Abstract
Purpose
We studied associations of magnetic resonance imaging (MRI)-measured plaque area and relative percent lumen reduction in the proximal superficial femoral artery with Walking Impairment Questionnaire (WIQ) scores and quality of life in people with lower extremity peripheral arterial disease (PAD).
Methods
Four-hundred forty-two participants with PAD underwent cross-sectional imaging of the proximal superficial femoral artery with MRI, and completed the WIQ and the Short-Form-12 mental and physical functioning questionnaires. Questionnaires were scored on a 0–100 scale (100=best). Results adjust for age, sex, race, the ankle brachial index (ABI), comorbidities, and other covariates.
Results
Adjusting for age, sex, race, ABI, comorbidities, and other covariates, higher mean plaque area was associated with poorer WIQ distance scores (1st quintile (least plaque)-44.8, 2nd quintile-43.3, 3rd quintile-38.9, 4th quintile-34.6, 5th quintile (greatest plaque)-30.6, p trend <0.001) and poorer WIQ speed scores (1st quintile-40.6, 2nd quintile-39.6, 3rd quintile-39.5, 4th quintile-32.8, 5th quintile-33.0, p trend =0.019). Similar associations of higher maximum plaque area, mean lumen reduction, and maximum lumen reduction with poorer WIQ distance and speed scores were observed. Plaque measures were not associated with WIQ stair climbing scores or SF-12 scores.
Conclusion
Among participants with PAD, greater plaque burden and smaller lumen area in the proximal superficial femoral artery are associated with poorer walking endurance and slower walking speed as measured by the WIQ, even after adjusting for the ABI.
Keywords: Peripheral arterial disease, intermittent claudication, physical functioning, atherosclerotic plaque
Lower extremity peripheral arterial disease (PAD) is common among older men and women and will be increasingly common as the United States population survives longer with chronic disease [1]. Mechanisms of functional impairment in patients with PAD are not fully understood. Although people with PAD have greater functional impairment and faster functional decline compared to those without PAD [2–4], the specific contribution of lower extremity atherosclerosis to functional impairment and functional decline in PAD is not fully defined. For example, the ankle brachial index (ABI), a measure of PAD severity, has not consistently correlated with the degree of functional impairment and functional decline in patients with PAD [5–8].
High resolution magnetic resonance imaging (MRI) has emerged as a promising non-invasive modality for direct imaging of atherosclerotic plaque [9]. We used MRI of the superficial femoral artery to determine whether higher plaque area and smaller lumen area were associated with greater patient-reported walking impairment and poorer quality of life among participants with PAD. We hypothesized that greater plaque area and smaller lumen area in the superficial femoral artery would be associated with greater patient-reported walking impairment and poorer quality of life in individuals with PAD. Given that 10–15% of community-dwelling men and women age 65 and older have PAD [1], these findings are relevant to a large proportion of older men and women.
METHODS
Enrollment occurred between 12/1/07 and 12/31/09. Participants were recruited as part of the Walking and Leg Circulation Study (WALCS) III cohort, a prospective, observational study designed to establish associations of MRI-measured plaque characteristics in the superficial femoral artery with functional impairment and functional decline in PAD [10].
Participants were identified from among consecutive PAD patients in medical practices and/or the non-invasive vascular laboratories at Northwestern Medical Center and three additional Chicago-area medical centers. A small number of participants were identified from among men and women age 65 and older in Northwestern’s largest general internal medicine practice who were screened with the ABI and found to have an ABI <1.00. The protocol was Institutional Review Board-approved by Northwestern University Feinberg School of Medicine and all participating study sites. Participants gave informed consent.
Inclusion Criterion
The inclusion criterion was a lowest leg ABI < 1.00. This inclusion criterion was selected because truly normal ABI values are 1.10–1.40 [4,11–13] and because including participants with ABI < 1.00 ensured a broad range of severity of lower extremity atherosclerosis.
Exclusion Criteria
Potential participants with dementia and those with a mini-mental status examination (MMSE) score < 23 [14], were excluded because of concern that cognitive impairment might influence their questionnaire responses. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because of their severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded. Patients with contraindications to MRI testing were excluded. In addition, we aimed to exclude potential participants primarily limited by comorbidities other than PAD. Therefore, we excluded potential participants who required oxygen therapy, those who stopped a six-minute walk test due to shortness of breath, and those with severe knee osteoarthritis, measured by reported pain in or around the knee joint combined with a radiograph-measured osteoarthritis K/L score of four [15].
Ankle Brachial Index Measurement
After participants rested supine for five minutes, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to measure systolic pressures in this order: right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures [16]. Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg in at least one measurement set, since in such cases subclavian stenosis was possible [17]. For the dorsalis pedis and posterior tibial vessels, arteries with zero values and arteries that were incompressible were excluded from the ABI calculation. Lowest leg ABI was used in analyses.
Magnetic Resonance Imaging
We imaged the superficial femoral artery because it is the most common site of lower extremity atherosclerosis [18,19] and because it supplies calf muscle, which is classically symptomatic in patients with PAD. MRI data were obtained with a 1.5 Tesla (Siemens) platform using four-element phased-array surface coils and a dedicated leg coil from Nova Medical. We imaged the proximal region of the SFA because its superficial location was more amenable to high quality images than the more deeply located distal SFA. The bifurcation of the common femoral artery served as the reference point. Images were collected with a standard, proton density weighted (TR/TE= 2160 milliseconds/8 milliseconds) Turbo Spin Echo acquisition, including a chemically selective fat saturation pre-pulse for suppression of peri-adventitial fat and regional saturation of flowing blood. Twelve consecutive 2.5 millimeter cross-sectional images were obtained, beginning at the site of bifurcation of the common femoral artery into the superficial femoral artery and moving distally using 2-dimensional bright blood time-of-flight and proton-density weighted (PD) images. Fat suppression was applied in black-blood sequences to improve image quality. Previous study shows that this method has excellent test re-test reliability [20].
CASCADE software (Seattle, WA) was used by two physician readers to trace the outer boundary and the lumen of each cross-sectional artery image. Images for each participant were assigned to one primary reviewer, and arterial tracings were re-reviewed by the second reviewer to ensure accuracy. CASCADE software quantified the plaque area based on the tracings. To adjust for the fact that a given plaque area will have a different effect on different sized arteries, plaque measurements were normalized for artery size. Mean plaque area was normalized by dividing the average plaque area by the median of the outer wall area [21]. Maximum plaque area was normalized by dividing maximum plaque area by the median of the outer wall area. Similarly, mean and minimum percent lumen area were normalized by dividing the mean and minimum lumen area by the outer wall area for each arterial slice. A six percent subsample of participants returned on a second day within six months of the original scan for test re-test reliability assessment of MRI measures. The coefficient of variation percent values for these test re-test reliability assessments were 5.69 for mean plaque area, 8.90 for maximum plaque area, 7.9 for mean percent lumen area and 12.9 for minimum percent lumen area. Mean inter-class correlation coefficients were 0.93, 0.84, 0.97, and 0.95 and mean differences between repeated tests were 0.001, 0.002, 0.002, and 0.003 for mean plaque area, maximum plaque area, mean percent lumen area, and minimum percent lumen area, respectively.
Walking Impairment Questionnaire
The Walking Impairment Questionnaire (WIQ) measures patient-perceived walking impairments in patients with PAD and includes three domains: walking distance, walking speed, and stair climbing [22]. Each domain is scored on a 0–100 scale, where 0 represents the most severe limitation and 100 represents absence of any impairment [22]. The WIQ distance score is measured by asking participants about difficulty walking distances ranging from across a room to a distance of ¼ mile. Participants rank the degree of difficulty on a Likert scale ranging from inability to walk the specified distance to no difficulty walking the distance. To calculate the WIQ distance score, the Likert score selected for each distance is multiplied by the corresponding distance. These products are summed and a percent score is calculated. The WIQ speed score is measured by asking participants about difficulty walking specific speeds. Participants rank the degree of difficulty for each speed and their Likert Scale response is multiplied by a weight assigned to each speed question [22]. Products are summed and a percent score is calculated ranging from 0 to 100. Similar methods are used for the WIQ stair-climbing score.
Short-Form 12
We used the Medical Outcomes Study Short-Form 12 (SF-12) to assess quality of life in the physical and mental domains [23]. Scores range from 0 to 100, where 100 represents the best quality of life.
Comorbidities
Algorithms developed for the Women’s Health and Aging Study and the Cardiovascular Health Study were used to document comorbidities [24]. These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire [25]. Comorbidities assessed were angina, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, and disk disease. Criteria from the American College of Rheumatology were used to diagnose knee and hip osteoarthritis [15,25].
Leg Symptoms
We used the San Diego claudication questionnaire to classify participants into one of five mutually exclusive leg symptom categories, based on prior study [26]: 1) Intermittent claudication (exertional calf pain that does not begin at rest, causes the participant to stop walking, and resolves within ten minutes of rest); 2) Atypical exertional leg pain/carry on; 3) Atypical exertional leg pain/stop; 4) Leg pain on exertion and rest; 5) Asymptomatic (no exertional leg symptoms) [26].
Other Measures
Height and weight were measured at the study visit. Body mass index (BMI) was calculated as weight (kg)/(height (meters))2. Cigarette smoking history was measured with self-report. Participants brought their medication bottles or a list of medications to their study visit. Medication names were recorded. The study principal investigator (MMM) identified which participants were taking statin medications, blinded to all other participant characteristics.
Statistical Analyses
Analyses were performed on an individual (per participant) basis. Correlations between each plaque measure and the WIQ scores and between plaque volume and lumen reduction were made using the Pearson method. Quintiles of maximum plaque area, mean plaque area, minimum percent lumen area, and mean percent lumen area in the superficial femoral artery were defined. Differences in WIQ and SF-12 scores were compared across quintiles using analyses of co-variance, adjusting for age, race, sex, smoking, BMI, statins, leg symptoms, and comorbidities (Model 1). These analyses were repeated with additional adjustment for the ABI (Model 2). Pair-wise comparisons were made between the highest and the lower quintiles. Analyses were performed using SAS Statistical Software version 9.0 (SAS Inc, Cary, NC).
RESULTS
Participation rates and exclusion criteria for the WALCS III cohort have been reported [10]. A total of 468 participants with PAD were identified from Chicago-area vascular laboratories. An additional five PAD participants were identified from among patients in the general internal medicine practice who were screened with the ABI. Of these 473 PAD participants, 16 had poor quality MR images, two were missing covariate data, and 13 were missing data for both the WIQ and SF-12 questionnaires, leaving 442 PAD participants for analyses.
Unadjusted correlation coefficients between the ABI and the WIQ scores were 0.24 (P<0.001), 0.18 (P<0.001), and 0.07 (P=0.15) for the distance, speed, and stair climbing domains of the WIQ. Unadjusted correlation coefficients were −0.897 (P<0.001) between mean plaque area and mean lumen area and −0.690 (P<0.001) between maximum plaque area and minimum lumen area.
Adjusting for age, sex, race, BMI, smoking, leg symptoms, comorbidities, and statin use, greater mean plaque area was associated with lower WIQ distance scores (P trend < 0.001) and lower WIQ speed scores (P trend<0.001) (Figure 1, Model 1). Associations of greater mean plaque area with lower WIQ distance and lower WIQ speed scores remained statistically significant even after additional adjustment for the ABI (P trend<0.001 and P trend=0.019, respectively) (Figure 1, Model 2). Adjusting for age, sex, race, BMI, smoking, leg symptoms, comorbidities, and statin use, smaller mean percent lumen area was associated with lower WIQ distance scores (P trend<0.001) and lower WIQ speed scores (P trend<0.001) (Figure 1, Model 1). These associations remained statistically significant even after additional adjustment for the ABI (P trend=0.002 and P trend=0.016, respectively) (Figure 1, Model 2).
Figure 1.
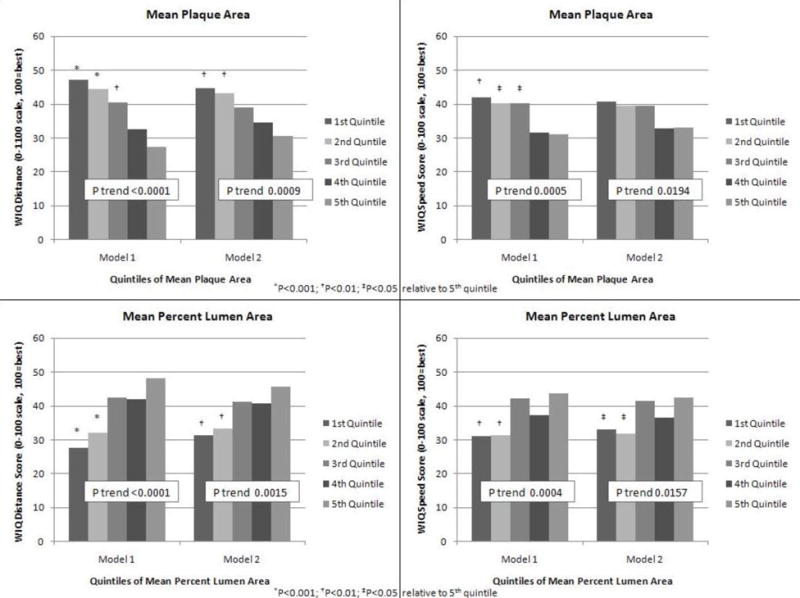
Adjusted Associations of Mean Plaque area and Mean Percent Lumen Area in the Proximal Superficial Femoral Artery with Walking Impairment Questionnaire Distance and Speed Scores in Peripheral Arterial Disease Participants (n=442)
Model 1- Analyses adjust for age, sex, race, body mass index, comorbidities, smoking, leg symptoms, and statin use. Model 2- Analyses adjust for covariates in Model 1 and the ankle brachial index.
Adjusting for age, sex, race, BMI, smoking, comorbidities, leg symptoms, and statin use, greater maximum plaque area was associated with lower WIQ distance scores (P trend<0.001) and lower WIQ speed scores (P trend<0.001) (Figure 2, Model 1). These associations remained statistically significant even after additional adjustment for the ABI (P trend<0.001 and P trend=0.015, respectively) (Figure 2, Model 2). Adjusting for age, sex, race, BMI, smoking, leg symptoms, comorbidities, and statin use, smaller minimum percent lumen area was associated with lower WIQ distance scores (P trend < 0.001) and lower WIQ speed scores (P trend=0.001) (Figure 2, Model 1). These associations remained statistically significant even after additional adjustment for the ABI (P trend<0.001 and P trend=0.033, respectively) (Figure 1, Model 2).
Figure 2.
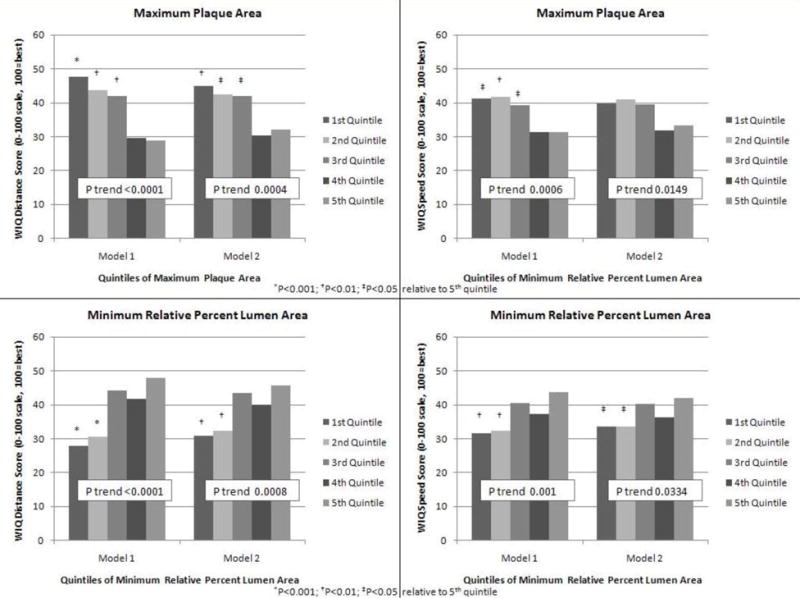
Adjusted Associations of Maximum Plaque Area and Minimum Percent Lumen Area in the Proximal Superficial Femoral Artery with Walking Impairment Questionnaire Distance and Speed Scores in Peripheral Arterial Disease Participants (n=442)
Model 1- Analyses adjust for age, sex, race, body mass index, comorbidities, smoking, leg symptoms, and statin use.
Model 2- Analyses adjust for covariates in Model 1 and the ankle brachial index.
Greater mean and maximum plaque area and greater mean and minimum relative percent lumen reduction were not associated with the WIQ stair climbing score, the SF-12 physical functioning score, or the SF-12 mental functioning score (Table 2).
Table 2.
Associations of Plaque Characteristics with the Walking Impairment Questionnaire Stair-Climbing Score and Quality of Life in Men and Women with Peripheral Arterial Disease (n=442)
| Quintile 1 (0.38–0.56) |
Quintile 2 (0.56–0.62) |
Quintile 3 (0.62–0.67) |
Quintile 4 (0.67–0.76) |
Quintile 5 (0.76–1.22) |
Trend P value | ||
|---|---|---|---|---|---|---|---|
| Mean Plaque area | |||||||
| WIQ Stair-Climbing Score | Model 1 | 45.98 | 53.47† | 48.42 | 43.87 | 41.88 | 0.0622 |
| Model 2 | 45.36 | 53.20† | 48.03 | 44.36 | 42.71 | 0.1757 | |
| SF-12 Physical Functioning Component | Model 1 | 40.25 | 40.30 | 41.14 | 40.67 | 40.36 | 0.8001 |
| Model 2 | 40.06 | 40.21 | 41.03 | 40.82 | 40.59 | 0.4603 | |
| SF-12 Mental Functioning Component | Model 1 | 40.20 | 39.47 | 38.87 | 38.63 | 38.47 | 0.0451 |
| Model 2 | 40.13 | 39.44 | 38.83 | 38.69 | 38.55 | 0.0876 | |
| Maximum Plaque area | |||||||
| WIQ Stair-Climbing Score | Model 1 | 46.64 | 54.95‡ | 48.16 | 39.54 | 44.20 | 0.0364 |
| Model 2 | 45.94 | 54.56‡ | 48.22 | 39.75 | 45.05 | 0.0993 | |
| SF-12 Physical Functioning Component | Model 1 | 40.03 | 40.23 | 41.41 | 40.80 | 40.25 | 0.6781 |
| Model 2 | 39.83 | 40.12 | 41.42 | 40.88 | 40.47 | 0.4051 | |
| SF-12 Mental Functioning Component | Model 1 | 40.49 | 39.20 | 38.63 | 38.44 | 38.88 | 0.0717 |
| Model 2 | 40.41 | 39.16 | 38.64 | 38.47 | 38.97 | 0.129 | |
| Mean Percent Lumen Area | |||||||
| WIQ Stair Climbing Score | Model 1 | 42.28 | 41.67 | 52.83 | 49.82 | 47.06 | 0.0557 |
| Model 2 | 43.06 | 41.91 | 52.57 | 49.58 | 46.57 | 0.1638 | |
| SF-12 Physical Functioning Score | Model 1 | 40.12 | 40.95 | 41.27 | 40.44 | 39.93 | 0.7071 |
| Model 2 | 40.40 | 41.05 | 41.19 | 40.33 | 39.74 | 0.3632 | |
| SF-12 Mental Functioning Score | Model 1 | 38.87 | 38.41 | 38.81 | 40.10 | 39.45 | 0.1769 |
| Model 2 | 39.03 | 38.47 | 38.76 | 40.04 | 39.34 | 0.3253 | |
| Minimum Relative Percent Lumen Area | |||||||
| WIQ Stair Climbing Score | Model 1 | 41.42 | 44.73 | 52.23 | 46.79 | 48.42 | 0.088 |
| Model 2 | 42.25 | 45.20 | 52.09 | 46.31 | 47.77 | 0.2398 | |
| SF-12 Physical Functioning Score | Model 1 | 40.78 | 39.69 | 42.68* | 40.15 | 39.45 | 0.3722 |
| Model 2 | 41.02 | 39.84 | 42.63* | 40.02 | 39.25 | 0.1522 | |
| SF-12 Mental Functioning Score | Model 1 | 38.30‡ | 38.71 | 38.61 | 39.76 | 40.24 | 0.0227 |
| Model 2 | 38.36 | 38.75 | 38.60 | 39.73 | 40.19 | 0.045 | |
P<0.001;
P<0.01;
P<0.05. Model 1 adjusts for age, sex, race, smoking, body mass index, statins, leg symptoms, and comorbidities (angina, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, and disk disease). Model 2 adjusts for covariates in Model 1 and the ankle brachial index. P trend tests the degree to which associations across the plaque quintiles are linear.
Our results were not substantially changed when analyses were repeated among participants with an ABI < 0.90.
DISCUSSION
Among 442 participants with PAD, we found that greater plaque area and smaller percent lumen area in the proximal superficial femoral artery were associated with poorer WIQ distance and speed scores, even after adjustment for covariates including age, sex, race, leg symptoms, comorbidities, and the ABI. These findings suggest that greater atherosclerotic plaque burden and smaller percent lumen area are associated with greater patient-perceived walking impairment in distance and speed among men and women with PAD. In contrast, we found no significant associations of plaque area or percent lumen area with the WIQ stair-climbing score, or the SF-12 physical and mental functioning domains.
Reasons for the lack of association of plaque characteristics with the WIQ stair-climbing score and quality of life cannot be discerned from this study. However, mechanisms of impairments in walking endurance and walking speed may differ from mechanisms of impairments in stair-climbing and quality of life in people with PAD. Our findings suggest that among PAD participants, plaque burden in the superficial femoral artery may be more important for patient-perceived impairments in walking endurance and walking speed, while other characteristics such as impaired cardiorespiratory fitness or reduced leg strength may contribute to impaired stair-climbing ability and impaired quality-of-life, respectively, in people with PAD.
To our knowledge, no prior studies have reported associations of MRI-measured atherosclerotic plaque characteristics in the superficial femoral artery with patient-perceived walking impairment or quality of life. Anderson et al reported that among 85 men and women with PAD, six-minute walk distance was modestly correlated with plaque area in the proximal superficial femoral artery (r=−0.30, p<0.01). However, analyses did not adjust for confounders such as age, the ABI, or comorbidities.
Calf skeletal muscle oxygenation may be affected by atherosclerotic blockages ranging in location from the aorto-iliac vessels to the distal superficial femoral and popliteal arteries. In WALCS III, plaque measures were obtained from the proximal segment of the superficial femoral artery. Our results suggest that among PAD participants, plaque area and percent lumen area in the proximal superficial femoral artery of the leg with lowest ABI may be surrogate measures of total lower extremity plaque burden and lumen area. It is important to point out that the ABI measurement is a more cost-effective measure of lower extremity plaque burden than MRI testing.
Because of expansive re-modeling, in which plaque burden expands outward before encroaching on an arterial lumen [27], it is conceivable that only lumen area, but not plaque burden, would be associated with impaired lower extremity muscle oxygenation and functional impairment. However, our findings indicate that both plaque burden and lumen reduction are associated with the WIQ scores in participants with PAD. Our results also show that plaque area and lumen reduction are highly correlated. Previous work demonstrates that more adverse calf muscle characteristics, poorer calf mitochondrial function, poorer leg strength, and higher levels of inflammatory biomarkers are associated with greater functional impairment in men and women with PAD [21,28–30]. Thus, lower extremity functional impairment in patients with PAD is likely multi-factorial.
This study has limitations. First, data are cross-sectional. Causal associations cannot be inferred from data presented here. Second, potential participants with contraindications to MRI testing were excluded. Thus, our findings may not be generalizable to PAD patients with contraindications to MRI testing. Third, only the leg with lowest ABI was scanned. Fourth, we did not collect data on presence or severity of aorto-iliac disease, which may have influenced our results. Fifth, the coefficient of variation percent values for our plaque measures from 5.7% to 12.9%. It is conceivable that some associations may be even stronger with lower coefficient of variation percent values.
In conclusion, greater plaque area and smaller percent lumen area in the proximal superficial femoral artery are associated with poorer WIQ distance and speed scores in men and women with PAD. Further study is needed to identify mediators of the associations reported here. Further study is also needed to determine whether atherosclerotic plaque burden in other lower extremity arteries is associated with poorer WIQ scores.
Table 1.
Baseline Characteristics of Participants with Peripheral Arterial Disease (N=442)
| Study Characteristic | Mean (Standard Deviation) |
|---|---|
| Age (years) | 69.4 (9.9) |
| Male Sex (%) | 65.6 |
| Black Race (%) | 33.3 |
| Ankle Brachial Index | 0.67 (0.17) |
| Body mass index (kg/M2) | 29.3 (6.0) |
| Current Smoking (%) | 24.4 |
| Diabetes mellitus (%) | 38.7 |
| Angina (%) | 22.4 |
| Myocardial Infarction (%) | 21.3 |
| Heart Failure (%) | 13.1 |
| Statin use (%) | 74.7 |
| Leg symptoms | |
| Intermittent claudication (%) | 23.3 |
| Asymptomatic (%) | 20.4 |
| Atypical exertional leg symptoms/carry on (%) | 9.9 |
| Atypical exertional leg symptoms/stop (%) | 19.9 |
| Leg pain on exertion and rest (%) | 26.5 |
| WIQ distance score | 38.5 (31.5) |
| WIQ speed score | 37.1 (26.1) |
| WIQ Stair Climbing Score | 46.8 (28.8) |
| Physical Component Summary (PCS) from SF12 | 40.5 (6.2) |
| Mental Component Summary (MCS) from SF12 | 39.1 (6.1) |
| Mean vessel plaque area | 0.70 (0.17) |
| Mean percent lumen reduction | 0.32 (0.15) |
| Maximum percent lumen reduction | 0.24 (0.14) |
| Maximum vessel plaque area | 0.92 (0.35) |
Acknowledgments
Supported by the National Heart Lung and Blood Institute (R01-HL083064), the Intramural Research Program of the National Institute on Aging, and the Jesse Brown VA Medical Center. Chun Yuan receives research support from VP Diagnostics and from Philips Healthcare. Christopher M. Kramer receives research support from Siemens Healthcare.
Sponsor’s Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis or preparation of the manuscript.
Footnotes
Author Contributions: See cover letter.
References
- 1.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Guralnik JM, Tian L, et al. Associations of borderline and low-normal ankle brachial index values with functional decline at 5-year follow-up: The WALCS (Walking and Leg Circulation Study) J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner AW, Montgomery PS, Killewich LA. Natural history of physical function in older men with intermittent claudication. J Vasc Surg. 2004;40:73–78. doi: 10.1016/j.jvs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Ferrucci L, Guralnik JM, et al. The ankle brachial index is associated with the magnitude of impaired walking among men and women with peripheral arterial disease. Vascular Medicine. 2010;15:251–257. doi: 10.1177/1358863X10365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Hargarten ME, Rutherford RB, et al. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 8.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vascular Medicine. 2006;11:155–163. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 9.Meissner OA, Rieger J, Rieber J, et al. High-resolution MR imaging of human atherosclerotic femoral arteries in vivo: Validation with intra-vascular ultrasound. J Vasc Interv Radiol. 2003;14:227–231. doi: 10.1097/01.rvi.0000058325.82956.63. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Liu K, Carr J, et al. Superficial femoral artery, the ankle brachial index, and leg symptoms in peripheral arterial disease. Circulation Cardiovascular Imaging. 2011 Mar; doi: 10.1161/CIRCIMAGING.110.962183. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Brit J Surg. 1969;56:676–9. doi: 10.1002/bjs.1800560910. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 13.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Altman R, Ashe E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Criqui MH, Liu K, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 17.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Hyvarinen S. Arteriographic findings of claudication patients. Ann Clin Res. 1984;16:1–45. [PubMed] [Google Scholar]
- 19.Mavor GE. The pattern of occlusion in atheroma of the lower limb arteries. The correlation of clinical and arteriographic findings. Br J Surg. 1956;43:352–358. doi: 10.1002/bjs.18004318003. [DOI] [PubMed] [Google Scholar]
- 20.Isbell DC, Meyer CH, Rogers WJ, et al. Reproducibility and reliability of atherosclerotic plaque area measurements in peripheral arterial disease with magnetic resonance imaging. J Cardiovasc Magn Reson. 2007;9:71–76. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JD, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regensteiner JG, et al. Evaluation of Walking Impairment by Questionnaire in Patients with Peripheral Arterial Disease. Journal of Vascular Medicine and Biology. 1990;2:142–152. [Google Scholar]
- 23.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 25.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606.25. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 27.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Ferrucci L, Guralnik JM, et al. Pathophysiologic changes in calf muscle predict mobility loss at two year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Liu K, Ferrucci L, et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;56:1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott MM, Tian L, Ferrucci L, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2008;56:724–729. doi: 10.1111/j.1532-5415.2008.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


