Abstract
Objective
The present study examined differences in neurocognitive outcomes among non-Hispanic Black and White stroke survivors utilizing the NIH Toolbox-Cognition Battery (NIHTB-CB), and investigated the roles of healthcare variables in explaining racial differences in neurocognitive outcomes post-stroke.
Method
One-hundred-seventy adults (91 Black; 79 White), who participated in a multisite study were included (Age: M=56.4, SD=12.6; Education: M=13.7, SD=2.5; 50% male; Years post-stroke: 1–18; Stroke type: 72% ischemic, 28% hemorrhagic). Neurocognitive function was assessed with the NIHTB-CB, using demographically-corrected norms. Participants completed measures of socio-demographic characteristics, health literacy, and healthcare use and access. Stroke severity was assessed with the modified Rankin Scale.
Results
An independent samples t-test indicated Blacks showed more neurocognitive impairment (NIHTB-CB Fluid Composite T-score: M=37.63 SD=11.67) than Whites (Fluid T-score: M=42.59, SD=11.54; p=.006). This difference remained significant after adjusting for reading level (NIHTB-CB Oral Reading), and when stratified by stroke severity. Blacks also scored lower on health literacy, reported differences in insurance type, and reported decreased confidence in the doctors treating them. Multivariable models adjusting for reading level and injury severity showed that health literacy and insurance type were statistically significant predictors of the Fluid cognitive composite (p<.001 and p=.02, respectively) and significantly mediated racial differences on neurocognitive impairment.
Conclusion
We replicated prior work showing that Blacks are at increased risk for poorer neurocognitive outcomes post-stroke than Whites. Health literacy and insurance type might be important modifiable factors influencing these differences.
Keywords: cerebrovascular accident, insurance coverage, cognition, healthcare disparities, health literacy, African Americans
Stroke is one of the leading causes of long-term disability and death in the United States (Mozaffarian et al., 2016). Every year, approximately 795,000 people in the United States suffer strokes (Mozaffarian et al., 2016), and by 2050, the total number of incident strokes is expected to increase more than 2-fold compared to 2010 (Howard & Goff, 2012). This projected increase is due primarily to the increased older adult population size and diversity within the older adult population, as “baby boomers” and ethnic/racial minorities, which once comprised a large proportion of the younger population, are reaching stroke-prone ages (i.e., 45 years and older).
Blacks/African-Americans are the second largest racial/ethnic minority group in the United States, representing 13.2 % of the total population (Colby & Ortman, 2015), and are disproportionally affected by stroke. Blacks are approximately twice as likely as non-Hispanic (NH) Whites to experience a first-ever ischemic (White et al., 2005) or hemorrhagic stroke (Kleindorfer et al., 2010), as well as recurrent strokes (Sheinart et al., 1998). Compared to NH Whites, Blacks also reportedly have more severe strokes (Jones et al., 2000; Stansbury, Jia, Williams, Vogel & Duncan, 2005), worse functional recovery during hospitalization due to stroke as assessed by Functional Independence Measures (FIM) scores (Ottenbacher et al., 2008), and greater rates of mortality across all stroke sub-types (Ayala et al., 2001).
Stroke and stroke symptoms are associated with neurocognitive impairment (NCI; Desmond, 2000, 2002; Levine, 2015; Tatemichi, 1993, 1994; Wadley et al., 2007). However, specific rates of NCI among stroke survivors vary widely depending on a variety of factors, including the methods used to evaluate neurocognitive functioning, test score cutoffs used to indicate NCI, as well as the severity of stroke, the presence of vascular risk factors, and the age and education level of stroke survivors, among others (Sun, Lan, & Yu, 2014). For example, Wadley et al. (2007) found that older age, male gender, Black race, and less than a high school education were all associated with impaired neurocognitive status. In addition, among Blacks, the risk of developing incident cognitive impairment has also been associated with reports of stroke symptoms and transient ischemic attacks (Kelley et al., 2013). While NCI post-stroke is frequently mild (Sun et al., 2014; Tatemichi, 1994), even its milder forms have been linked to significant everyday cognitive functioning difficulties, including problems with medical decision making, financial management, and other activities of daily living (e.g., shopping, housework, driving, using public transportation) (Aretouli & Brandt, 2010; Baum et al., 2008; Edwards, Hahn, Baum, & Dromerick, 2006; Pereira et al., 2010; Schmitter-Edgecombe & Parsey, 2014; Stephens et al., 2005). Problems in everyday functioning can compromise the independence, safety, and quality of life of stroke survivors as well as increase caregiver burden and community expenses (Aretouli & Brandt, 2010).
Even though NCI post stroke is common and impactful, and despite the disproportionate impact of stroke among Blacks, few studies have investigated racial differences in NCI among stroke survivors. The existing literature suggests that Blacks might be at increased risk for NCI (Patel, Coshall, Rudd, & Wolfe, 2002), reduced cognitive recovery (Newman, Bang, Hussain, & Toole, 2007) and increased incident dementia (Desmond et al., 2002) following stroke. In general, most of these studies have consisted of relatively large sample sizes, including large subgroups of Blacks. Cross-sectional studies examining neurocognition 3 months post stroke found increased NCI (defined as Mini-Mental State Examination [MMSE]< 24) after first ever stroke (Patel et al., 2002) and increased dementia (determined via clinical review based on a comprehensive neurocognitive battery and assessment of everyday functioning; Desmond et al., 2000) among Black stroke survivors compared to their NH White counterparts. A longitudinal study that examined neurocognition within 3 months of stroke and at annual follow-up assessments found significantly less neurocognitive recovery over 2 years (Newman et al., 2007) among Blacks compared to NH Whites. Another longitudinal study found 47% of Blacks and 25% of Whites developed dementia over the course of the study (median follow-up=21.1 months) (Desmond et al., 2002). While this racial difference was not significant in the multivariable models reported, the study was not focused on examining racial differences, and thus statistical models were not specifically constructed to address this question.
Overall, findings from these prior studies indicating worse outcomes post-stroke among Blacks highlight the importance of understanding the underlying mechanisms of racial differences. Neurocognitive studies to date, however, have used a single, screening measure of cognition (i.e., MMSE) or examined for dementia. Thus, they are unlikely to capture the milder, more prevalent forms of NCI. In addition, the MMSE has been found to be a suboptimal measure of NCI post-stroke (Nys et al., 2005), and most prior studies lack adequate normative data or estimates of pre-morbid functioning, which are necessary to disentangle the impact of potential pre-stroke neurocognitive functioning. Importantly, none of the identified studies focused on determining factors that might help “explain” racial differences in NCI post stroke. Identifying these factors would be a major step towards the development and implementation of interventions aimed at reducing racial disparities post-stroke.
Differences in stroke characteristics, cardiovascular risk factors and premorbid functioning are likely to play a role in racial differences on NCI post-stroke. Additionally, poor healthcare, including suboptimal access, use and quality of health services, as well as worse health literacy, are potentially modifiable variables, which might exacerbate racial differences post-stroke. Previous research suggests that these healthcare variables are associated with differences in a number of health outcomes, and are important contributors to racial health disparities (Fiscella, Franks, Gold, & Clancy, 2000; Paasche-Orlow et al., 2005; Waidmann & Rajan, 2000). Racial disparities in health literacy are well-documented (Kutner, Greenburg, Jin & Paulsen, 2006), and poor health literacy has been associated with worse health outcomes, including cognitive functioning among older adults (Federman, Sano, Wolf, Siu, & Halm, 2009; Kobayashi, Wardle, Wolf & Wagner, 2015). Yet the role that health literacy might play in explaining racial disparities in NCI post-stroke has not been investigated. While the mechanisms linking health literacy and poor cognitive function are likely to be varied and bidirectional, among persons with stroke, worse health literacy might lead to worse healthcare practices pre-stroke and post-stroke, which in turn might impact neurocognitive recovery.
The objectives of the current study were twofold: 1) to examine post-stroke differences in neurocognitive outcomes among stroke survivors who identify as either NH Black or NH White, utilizing the NIH Toolbox-Cognition Battery (NIHTB-CB), which has extensive demographically-adjusted normative data (including for race/ethnicity), includes estimates of premorbid functioning/quality of education, and allows for identification of milder deficits in cognitive domains and overall cognition; and 2) to investigate the potential roles of healthcare variables (i.e., health literacy, and healthcare access, use, and quality) in explaining any racial differences in neurocognitive outcomes post-stroke. We hypothesized that NH Blacks would show worse neurocognitive functioning than NH Whites, even after adjusting for stroke severity and estimates of premorbid functioning. Further, we hypothesized that this difference in neurocognitive outcomes would be at least partly mediated by lower healthcare use and access, and lower health literacy among NH Blacks.
Methods
Participants
Participants included in this analysis include 91 NH Black and 79 NH White adults with stroke who were enrolled in a multi-site study of medical rehabilitation outcomes among stroke, traumatic brain injury, and traumatic spinal cord injury survivors. Participants completed a comprehensive, two-day assessment at two academic medical centers and one free-standing rehabilitation hospital in the Midwestern United States (located in St. Louis, Chicago, Ann Arbor). Participants were recruited via community outreach networks, registries, and flyers. Inclusion criteria for the multi-site study included medically documented diagnosis of stroke at least one year prior to enrollment; being 18 to 85 years of age; and being able to comprehend (5th grade reading level) and speak English. Stroke survivors with significant aphasia as assessed by The Frenchay Aphasia Screening Test (Enderby, Wood, Wade, & Hewer, 1987) were excluded. Eligibility for the current analysis required having a stroke diagnosis; self-identifying as NH Black or NH White; and having neurocognitive data available. All participants provided informed consent in compliance with the human subjects research institutional review boards at collaborating sites. Participants received an honorarium to acknowledge their contribution to the research.
Materials and Procedures
Subjects completed comprehensive neurocognitive, stroke, and psychosocial assessments.
Neurocognitive Assessment
Neurocognitive function was measured using the NIHTB-CB. The details of the battery have been described elsewhere (Casaletto et al., 2015; Heaton et al 2014; Weintraub et al., 2013). In short, the battery includes five tests of “fluid cognition” assessing multiple cognitive domains that are vulnerable to acquired brain dysfunction (i.e., Picture Sequence Memory Task = episodic memory, Dimensional Change Card Sort Task = executive function/flexibility, Pattern Comparison Task = processing speed, Flanker Inhibitory Control and Attention Task = executive function/inhibitory control, and List Sorting Task = working memory). In addition, the battery includes two tests of “crystallized cognition” that are less sensitive to acquired brain dysfunction and reflect past learning experiences (Oral Reading Recognition and Picture Vocabulary). The NIHTB-CB has extensive population-based normative data, and demographically adjusted T-scores (TS), which correct for age, gender, education, language, and race/ethnicity (Casaletto et al., 2015). We used demographically-adjusted Fluid Composite TS as our measure of global neurocognitive functioning or post-stroke functioning. This composite includes scores from all fluid tests, which are the most likely to be impacted by neurological conditions, such as stroke (Akshoomoff et al., 2013; Heaton et al., 2014). Additionally, we used the Oral Reading Recognition TS as an indicator of reading level and estimate of premorbid functioning/quality of education (Weintraub et al., 2013).
Stroke characteristics
Stroke severity was classified with the Functional Independence Measure (Keith, Granger, Hamilton, & Sherwin, 1987) and the NIH Stroke Scale (NIHSS) (Brott et al., 1989), and converted to the Modified Rankin Scale (MRS) to standardize classifications across measures. Strokes were classified as mild (scores of 1–2), moderate (scores of 3), or severe (scores of 4). Information on type of stroke (hemorrhagic/ischemic), post-stroke weakness, age at time of stroke, and family history of stroke was obtained via available medical records and/or self-report (see Table 1). Additionally, data on side of stroke (left or right cerebral hemisphere) and history of prior stroke was collected only at one of the sites (i.e., Washington University) via available medical records.
Table 1.
Socio-Demographic, Stroke and Psychiatric Characteristics of the Study Cohort by Racial Group
| Black (n=91) | White (n=79) | pa | |
|---|---|---|---|
| Socio-Demographic Characteristics | |||
| Age (years), M(SD) | 55.16 (11.18) | 57.73 (13.97) | .19 |
| Gender, % Male | 59.49 | 41.76 | .02 |
| Education, M(SD) | 13.03 (2.30) | 14.44 (2.58) | <.001 |
| NIH TB- CB Oral Reading T, M(SD) | 47.20 (10.03) | 50.65 (11.55) | .04 |
| Handedness, % Right | 85.71 | 81.01 | .41 |
| Marital status % | .007 | ||
| Married/Unmarried couple | 24.72 | 50.00 | |
| Other | |||
| Separated | 8.99 | 1.39 | |
| Widowed | 10.11 | 9.72 | |
| Divorced | 22.47 | 20.83 | |
| Never been married | 33.71 | 18.06 | |
| Personal incomeb % | .11 | ||
| $0 –$14,999 | 47.89 | 39.44 | |
| $15,000 – $34,999 | 35.21 | 29.58 | |
| $35,000 - $74,999 | 11.27 | 16.90 | |
| $75,000 – or more | 5.63 | 14.08 | |
| Study Site | <.0001 | ||
| Rehabilitation Institute of Chicago, n(%) | 32 (72.72) | 12 (27.27) | |
| University of Michigan, n(%) | 1 (7.67) | 14 (93.33) | |
| Washington University, n(%) | 58 (52.73) | 52 (47.27) | |
| Stroke Characteristics | |||
| Years since injury M(SD) | 2.78 (2.02) | 2.60 (2.44) | .61 |
| Age at injury M(SD) | 51.73 (11.25) | 53.96 (14.47) | .27 |
| Family history of stroke, % Yes | 52.33 | 45.33 | .33 |
| Stroke severityc % | .29 | ||
| Mild | 29.67 | 35.44 | |
| Moderate | 23.08 | 29.11 | |
| Severe | 47.25 | 35.44 | |
| Type of Stroke % | .54 | ||
| Ischemic | 74.16 | 69.86 | |
| Hemorrhagic | 25.84 | 30.14 | |
| Weakness % | .33 | ||
| No weakness | 14.44 | 21.33 | |
| Left | 43.33 | 30.67 | |
| Right | 38.89 | 42.67 | |
| Both | 3.33 | 5.33 | |
| Emotional Characteristicsd | |||
| Psychological Well-being M(SD) | 44.76 (10.29) | 46.12 (12.40) | .45 |
| Negative Affect M(SD) | 52.53 (11.38) | 50.08 (11.02) | .16 |
| Social Satisfaction M(SD) | 45.70 (12.60) | 47.26 (12.43) | .43 |
Note.
Results from independent sample t-tests and Chi-Square tests;
Black: n=71; White: n=71; 19 participants (12 NH Black and 7 NH White) chose not to provide information on their income and 9 participants (8 NH Black and 1 NH White) did not know their income;
based on modified Rankin Scale;
based on NIH Toolbox Emotion Battery.
Cardiovascular Risk
Data on cardiovascular risk factors (i.e., diabetes, hypertension, history of smoking) at the time of hospitalization for stroke was collected for the participants recruited at Washington University (54 NH Black and 44 NH White), and was ascertained via self-report and review of available medical records.
Socio-demographic characteristics
Socio-demographic data, including age, gender, education, race/ethnicity, handedness, marital status, and income, were ascertained via self-report. Race/ethnicity was ascertained following NIH guidelines and consistent with the US Census Bureau and National Institutes of Health methodology (Evans, 2015).
Health Literacy
Health literacy was measured with the short form version of the Talking Touchscreen Technology (Health LiTT) assessment (Hahn, Choi, Griffith, Yost, & Baker, 2011; Yost et al., 2010). Subjects respond to 3 types of health related multiple-choice questions: prose (reading comprehension), document (identify and interpret information presented in charts, graphs, or tables), and quantitative (perform arithmetic operations). This assessment is in a self-administered, multimedia, multiple-choice format with one correct response. The health LiTT assessment has acceptable internal consistency (Cronbach’s alpha = 0.73) and scores on the assessment have adequate convergent validity with scores on other commonly used measures of health literacy (i.e., Rapid Estimate of Adult Literacy in Medicine, Test of Functional Health Literacy in Adults, and Newest Vital Sign) (Davis et al., 1991; Parker, Baker, Williams, & Nurss, 1995; Weiss et al., 2005).
Healthcare Access, Use and Quality
Healthcare data, including items regarding healthcare access, use, and quality, were ascertained via self-report. The items comprising the healthcare assessments were informed by the Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey item content (Dyer, Sorra, Smith, Cleary, & Hays, 2012; Hays et al., 1999; Lee Hargraves, Hayes, & Cleary, 2003). In the current study a limited number of items were included; thus, it is not fully representative of the CAHPS survey. Please refer to Table 2 for specific items and response options. Items assessing healthcare access examined content such as the type of insurance benefits held by participants, the degree to which participants felt they had a choice in selecting their medical care and whether participants neglected to seek care for a medical problem or needed care but could not afford it in the year prior to enrollment.
Table 2.
Healthcare Characteristics of the Study Cohort by Racial Group
| Black (n=91) |
White (n=79) |
pa | ||||
|---|---|---|---|---|---|---|
| Health Literacy | ||||||
| HealthLiTT T, M(SD) | 51.10 (8.49) | 59.59 (6.49) | <.001 | |||
| Healthcare Access | ||||||
| Current Insurance Benefits | <.001 | |||||
| SSI, SDI, or SS | 29.21 | 24.05 | ||||
| Medicaid | 34.83 | 11.39 | ||||
| Medicare | 16.85 | 31.64 | ||||
| Services from independent living center | 0.00 | 1.27 | ||||
| Other (including private) | 19.10 | 31.65 | ||||
| Degree of choice of medical care | .66 | |||||
| A great deal of choice | 64.04 | 68.83 | ||||
| Some choice | 22.47 | 16.88 | ||||
| Very little choice | 13.48 | 14.29 | ||||
| Did not seek care for medical problem (Past 12 months) | 17.98 | 28.57 | .11 | |||
| Needed care but couldn’t afford it (Past 12 month) | 47.19 | 35.06 | .11 | |||
| Healthcare Use | ||||||
| Time since last contact with doctor | .12 | |||||
| Less than 6 months | 96.59 | 89.61 | ||||
| 6 months - 2years | 3.41 | 10.39 | ||||
| Visit to doctor/clinic/ER (Past 12 month, Yes) | 98.88 | 94.81 | .18 | |||
| Has regular source of care (Yes) | 98.88 | 96.10 | .34 | |||
| Type of care | <.001 | |||||
| Private clinic | 49.44 | 81.82 | ||||
| Public clinic | 29.21 | 11.69 | ||||
| Emergency room | 3.37 | 2.60 | ||||
| Hospital outpatient department | 16.85 | 2.60 | ||||
| No regular | 0.00 | 1.30 | ||||
| Other | 1.12 | 0.00 | ||||
| Quality of Healthcare (Last doctor’s visit)b | ||||||
| Doctor listened to | .33 | |||||
| Everything you said | 87.64 | 80.52 | ||||
| Most of what you said | 11.25 | 15.58 | ||||
| Some of what you said | 1.12 | 3.90 | ||||
| Patient understood | .49 | |||||
| Some of what was said | 2.25 | 3.90 | ||||
| Most of what was said | 19.10 | 12.99 | ||||
| Everything that was said | 78.65 | 83.12 | ||||
| Patient had questions to discuss, but didn’t | 16.85 | 11.69 | .34 | |||
| Great deal of confidence in doctor (Yes) | 65.17 | 79.22 | .04 | |||
| Treated with great deal of respect and dignity (Yes) | 88.76 | 92.21 | .45 | |||
| Doctor involved you in decisions affecting your health | .22 | |||||
| Less than wanted | 8.99 | 3.90 | ||||
| Almost as much as wanted or more | 91.01 | 96.10 | ||||
| Time spent with doctor | .17 | |||||
| Less than wanted | 12.36 | 5.19 | ||||
| Almost as much as wanted or more | 87.64 | 94.81 | ||||
| Satisfaction with Healthcare (Past 2 years) | .07 | |||||
| Very dissatisfied | 2.25 | 5.19 | ||||
| Somewhat dissatisfied | 5.62 | 3.90 | ||||
| Somewhat satisfied | 31.46 | 15.58 | ||||
| Very satisfied | 60.67 | 75.32 |
Note. Values represent % unless otherwise noted.
Results from independent sample t-tests and Chi-Square tests (or Fisher’s Exact).
Quality of healthcare items concern the last doctor’s visit (i.e., “Last time you saw a doctor”). ER = Emergency room. SDI = State Disability. SS = Social Security. SSI = Supplemental Security Income.
Participants were provided six response options to the type of insurance benefits item: 1) Supplemental Security Income (SSI), State Disability Insurance (SDI), or Social Security (SS); 2) Medicaid; 3) Medicare; 4) services from an independent living center; 5) other; and 6) I prefer not to answer. We consolidated the latter two options into one category that we termed “Other (including private)” because of the type of response options and the low numbers of responses in the last category (n=2). Items examining healthcare use assessed participants’ use of healthcare, including how long it had been since their last doctor visit, whether they had seen a doctor in the 12 months prior to assessment, whether they had a regular source of care, and if so, the type of facility from which they receive care. Healthcare quality items assessed participants’ perceptions of healthcare quality indicators from their last doctor’s visit, including how much the doctor listened to them, how much they understood what the doctor expressed, whether they had questions they refrained from asking, the degree of confidence they had in their doctor, their satisfaction with the degree of involvement they had in decisions pertaining to their health, and their satisfaction with the amount of time the doctor spent with them.
NIH Toolbox Emotion Battery
Emotional functioning was assessed with the Toolbox Emotion Battery. The emotion battery was created to develop a set of measures that is sensitive to changes in health status and function over time (Salsman et al., 2013). Through self-report surveys, the battery assesses multiple emotional domains: with factor analysis-derived composites of psychological well-being, negative affect, and social satisfaction (Babakhanyan, Casaletto, & Heaton, 2015; Babakhanyan, McKenna, Casaletto, & Heaton, 2016; Salsman et al., 2013).
Statistical Analyses
We contrasted groups on socio-demographic, stroke, psychiatric and healthcare characteristics via independent sample t-tests for continuous variables and Chi-Square tests (or Fisher’s exact) for categorical data. To examine racial differences in neurocognitive function post-stroke, we first ran a series of independent sample t-tests by race on demographically corrected (including for race/ethnicity differences in normals) Fluid Composite T-scores and for each of the individual tests comprising this composite score. To investigate whether any racial differences in neurocognitive function might be accounted for by differences in other socio-demographic, stroke characteristics or emotional functioning, we examined the effects of potential covariates by running a multivariable linear regression model on Fluid T-scores with race (NH Black/White) as a predictor and adjusting for covariates that differed significantly between groups (listed in Table 1) and were associated with Fluid T-scores at p<.05. Given that Fluid T-scores already are adjusted for age, gender, and education, as well as race/ethnicity, these variables were not included as covariates.
In order to investigate the impact of healthcare characteristics (health literacy, healthcare use and access, quality of healthcare and satisfaction with healthcare) we first ran separate multivariable regression models on Fluid T-scores including race, and each of the healthcare variables that differed between groups, and adjusting for significant covariates. For the healthcare variables that were significantly associated with Fluid T-scores in these analyses (i.e. health literacy and insurance type), we investigated whether they might help explain or mediate racial differences in neurocognitive function. In order to do so, we conducted separate path analyses for health literacy and insurance type, while adjusting for significant covariates. The path analysis for health literacy also considered the effect of insurances status, and vice versa. We used estimation methods described by Preacher and Hayes for continuous mediators (i.e., health literacy) and causal mediation analyses by Imai et al. for binary mediators (i.e., insurance type; Imai, Keele & Tingley, 2010; Preacher & Hayes, 2004; Tingley, Yamamoto, Hirose, Keele & Imai, 2014). The first approach estimated the path coefficients in a mediator model and generated bootstrap confidence intervals (for bootstrapping, 1000 samples were requested) for total and specific indirect effects of race on neurocognitive function through health literacy, adjusting for insurance type, cognitive reserve (Oral Reading) and stroke severity. The latter approach used bootstrap confidence intervals (1000 samples) for the total, direct, and specific indirect effects of race on neurocognitive function through insurance type, adjusting for health literacy, cognitive reserve, and stroke severity.
Results
Socio-demographic, Assessment Location, Stroke and Psychosocial Characteristics of the Study Cohort by Racial Group
Table 1 shows socio-demographic, assessment location, stroke and psychosocial/emotion characteristics of the study cohort by racial group. There were significant differences in the racial composition of groups recruited across sites. While the sample collected at Washington University in St. Louis was nearly half NH Black and half NH White, only a quarter of the participants recruited at the Rehabilitation Institute of Chicago were NH Whites and virtually all participants from the University of Michigan were NH Whites. NH Blacks were more likely than NH Whites to be male, unmarried, have fewer years of education, and lower reading level (estimated by Oral Reading T-score). Racial groups were comparable in age and handedness. There were no significant differences in stroke or psychosocial characteristics between racial groups: both groups primarily had ischemic strokes and on average reported mildly reduced psychological well-being and social satisfaction. Results on a subset of participants (NH Blacks: n=54; NH Whites: n=44) with available data showed that NH Blacks were more likely to report a history of hypertension (Black=81%, White=57%; p<.01) and smoking (NH Black=57%, NH White=20%; p<.001) than NH Whites. NH Blacks were less likely to report a history of previous transient ischemic attack than NH Whites (TIA; NH Black=4%, NH White=18%; p=.04), but there were no racial differences on reported prior history of stroke (NH Black=30%, NH White=32%; p=.82).
Neurocognitive Performance by Racial Group
An independent samples t-test indicated NH Blacks demonstrated lower neurocognitive performance (NIHTB-CB Fluid Composite T-scores: M=37.63 SD=11.67) than NH Whites (Fluid TS: M=42.59, SD=11.54; p=.006; Cohen’s d=0.43) (Table 3). Further independent sample t-tests examining neurocognitive outcomes on specific NIHTB-CB tasks revealed that NH Blacks showed lower neurocognitive scores than NH Whites on the Dimensional Change Card Sorting (Cohen’s d=0.34), Flanker Inhibitory Control and Attention (Cohen’s d=0.42), and List Sorting Working Memory tasks (Cohen’s d=0.38) (Table 3). In addition, there was a trend towards significance for the Picture Sequence Memory task, wherein NH Blacks showed lower neurocognitive scores than NH Whites (Cohen’s d=0.30).
Table 3.
Neurocognitive Performance by Racial Group
| Black (n=91) M (SD) |
White (n=79) M (SD) |
pa | Cohen’s d | % Impaired
|
||
|---|---|---|---|---|---|---|
| Black | White | |||||
| Fluid Composite T | 37.63 (11.67) | 42.59 (11.54) | <.01 | .43 | 64% | 39% |
| Dimensional Change Card Sort Task T | 40.34 (9.25) | 43.60 (10.13) | .03 | .34 | 52% | 32% |
| Picture Sequence Memory Task T | 41.25 (10.80) | 44.72 (12.21) | .05 | .30 | 51% | 38% |
| List Sorting Working Memory Task T | 41.90 (9.93) | 45.66 (10.10) | .02 | .38 | 48% | 30% |
| Flanker Inhibitory Control and Attention Task T | 39.87 (10.00) | 44.10 (10.22) | <.01 | .42 | 51% | 38% |
| Pattern Comparison Processing Speed Test T | 46.55 (11.99) | 47.86 (11.48) | .47 | .11 | 29% | 28% |
Note.
Results from independent sample t-tests. Impairment = T-score of 40 or below (1 SD below the mean)
Results from a multivariable regression model adjusting for reading level, as assessed by the NIHTB-CB Oral Reading T-scores (this was the only variable among our list of potential covariates that differed significantly between groups and was associated with Fluid TS), showed that the racial difference in Fluid Composite T-scores remained significant (NH Black: Estimate =−3.29, SE=1.65, p<.05). In this model, higher Oral Reading T-scores were also an independent significant predictor of higher Fluid TS (p<.001). Stroke characteristics (i.e., stroke severity and type of stroke) did not differ between groups, but because of the known impact of these factors on neurocognitive function, we further investigated their role as potential covariates in follow-up analyses. In order to do so, we first ran two separate univariable linear regression models on Fluid T-scores and found that increased stroke severity was significantly associated with worse neurocognitive function (p<.001) but type of stroke was not (p=.93). We then ran a multivariable model including race and stroke severity as predictors of Fluid T-scores and found that there were independent effects of both race and stroke severity (ps<.01). Because both Oral Reading and stroke severity were independently associated with Fluid T-scores in the models presented above, they were included as significant covariates in further analyses examining the impact of healthcare characteristics on racial differences in neurocognitive function.
In the subset of participants with available data (n=98), we found that having a history of hypertension and smoking were both significantly associated with lower neurocognitive function (ps<.04), and having a history of a prior TIA was not (p=.18). Given the relatively small sample size, these variables were not considered for further multivariable models examining the role of healthcare characteristics in explaining racial differences on neurocognitive outcomes post-stroke.
Impact of Healthcare Characteristics on Racial Differences in Neurocognitive Performance
As compared to NH Whites, NH Blacks scored lower on health literacy, showed lower healthcare use and access in two of the variables assessed (i.e., insurance type and healthcare type), and reported less confidence in doctors treating them (see Table 2).
Separate multivariable models investigating the impact of each of these factors on racial differences in Fluid T-scores, and adjusting for Oral Reading and injury severity, showed that health literacy and insurance type were significant predictors (shown in Table 4), while healthcare type and confidence in doctors were not (p=.18 and p=.97, respectively). Review of the variance inflation factors (VIFs) for each model indicated that multicollinearity was not a concern.
Table 4.
Impact of health literacy and insurance type on racial differences in Fluid Composite T-Scores post-strokea
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Estimate (SE) | p | Estimate (SE) | p | |
| Race (Black)b | 0.36 (1.75) | .84 | −1.26 (1.65) | .45 |
| Oral Reading T | 0.30 (0.08) | <.001 | 0.44 (0.07) | <.001 |
| Stroke Severity (Severe)c | −5.12 (1.56) | .001 | −4.61 (1.61) | .005 |
| Health literacy | 0.43 (0.11) | <.001 | — | — |
| Insurance typed | — | — | — | .007 |
| Medicaid | — | — | 0.69 (2.21) | .76 |
| Medicare | — | — | 6.54 (2.21) | .004 |
| Independent Living Center | — | — | 1.37 (10.17) | .89 |
| Other (including private) | — | — | 6.56 (2.18) | .003 |
Results based on two separate multivariable linear regression models, both adjusting for similar covariates (Model 1: F[4,164] = 19.53, p<.001, Adjusted R2 =0.31; Model 2: F[7, 159] = 11.06, p<.001, Adjusted R2 = 0.30);
Reference group = White;
Reference group = Mild to moderate;
Reference group = Supplemental Security Income (SSI), State Disability Insurance (SDI), or Social Security (SS).
We then conducted path analyses to investigate whether health literacy might explain racial differences in neurocognitive function post-stroke, after adjusting for significant insurance type. Raw score (unstandardized) coefficients for all of the paths appear in Figure 1a. The overall multivariable model on neurocognitive function (Fluid Composite T-score) including terms for race, health literacy, insurance type, reading level and injury severity, was significant (F[5,161] =19.09, p<.001, Adj R2=0.37). The association between race and neurocognitive functioning not controlling for the other variables (path c) was significant and indicated that NH Blacks performed significantly worse than Whites. After adjusting for health literacy and other significant covariates, the association of race with neurocognitive function was no longer significant (path c’). The path through health literacy showed that its 95% CI (−4.62 to −1.11) obtained by bootstrapping did not include 0, indicating that it was statistically significant; in other words, after considering insurance type, reading level and injury severity, health literacy explained or mediated remaining significant racial differences in cognitive outcomes. Figure 1b demonstrates path analysis investigating whether insurance type might also explain racial differences in neurocognitive function post-stroke, adjusting for significant covariates (reading level and injury severity) and health literacy. The path through insurance type showed that 95% CI for indirect effect (−0.187 to −0.002) obtained by bootstrapping did not include 0, indicating that, after adjusting for health literacy, reading level, and injury severity, the path was statistically significant; that is, insurance type mediated the remaining significant racial difference in neurocognitive function.
Figure 1a.
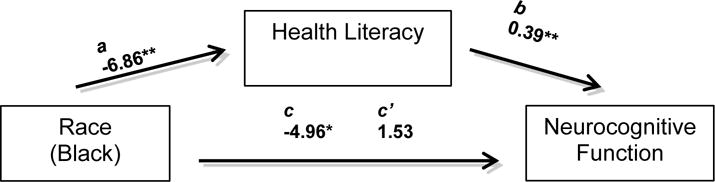
Model examining the association between race and neurocognitive function (NIH TB Fluid Composite TS), and whether health literacy might account for this association after adjusting for reading level, stroke severity and insurance type. The c coefficient represents the total relationship between race and neurocognitive function (not controlling for health literacy, insurance type or significant covariates). The c’ coefficient represents the strength of the association between race and neurocognitive function after controlling for the proposed intermediate factor (health literacy), significant covariates (reading level and stroke severity), and insurance type. The Coefficient for the direct effect (c’), was estimated directly from the models (Preacher & Hayes). The a and b paths represent the indirect path involving health literacy. The a path shows the coefficient for the association of race to health literacy based on a linear regression model adjusting for reading level, stroke severity and insurance type. The b path shows the coefficient for the association of health literacy to neurocognitive function when included in a multivariable regression model along with other variables.
*p < .01, **p < .001.
Figure 1b.
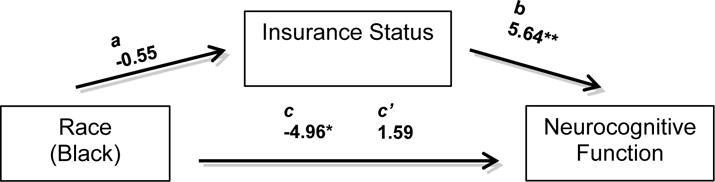
Model examining the association between race and neurocognitive function (NIH TB Fluid Composite TS), and whether insurance type might account for this association after adjusting for reading level, stroke severity and health literacy. The c coefficient represents the total relationship between race and neurocognitive function (not controlling for insurance type, or significant covariates). The c’ coefficient represents the strength of the association between race and neurocognitive function after controlling for the proposed intermediate factor (insurance type) and reading level, stroke severity and health literacy. The Coefficient for the direct effect, c’, was estimated using the bootstrap approximations from causal mediation analysis (Imai et al.). The a and b paths represent the indirect path involving insurance type. The a path shows the coefficient for the association of race to insurance type based on a linear regression model adjusting for covariates (reading level, stroke severity and health literacy). The b path shows the coefficient for the association of insurance type to neurocognitive function when included in a multivariable regression model along with other variables.
*p < .01, **p < .001.
Follow-up Analysis: Potential Impact of study site
As noted above, the participants who were assessed across the three study sites were not balanced with respect to racial composition; the University of Michigan participants were virtually all NH White, whereas 73% of the Chicago group and 53% of the St. Louis group were Black. The possibility was considered that overall racial differences in Fluid Cognition might have resulted from site-related cohort effects, possibly influenced by geographic differences in access to or quality of healthcare. In order to address this question, we ran a linear regression model on Fluid T, with predictors being race, site and their interaction, including only participants from the Rehabilitation Institute of Chicago and Washington University. Results showed that the interaction was not significant (p=.79). The interaction term was then removed, and the resulting model showed significant independent effects of race (p=.03) and site (p<.01), such that Blacks and participants from Rehabilitation Institute of Chicago performed worse.
Discussion
Consistent with prior findings (Desmond et al., 2000; Newman et al., 2007; Patel et al., 2002), results from the present study showed worse neurocognitive outcomes among NH Black stroke survivors than their NH White counterparts. The present study extends the existing literature by showing these racial differences extend to multiple neurocognitive domains, and that possible pre-existing racial differences in quality of education/premorbid functioning and stroke severity do not fully account for these racial differences in stroke outcomes. Furthermore, they indicate that healthcare variables might be important factors underlying these racial differences.
Present findings showing racial differences in a cognitive composite score (NIHTB-CB Fluid TS) among stroke survivors are consistent with our hypothesis that NH Blacks might have worse neurocognitive outcomes post-stroke than Whites. Our measure of cognition included adjustments for age, education, gender, and race/ethnicity, and, in our models, we adjusted for other significant socio-demographic variables, including an estimate of premorbid functioning (i.e., NIHTB-CB Oral Reading). Thus, racial differences in post-stroke cognitive outcomes do not appear to be explained by pre-existing differences in neurocognitive function (Rushton & Jensen, 2005). In addition, groups scored comparably on the Toolbox Emotion Battery composites (negative affect, psychological well-being, and social satisfaction), which suggests that group differences in neurocognitive outcomes were not influenced by social or emotional problems associated with stroke. There are documented racial differences in stroke characteristics among NH Blacks and Whites, which could account for racial differences in NCI post-stroke. While information on stroke characteristics was fairly limited in our study, there were no significant racial differences on the variables assessed. Stroke severity was associated with neurocognitive function, yet it did not fully account for racial differences post-stroke.
The racial differences in global neurocognitive function appeared to result from compromised performance on individual NIHTB-CB Fluid tests assessing cognitive flexibility (Dimensional Change Card Sort Task), inhibitory control (Flanker Inhibitory Control and Attention Task) working memory (List Sorting Working Memory Task) and episodic memory (Picture Sequence Memory Task). Deficits in these domains are linked to problems in everyday functioning post-stroke, particularly driving (Marshall et al., 2007) and managing a complex medication regimen (Zinn, Bosworth, Hoenig, & Swartzwelder, 2007). This suggests that the observed racial differences in neurocognitive deficits are likely to have real-world consequences in the lives of stroke patients.
Importantly, we examined racial differences in cognition at least one-year post-stroke, which is likely representative of the long-term effect of stroke on neurocognitive function rather than acute effects. In addition, given that NH Blacks have a higher risk of mortality following stroke, requiring at least one year of recovery following stroke for enrollment might have produced a resilient group of NH Black stroke survivors. It is also worth noting that interactions between potentially explanatory variables that did not differ between racial groups or were not associated with the outcome were not explored. Future studies, especially those with larger sample sizes, might usefully examine such interactions.
In our sample of stroke survivors, NH Blacks showed lower health literacy than NH Whites, which is consistent with findings in the general population (Kutner et al., 2006; Morrow et al., 2006; Rushton & Jensen, 2005). In the present study, health literacy helped explain racial differences in neurocognitive function, even after adjusting for the effects of estimated premorbid function, stroke severity, and insurance type. Health literacy affects a variety of health-related risks and behaviors. Low health literacy has been associated with decreased ability to interpret medical messages, read and comprehend medicine labels, and demonstrate proper medication consumption (Berkman et al., 2011). In addition, low health literacy has been associated with worse global health status and higher mortality rates (Berkman et al., 2011). In the context of stroke, health outcomes are highly dependent on the ability of stroke survivors to comprehend medical information and follow their treatment plan. Individuals with low health literacy might be more likely than those with higher health literacy to have problems with compliance to stroke care because of issues with written and oral communication, understanding and following directions, self-empowerment, and trusting new information, among others (Cruz-Flores et al., 2011). In addition, stroke survivors with low health literacy might be less likely to engage in positive health behaviors, such as adhering to and refilling medications and maintaining a healthy diet (Gazmararian et al., 2006; Murray et al., 2004; Zoellner et al., 2011). Among stroke survivors, Blacks are less likely than NH Whites with a history of stroke to recognize stroke symptoms and the need for urgent medical attention (Ellis & Egede, 2008). Future research might evaluate whether the role of health literacy in explaining racial differences in neurocognitive function is due to the decreased ability of NH Blacks to understand and thus adhere to treatment (Cruz-Flores et al., 2011; Ettenhofer, Foley, Castellon, & Hinkin, 2010; McDonald, Garg & Haynes, 2002). Alternatively, worse neurocognitive functioning among NH Blacks might contribute to worse health literacy. Given the cross-sectional nature of our study, we cannot ascribe specific causal inference to these findings. Yet, while non-conclusive, findings showing worse health literacy among Blacks in the general population, suggest that racial differences in health literacy might be, at least in part, premorbid. Furthermore, the relation between health literacy and neurocognitive function, regardless of the causation, highlights the need for appropriate interventions targeting health literacy, and the importance of matching provider communications to stroke survivors’ literacy and learning needs (Magasi, Durkin,Wolf, & Deutsch, 2009).
Insurance type also helped explain racial differences in neurocognitive function, even after adjusting for the effects of estimated premorbid function, stroke severity, and health literacy. Consistent with prior literature (National Center for Health Statistics, 2011), racial differences in insurance type were driven by the disproportionate number of NH Blacks compared to NH Whites with Medicaid coverage and the higher percentage of NH Whites compared to NH Blacks with Medicare coverage and “other” coverage (including private insurance). Given that the administrative burden and low reimbursement rates associated with Medicaid might decrease the incentives and likelihood of physicians to participate in Medicaid programs, this effect could influence differences in access to care among Medicaid beneficiaries compared to Medicare enrollees (Cunningham & O’Malley, 2009). Prior research examining the proportion of physicians accepting Medicaid patients suggests an oversaturation of the program (Cunningham & May, 2006). Moreover, while there has been an increase in the number of Medicaid enrollees, there has been a decrease in the number of physicians accepting Medicaid beneficiaries (Cunningham & May, 2006). Consistent with our finding that NH Blacks were less likely than NH Whites to receive treatment at a private clinic, prior research suggests that Medicaid beneficiaries are more likely to be treated at a federally qualified health institution (i.e., community clinic) than a private clinic (Rothkopf, Brookler, Wadhwa, & Sajovetz, 2011). Community clinic patients are less likely than private clinic patients to take advantage of medical services for a preventable disturbance, which could be viewed as an indicator of quality of care (Rothkopf et al., 2011).
Given the absence of data on healthcare access at the time of stroke, we cannot rule out the possibility that there were changes in insurance between the time of stroke and the time of neuropsychological testing, which could have influenced the associations reported here. More specifically, we cannot rule out the possibility that worse healthcare coverage and access may have occurred in some people because of post-stroke unemployment. In this regard, it is worth noting that 65% of participants reported being unemployed post-stroke and NH Blacks were more likely than their NH White counterparts to report being unemployed post-stroke (72.73% of NH Blacks, 55.84% of NH White; p=.02).
The present study has other limitations as well. Although we found that a history of hypertension and smoking were both more prevalent among NH Blacks, and were associated with neurocognitive outcomes, these data were only available in a subset of participants; thus, we did not examine their role in explaining racial differences in neurocognitive outcomes post-stroke. The number of prior strokes was not consistently recorded across sites. Given that recurrent stroke has been associated with the increased risk of neurocognitive impairment and Blacks are at a higher risk for recurrent stroke, our findings could at least partially be due to more NH Blacks suffering recurrent strokes than NH Whites (Park & Ovbiagele, 2016; Pendlebury & Rothwell, 2009). Other stroke characteristics, such as the vascular territory of the infarction and the depth of infarction, might also play a role in explaining our findings, but these characteristics were not consistently recorded in the cohort. Available data on cardiovascular risk and stroke characteristics were collected via a combination of review of medical records, self-report, or report by informant. Unfortunately, we are not able to discern how these data were collected for each participant, limiting our ability to interpret findings based on these data.
Although the healthcare access, use, and quality assessments were developed using questions from validated surveys, only a subset of questions of the original surveys were included in the present study. Thus, we did not have composite measures of these variables. The present study also might have benefited from including objective measures of healthcare access, use, and quality; however, self-report assessments of healthcare utilization have been found to be comparable to objective measures (Gordon, Wortley, Singleton, Lin, & Bardenheier, 2008; Reijneveld & Stronks, 2001). Given that these racial differences were observed in a community-dwelling NH Black population living in the Midwestern United States, our findings might not be generalizable to Blacks living in other areas of the United States, or more representative samples of the stroke populations (i.e., samples including patients from hospital and assisted living environments). Follow-up analyses investigating the potential influence of study site on fluid cognition indicated that there could be an independent effect of study site in addition to racial differences. However, the relatively small sample sizes warrant future examination of this issue. Also, the number of potential participants who were excluded because of significant aphasia was not recorded. The exclusion criteria of significant aphasia could well have excluded more severe cases of cognitive impairment that would be present in a representative sample of the stroke population.
Overall, the present study identified health literacy and insurance type as variables that might help “explain” differences in neurocognitive outcomes between NH Blacks and NH Whites post-stroke. Health literacy is a potentially modifiable risk factor; thus, intervention studies aimed at improving health literacy among individuals with inadequate health literacy might decrease this disparity. Although insurance type is a risk factor less amenable to modification, efforts can be made to decrease disparities in the access to and quality of healthcare.
Acknowledgments
Funding: This work was supported by the National Institute on Aging (5R25AG043364-02); the National Institute on Disability and Rehabilitation Research (H133B090024, H133F140037); the National Institute of Mental Health (K23 MH105297); and James S. McDonnell Foundation (JSMF 98.32 CRH-QUA.11).
Footnotes
Conflicts of Interest: The authors report no personal or financial conflicts of interest.
References
- Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, Heaton RK. VIII. NIH Toolbox Cognition Battery (CB): Composite scores of crystallized, fluid, and overall cognition. Monographs of the Society for Research in Child Development. 2013;78(4):119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretouli E, Brandt J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. International Journal of Geriatric Psychiatry. 2010;25(3):224–233. doi: 10.1002/gps.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala C, Greenlund KJ, Croft JB, Keenan NL, Donehoo RS, Giles WH, Marks JS. Racial/ethnic disparities in mortality by stroke subtype in the United States, 1995–1998. American Journal of Epidemiology. 2001;154(11):1057–1063. doi: 10.1093/aje/154.11.1057. [DOI] [PubMed] [Google Scholar]
- Babakhanyan I, Casaletto K, Heaton RK. NIH Toolbox Emotion Domain: Exploration of Emotion Summary Scores and Demographic Effects in the US Standardization Norming Study (2015, November). Archives of Clinical Neuropsychology; Poster presented at the 35th Annual Conference for the National Academy of Neuropsychology; Austin, TX. [Google Scholar]
- Babakhanyan I, McKenna B, Casaletto K, Heaton RK. NIH Toolbox Emotion Domain: Creation of Census Stratified Normative Data, Summary Scales and Base Rates for Distressed Emotional Functioning. Poster presented at the 44th Annual Meeting for the International Neuropsychological Society; Boston, MA. 2016. Feb, [Google Scholar]
- Baum CM, Connor LT, Morrison T, Hahn M, Dromerick AW, Edwards DF. Reliability, validity, and clinical utility of the Executive Function Performance Test: A measure of executive function in a sample of people with stroke. American Journal of Occupational Therapy. 2008;62(4):446–455. doi: 10.5014/ajot.62.4.446. [DOI] [PubMed] [Google Scholar]
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Annals of Internal Medicine. 2011;155(2):97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, Heaton RK. Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. Journal of the International Neuropsychological Society. 2015;21(05):378–391. doi: 10.1017/S1355617715000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. US Census Bureau. 2015:25–1143. Ed. [Google Scholar]
- Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Peterson E. Racial-ethnic disparities in stroke care: the American experience a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- Cunningham PJ, May J. Medicaid patients increasingly concentrated among physicians. Washington, DC: Center for Studying Health System Change; 2006. [PubMed] [Google Scholar]
- Cunningham PJ, O’Malley AS. Do reimbursement delays discourage Medicaid participation by physicians? Health Affairs. 2009;28(1):w17–w28. doi: 10.1377/hlthaff.28.1.w17. [DOI] [PubMed] [Google Scholar]
- Davis TC, Crouch MA, Long SW, Jackson RH, Bates P, George RB, Bairnsfather LE. Rapid assessment of literacy levels of adult primary care patients. Family Medicine. 1991;23(6):433–435. [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Paik MC, Sano M, Mohr JP, Aboumatar S, Hauser WA. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54(5):1124–1131. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of Dementia After Ischemic Stroke Results of a Longitudinal Study. Stroke. 2002;33(9):2254–2262. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- Dyer N, Sorra JS, Smith SA, Cleary P, Hays R. Psychometric properties of the Consumer Assessment of Healthcare Providers and Systems (CAHPS®) clinician and group adult visit survey. Medical Care. 2012;50(Suppl):S28. doi: 10.1097/MLR.0b013e31826cbc0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DF, Hahn M, Baum C, Dromerick AW. The impact of mild stroke on meaningful activity and life satisfaction. Journal of Stroke and Cerebrovascular Diseases. 2006;15(4):151–157. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ellis C, Egede LE. Ethnic disparities in stroke recognition in individuals with prior stroke. Public Health Reports. 2008:514–522. doi: 10.1177/003335490812300413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderby PM, Wood VA, Wade DT, Hewer RL. The Frenchay Aphasia Screening Test: a short, simple test for aphasia appropriate for non-specialists. International Rehabilitation Medicine. 1987;8(4):166. doi: 10.3109/03790798709166209. [DOI] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010;74(15):1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. 2015 Retrieved from https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html.
- Federman AD, Sano M, Wolf MS, Siu AL, Halm EA. Health literacy and cognitive performance in older adults. Journal of the American Geriatrics Society. 2009;57(8):1475–1480. doi: 10.1111/j.1532-5415.2009.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283(19):2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- Gazmararian JA, Kripalani S, Miller MJ, Echt KV, Ren J, Rask K. Factors associated with medication refill adherence in cardiovascular-related diseases: A focus on health literacy. Journal of General Internal Medicine. 2006;21(12):1215–1221. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NP, Wortley PM, Singleton JA, Lin TY, Bardenheier BH. Race/ethnicity and validity of self-reported pneumococcal vaccination. BMC Public Health. 2008;8(1):1. doi: 10.1186/1471-2458-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EA, Choi SW, Griffith JW, Yost KJ, Baker DW. Health literacy assessment using talking touchscreen technology (Health LiTT): a new item response theory-based measure of health literacy. Journal of health communication. 2011;16(sup3):150–162. doi: 10.1080/10810730.2011.605434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Shaul JA, Williams VS, Lubalin JS, Harris-Kojetin LD, Sweeny SF, Cleary PD. Psychometric properties of the CAHPS™ 1.0 survey measures. Medical Care. 1999;37(3):MS22–MS31. doi: 10.1097/00005650-199903001-00003. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Gershon R. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. Journal of the International Neuropsychological Society. 2014;20(06):588–598. doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Annals of the New York Academy of Sciences. 2012;1268(1):14–20. doi: 10.1111/j.1749-6632.2012.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological Methods. 2010;15(4):309. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith-Hammond CA, Oddone EZ. Racial variation in initial stroke severity. Stroke. 2000;31(3):563–567. doi: 10.1161/01.str.31.3.563. [DOI] [PubMed] [Google Scholar]
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure. Advances in Clinical Rehabilitation. 1987;1:6–18. [PubMed] [Google Scholar]
- Kelley BJ, McClure LA, Letter AJ, Wadley VG, Unverzagt FW, Kissela BM, Howard G. Report of stroke-like symptoms predicts incident cognitive impairment in a stroke-free cohort. Neurology. 2013;81(2):113–118. doi: 10.1212/WNL.0b013e31829a352e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Kissela BM. Stroke incidence is decreasing in whites but not in blacks a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41(7):1326–1331. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi LC, Wardle J, Wolf MS, Wagner C. Cognitive function and health literacy decline in a cohort of aging English adults. Journal of General Internal Medicine. 2015;30(7):958–964. doi: 10.1007/s11606-015-3206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner M, Greenburg E, Jin Y, Paulsen C. NCES 2006-483. National Center for Education Statistics; 2006. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. [Google Scholar]
- Lee Hargraves J, Hays RD, Cleary PD. Psychometric properties of the consumer assessment of health plans study (CAHPS®) 2.0 adult core survey. Health Services Research. 2003;38(6p1):1509–1528. doi: 10.1111/j.1475-6773.2003.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DA, Kabeto M, Langa KM, Lisabeth LD, Rogers MA, Galecki AT. Does Stroke Contribute to Racial Differences in Cognitive Decline? Stroke. 2015;46(7):1897–1902. doi: 10.1161/STROKEAHA.114.008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasi S, Durkin E, Wolf MS, Deutsch A. Rehabilitation consumers’ use and understanding of quality information: a health literacy perspective. Archives of Physical Medicine and Rehabilitation. 2009;90(2):206–212. doi: 10.1016/j.apmr.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Marshall SC, Molnar F, Man-Son-Hing M, Blair R, Brosseau L, Finestone HM, Wilson KG. Predictors of driving ability following stroke: a systematic review. Topics in Stroke Rehabilitation. 2007;14(1):98–114. doi: 10.1310/tsr1401-98. [DOI] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Morrow D, Clark D, Tu W, Wu J, Weiner M, Steinley D, Murray MD. Correlates of health literacy in patients with chronic heart failure. The Gerontologist. 2006;46(5):669–676. doi: 10.1093/geront/46.5.669. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Huffman MD. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2016;131(4):e29. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Murray MD, Morrow DG, Weiner M, Tu W, Deer MM, Brater DC, Weinberger M. A conceptual framework to study medication adherence in older adults. The American Journal of Geriatric Pharmacotherapy. 2004;2(1):36–43. doi: 10.1016/s1543-5946(04)90005-0. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (US), & National Center for Health Services Research. Health, United States. US Department of Health, Education, and Welfare, Public Health Service, Health Resources Administration, National Center for Health Statistics; 2011. [Google Scholar]
- Newman GC, Bang H, Hussain SI, Toole JF. Association of diabetes, homocysteine, and HDL with cognition and disability after stroke. Neurology. 2007;69(22):2054–2062. doi: 10.1212/01.wnl.0000280457.29680.9c. [DOI] [PubMed] [Google Scholar]
- Nys GMS, Van Zandvoort MJE, De Kort PLM, Jansen BPW, Kappelle LJ, De Haan EHF. Restrictions of the Mini-Mental State Examination in acute stroke. Archives of Clinical Neuropsychology. 2005;20(5):623–629. doi: 10.1016/j.acn.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Campbell J, Kuo YF, Deutsch A, Ostir GV, Granger CV. Racial and ethnic differences in postacute rehabilitation outcomes after stroke in the United States. Stroke. 2008;39(5):1514–1519. doi: 10.1161/STROKEAHA.107.501254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. Journal of General Internal Medicine. 2005;20(2):175–184. doi: 10.1111/j.1525-1497.2005.40245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Ovbiagele B. Association of black race with recurrent stroke risk. Journal of the Neurological Sciences. 2016;365:203–206. doi: 10.1016/j.jns.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults. Journal of General Internal Medicine. 1995;10(10):537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive Impairment after Stroke: Clinical Determinants and Its Associations with Long-Term Stroke Outcomes. Journal of the American Geriatrics Society. 2002;50(4):700–706. doi: 10.1046/j.1532-5415.2002.50165.x. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. The Lancet Neurology. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Pereira FS, Yassuda MS, Oliveira AM, Diniz BS, Radanovic M, Talib LL, Forlenza OV. Profiles of functional deficits in mild cognitive impairment and dementia: benefits from objective measurement. Journal of the International Neuropsychological Society. 2010;16(02):297–305. doi: 10.1017/S1355617709991330. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Reijneveld SA, Stronks K. The validity of self-reported use of health care across socioeconomic strata: a comparison of survey and registration data. International Journal of Epidemiology. 2001;30(6):1407–1414. doi: 10.1093/ije/30.6.1407. [DOI] [PubMed] [Google Scholar]
- Rothkopf J, Brookler K, Wadhwa S, Sajovetz M. Medicaid patients seen at federally qualified health centers use hospital services less than those seen by private providers. Health Affairs. 2011;30(7):1335–1342. doi: 10.1377/hlthaff.2011.0066. [DOI] [PubMed] [Google Scholar]
- Rushton JP, Jensen AR. Thirty years of research on race differences in cognitive ability. Psychology, Public policy, and Law. 2005;11(2):235. [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Lai JS. Emotion assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S76–S86. doi: 10.1212/WNL.0b013e3182872e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Parsey CM. Assessment of functional change and cognitive correlates in the progression from healthy cognitive aging to dementia. Neuropsychology. 2014;28(6):881. doi: 10.1037/neu0000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinart KF, Tuhrim S, Horowitz DR, Weinberger J, Goldman M, Godbold JH. Stroke recurrence is more frequent in Blacks and Hispanics. Neuroepidemiology. 1998;17(4):188–198. doi: 10.1159/000026172. [DOI] [PubMed] [Google Scholar]
- Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- Stephens S, Kenny RA, Rowan E, Kalaria RN, Bradbury M, Pearce R, Ballard CG. Association between mild vascular cognitive impairment and impaired activities of daily living in older stroke survivors without dementia. Journal of the American Geriatrics Society. 2005;53(1):103–107. doi: 10.1111/j.1532-5415.2005.53019.x. [DOI] [PubMed] [Google Scholar]
- Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Annals of Translational Medicine. 2014;2(8) doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Paik M, Figueroa M, Gropen TI, Stern Y, Mayeux R. Clinical determinants of dementia related to stroke. Annals of Neurology. 1993;33(6):568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57(2):202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis 2014 [Google Scholar]
- Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, Howard G. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Stroke. 2007;38(4):1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- Waidmann TA, Rajan S. Race and ethnic disparities in health care access and utilization: an examination of state variation. Medical Care Research and Review. 2000;57(4 suppl):55–84. doi: 10.1177/1077558700057001S04. [DOI] [PubMed] [Google Scholar]
- Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. The Annals of Family Medicine. 2005;3(6):514–522. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Fox NA. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and hispanics the northern manhattan study. Circulation. 2005;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- Yost KJ, Webster K, Baker DW, Jacobs EA, Anderson A, Hahn EA. Acceptability of the talking touchscreen for health literacy assessment. Journal of Health Communication. 2010;15(S2):80–92. doi: 10.1080/10810730.2010.500713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn S, Bosworth HB, Hoenig HM, Swartzwelder HS. Executive function deficits in acute stroke. Archives of Physical Medicine and Rehabilitation. 2007;88(2):173–180. doi: 10.1016/j.apmr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Zoellner J, You W, Connell C, Smith-Ray RL, Allen K, Tucker KL, Estabrooks P. Health literacy is associated with healthy eating index scores and sugar- sweetened beverage intake: findings from the rural Lower Mississippi Delta. Journal of the American Dietetic Association. 2011;111(7):1012–1020. doi: 10.1016/j.jada.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


