a regulatory mechanism operating at an upstream level of cell activation or perhaps a protein secretion mechanism provides major regulation of these responses to mitogen stimulation. An example of a feedback mechanism of cytokine regulation involving induction of IL-10 by all cytokines that utilize the common γ-chain as a component of their receptors has been described previously (23) and whether a mechanism similar to this but expanded in scope might be responsible is one such possibility. That these close direct relations among the cytokines produced are not unique to stimulation with Con A/PMA is shown by Hartel et al. (24), who reported a similar close direct relation between IL-10 and IL-4 following stimulation with PMA/ionomycin, by Gabrielsson et al. (25), who showed a direct relation between the percentage of cells producing IL-4 and IFN-γ in cord blood stimulated with PHA, and by Schaub et al. (26), who found a significant relation between IL-13 production and the proliferation response to PHA. The precise mechanisms responsible for these correlations remain to be elucidated.
We thought it important to consider that at least part of the correlation among cytokines produced upon stimulation might be present in the data as a result of technical procedures. To adjust for any systematic influence of culture or other technical issues on variation, we assessed correlation among the ratios of each of the Th2 cytokines and IL-10 in relation to IFN-γ production and found similar or even stronger correlation coefficients than those for the individual cytokines, thus supporting a biologic basis for the correlation. The correlation between the maternal ratios from samples taken at different times (Table V) provides further support that such a regulatory mechanism not only appears to exist but persists over time.
Comparing the Th1 and Th2 cytokine values and ratios not only between pre- and postpartum mothers but also between mothers and fathers provided the opportunity to address gender differences. IFN-γ and IL-4 production did not differ between maternal and paternal samples. IL-10 and IL-13 production during pregnancy was lower than that of the fathers and IL-5 was modestly but significantly increased in mothers prepartum compared with the fathers Multivariate regression analyses supported a gender-based difference for IL-10 and IL-5 in fathers compared with both pre- and postpartum samples, whereas the modest IL-13 relation to gender was evident pre- but not postpartum. Interestingly, Wang et al. (27) found that at least in vitro, sex hormones influence the production of IL-5 from human PBMCs. Whether such influences might contribute to the IL-5-related differences we observed requires further study. Also, recent studies have identified that, in addition to common regulatory mechanisms, there are additional transcriptional regulatory mechanisms that differ between the genes of IL-4 and IL-5 (28–30), thus providing possible mechanisms that could alter IL-5 production without affecting IL-4.
Our results do not address nor preclude the potential for deleterious effects on pregnancy for even greater ratios of IFN-γ:IL-4 than those we report and as have been reported in relation to spontaneous abortion and preeclampsia (3, 6). Nonetheless, our large population study does not support a general Th2 bias as being the mechanism by which the fetal allograft is maintained during pregnancy. It is possible that there may be a local Th2 bias in the placental tissue per se that might occur during normal pregnancy and might not be reflected in systemic cytokine production. However, it should be noted that T cells have been reported to be very sparsely represented among placental cells (31) and thus perhaps mechanisms related to the exclusion of T cells from placental tissue may be of importance regarding the pregnant-state acceptance of the fetal allograft.
One might ask about the suitability of our mitogen choice in assessing whether a Th2 bias occurs during pregnancy to aid in preventing fetal rejection. In support of our choice, we note the study by Bevan et al. (19) who demonstrated that stimulation with Con A (in contrast to PHA) induced proliferation preferentially in allogeneic-reactive CTLs. Since Con A also induced proliferation of noncytotoxic T cells, later shown to be CD4 cells (20), our choice of this mitogen appears especially appropriate. In addition, stimulation with the two allergens shown in Tables VI and VII similarly demonstrated a lack of a general Th2 bias in pregnancy. It was noted, though, that allergen stimulation revealed an increase in IL-4 responses in mothers prepartum compared with postpartum, but this was limited to skin test-negative mothers and was without effect on IL-13 responses. It was noted, though, that allergen stimulation revealed an increase in IL-4 responses in mothers prepartum compared with postpartum, but this was limited to skin test-negative mothers and was without effect on IL-13 responses.
No Th2 bias among infants at birth or at 3 mo was evident for IL-4, IFN-γ, or the IFN-γ:IL-4 ratio. The production of IL-4 and IL-5 at birth was below the level of detectability of the assays for a large proportion of the newborns. The median values of IL-4 and IFN-γ produced at birth were both ~10-fold lower than for the parents and the mean IFN-γ:IL-4 ratio at 3 mo was comparable to that of the parents, findings that argue against a general Th2 bias in the newborn or young infant. In contrast, a differential developmental pattern of IL-13 vs IL-4, IL-5, and IFN-γ production was evident for this early period of life. Indeed, although IFN-γ production was at least 10-fold lower than that of adults, IL-13 production was only approximately one-half that of the adults, yielding an IFN-γ:IL-13 ratio of the newborn that supports an early life cytokine production bias but one that is limited to the context of IL-13. Allergen stimulations revealed that IL-13 (but not IL4 or IFN-γ) responses at birth were more frequent than the responses at 3 mo for both allergens tested and at least equivalent to parental levels, further supporting IL-13-specific (but not a Th2 general) increased production at birth. Our data do not provide information about specific cell sources of cytokines. However, Jung et al. (32) demonstrated that at least among PBMCs in adults, the CD45RA+ (naive) T cells are capable of producing a large amount of IL-13 but not IL-4. Given that cord blood has been shown to have a much higher proportion of CD45RA+ cells compared with adults, (33), this might well account for the differences in relative production for IL-4 and IL-13 between infants and adults and is deserving of further study. Whether the allergen-induced responses at birth have relations to subsequent allergic phenotypes remains to be determined.
The close correlations found for cytokine production included IL-10. Although once regarded as a Th2 cytokine, IL-10 is known to be produced by monocytes and T regulatory cells and to have the capacity to inhibit cytokine production from other T cells and monocytes (34). Because of this inhibitory capacity of IL-10 and the recent report of increases in T regulatory cells in pregnant women (35), we had predicted that an inverse relation might be evident between IL-10 and the adaptive cytokines. However, our data instead demonstrate very strong direct relations between IL-10 and the other adaptive immune cytokines. The lack of any apparent inhibitory action may be explained in part by the reported temporal delay of IL-10 production compared with the other cytokines following stimulation (36). Also, MacNeil et al. (37) demonstrated a T cell antiapoptotic activity of IL-10. Whether such activity might contribute to the direct relations among the cytokine levels within individuals requires further study.
Our study provides what we believe to be the first demonstration of both mother-child and father-child influences on cytokine production. Although parental influences were not evident for cytokine production of the infant at birth, significant direct relations were found for the 3-mo samples. The correlations included mother-infant IFN-γ and IL-13 production and father-infant IFN-γ production. Thus, our study confirms the report by Larsson et al. (16), who identified correlation among the percentage of PBMCs producing IFN-γ, IL-4, and IL-10 in response to PHA stimulation between mother and child and extends these findings to show father-infant correlation. The intriguing possibility that the parent-child correlations have at least in part an environmental basis is raised as a result of the quite extensive correlation of cytokine production between mother and father. Our data do not point to the pathway(s) responsible but they do suggest that the appropriate controls for identifying differences in cytokine production related to a physiologic condition such as pregnancy are either the same subjects when not in the physiologic condition (i.e., in our study when nonpregnant) or individuals sharing the home environment (i.e., in our study the fathers) or both. Given that only 10 of the ~340 fathers were reported as not living in the same household, it was not possible to stratify our data on this basis.
In sum, this large population study of mitogen-induced cytokine production from stimulated mononuclear cells in parents and infants demonstrates very strong direct correlations among the cytokines produced by circulating immune cells that are suggestive of regulatory mechanisms maintaining a relative balance among Th1, Th2, and IL-10 cytokines and that are evident at different stages of life. Although a general Th2 bias in pregnancy is not evident in our study, prepartum vs postpartum samples nevertheless demonstrate a modestly reduced IFN-γ:IL-5 ratio to mitogen and increased IL-4 to allergen. In contrast, although IL-4 and IL-5 are not produced in detectable amounts in many newborns and IFN-γ production is produced in the greatest amounts (although diminished compared with adults), the capacity to produce IL-13, the ratios of IFN-γ:IL-13, and substantial IL-13 responses to allergen stimulation demonstrate a cytokine bias for the newborn that is restricted to IL-13. Intrafamily correlations that include parent-parent as well as parent-child correlations are evident at 3 mo after birth and suggest that a shared environment can influence cytokine production.
Supplementary Material
Acknowledgments
We are grateful to Heidi Erickson, RN, for subject enrollment and follow-up contacts and sample collection and to Susan Solomon, Stefania Scott, and David Spies for creating and maintaining the ARC data warehouse.
Footnotes
This work was funded in part by National Institutes of Health Grants AI 42268, AI 61811, and HL67672.
Abbreviations used in this paper: CBMC, cord blood mononuclear cell; LSM, lymphocyte separation medium.
The online version of this article contains supplemental material
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wegmann TG, Hin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 2.Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–458. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- 3.Raghupathy R, Makhseed M, Azizich F, Omu A, Gupta M, Farhat R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod. 2000;15:713–718. doi: 10.1093/humrep/15.3.713. [DOI] [PubMed] [Google Scholar]
- 4.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker AV. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem Biophys Res Commun. 1998;245:933–938. doi: 10.1006/bbrc.1998.8549. [DOI] [PubMed] [Google Scholar]
- 6.Saito S, Sakai Y, Sasaki K, Tanebe H, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0. Th1, Th2 and Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–555. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keski-Nisula L, Hirvonen MR, Roponen M, Heinonen S, Pekkanen J. Maternal and neonatal IL-4 and IFN-γ production at delivery and 3 months after birth. J Reprod Immunol. 2003;60:25–33. doi: 10.1016/s0165-0378(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 8.Rastogi D, Wang C, Lendor C, Rothman PB, Miller RL. T-helper type 2 polarization among asthmatics during and following pregnancy. Clin Exp Allergy. 2006;36:892–898. doi: 10.1111/j.1365-2222.2006.02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Gao Q, Field EH. Expansion of memory Th2 cells over Th1 cells in neonatal primed mice. Transplantation. 1999;60:1187–1193. [PubMed] [Google Scholar]
- 10.Vigano A, Esposito S, Arienti D, Zagliani A, Massironi E, Principi N, Clerici M. Differential development of type 1 and type 2 cytokines and β-chemokines in the ontogeny of healthy newborns. Biol Neonate. 1999;75:1–8. doi: 10.1159/000014071. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CB, Lewis DB. Basis and implications of selectively diminished cytokine production in neonatal susceptibility to infection. Rev Infect Dis. 1990;12:S410–S420. doi: 10.1093/clinids/12.supplement_4.s410. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CB, Penix L, Weaver WM, Melvin A, Lewis DB. Ontogeny of T lymphocyte function in the neonate. Am J Reprod Immunol. 1992;28:132–135. doi: 10.1111/j.1600-0897.1992.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 13.Hall AJ, Peckham CS. Infections in childhood and pregnancy as a cause of adult disease: methods and examples. Br Med Bull. 1997;53:10–23. doi: 10.1093/oxfordjournals.bmb.a011593. [DOI] [PubMed] [Google Scholar]
- 14.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25:S31–S35. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell NC, Davies PL, Kotecha S. Antenatal infection and inflammation: what’s new? Curr Opin Infect Dis. 2006;19:253–258. doi: 10.1097/01.qco.0000224819.42729.2e. [DOI] [PubMed] [Google Scholar]
- 16.Larsson AK, Nilsson C, Hoglind A, Sverremark-Ekstrom E, Lilja G, Troye-Blomberg M. Relationship between maternal and child cytokine responses to allergen and phytohaemagglutinin 2 years after delivery. Clin Exp Immunol. 2006;144:401–408. doi: 10.1111/j.1365-2249.2006.03083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landesburg R, Fallon M, Insel R. Alterations in helper-induced and suppressor-induced T-cell subsets in human neonatal blood. Immunology. 1988;65:323–325. [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers IMH, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 19.Bevan MJ, Langman RE, Cohn M. H-2 antigen-specific cytotoxic T cells induced by concanavalin A: estimation of their relative frequency. Eur J Immunol. 1976;6:150–156. doi: 10.1002/eji.1830060303. [DOI] [PubMed] [Google Scholar]
- 20.Adriaansen HJ, Osman C, Van Dongen JJ, Wijdenes-de Bresser JH, Kappetijn-van Tilborg CM, Hooijkaas H. Immunological marker analysis of mitogen-induced proliferating lymphocytes using BrdU incorporation or screening of metaphases: staphylococcal protein A is a potent mitogen for CD4+ lymphocytes. Scand J Immunol. 1990;32:687–694. doi: 10.1111/j.1365-3083.1990.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 21.Chipeta J, Komada Y, Zhang XL, Deguchi T, Sugiyama K, Azuma E, Sakurai M. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type1 cell populations with age. Cell, Immunol. 1998;183:149–156. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 22.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyere M. Development and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13:215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SBA, Parry SL, Feldmann M, Foxwell B. Autocrine and paracrine regulation of human T cell IL-10 production. J Immunol. 1997;158:5596–5602. [PubMed] [Google Scholar]
- 24.Hartel C, Adam N, Strunk T, Temming P, Muller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrielsson S, Soderlund A, Nilsson C, Lilja G, Nordlung M, Troye-Blomberg M. Influence of atopic heredity on IL-4-, IL-12- and IFN-γ-producing cells in in vitro activated cord blood mononuclear cells. Clin Exp Immunol. 2001;126:390–396. doi: 10.1046/j.1365-2249.2001.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaub B, Tantisira KG, Gibbons FK, He H, Litonjua AA, Gillman MW, Weiss S, Perkins DL, Gold DR, Finn PW. Fetal cord blood: Aspects of heightened immune responses. J Clin Immunol. 2005;25:329–227. doi: 10.1007/s10875-005-4180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Campbell HD, Young IG. Sex hormones and dexamethasone modulate interleukin-5 gene expression in T lymphocytes. J Steroid Biochem Mol Biol. 1993;44:203–210. doi: 10.1016/0960-0760(93)90080-g. [DOI] [PubMed] [Google Scholar]
- 28.Schwenger GTF, Fournier R, Kok CC, Mordvinov VA, Yeoman D, Sanderson CJ. GATA-3 has dual regulatory functions in human interleukin-5 transcription. J Biol Chem. 2001;276:48502–48509. doi: 10.1074/jbc.M107836200. [DOI] [PubMed] [Google Scholar]
- 29.Han S, Lu J, Zhang Y, Cheng C, Han L, Wang X, Li L, Liu C, Huang B. Recruitment of histone deacetylase 4 by transcription factors represses interleukin-5 transcription. Biochem J. 2006;400:439–448. doi: 10.1042/BJ20061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DH, Yang L, Ray A. Cutting edge: differential responsiveness of the IL-5 and IL-5 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–3821. [PubMed] [Google Scholar]
- 31.Starkey PM, Sargent IL, Redman CWG. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988;65:129–134. [PMC free article] [PubMed] [Google Scholar]
- 32.Jung T, Wijdenes J, Neumann C, de Vries JE, Yssel H. Interleukin-13 is produced by activated human CD45RA+ and CD45RO+ T cells: modulation by interleukin-4 and interleukin-12. Eur J Immunol. 1996;26:571–577. doi: 10.1002/eji.1830260311. [DOI] [PubMed] [Google Scholar]
- 33.Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyere M. Development and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13:215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 34.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHugh S, Deighton J, Rifkin I, Ewan P. Kinetics and functional implications of Th1 and Th2 cytokine production following activation of peripheral blood mononuclear cells in primary culture. Eur J Immunol. 1996;26:1260–1265. doi: 10.1002/eji.1830260612. [DOI] [PubMed] [Google Scholar]
- 37.MacNeil IA, Suda T, Moore K, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


