ABSTRACT
The res (restored cell structure by salinity) mutant, recently identified as the first tomato mutant accumulating jasmonate (JA) without stress, exhibited important morphological alterations when plants were grown under control conditions but these disappeared under salt stress. Since the defense responses against stresses are activated in the res mutant as a consequence of the increased expression of genes from the JA biosynthetic and signaling pathways, the mutant may display a tolerance response not only to salt stress but also to multiple stresses. Here, we show that when res mutant plants are grown under the summer natural conditions of the Mediterranean area, with high temperatures and low relative humidity, the characteristic leaf chlorosis exhibited by the mutant disappears and leaves become dark green over time, with a similar aspect to WT leaves. Moreover, the mutant plants are able to achieve chlorophyll and fluorescence levels similar to those of WT. These results hint that research on res tomato mutant may allow very significant advances in the knowledge of defense responses activated by JA against multiple stresses.
KEYWORDS: Growth-defense tradeoff, multiple stresses, phenotyping, semi-arid conditions, Solanum lycopersicum
Agriculture is probably facing its biggest challenge in human history due to world climate change, as the average global Earth surface temperature is significantly rising, drastically affecting global agricultural systems, especially in arid and semi-arid areas, which represent about one-third of the planet surface. The climate change is already causing the surge of major abiotic stresses and will also influence biotic stresses impact on plants and, therefore, it is necessary to develop a resilient agricultural system in order to uphold the increasing food demand worlwide.1,2 Moreover, the problem is more complex due to the fact that plant response to multiple stresses is different from that for individual stresses, and the molecular signaling pathways controlling biotic and abiotic stress responses may interact and antagonize each other.3,4 The responses to biotic and abiotic stresses are largely controlled by different hormone signaling pathways, being JA a clear example.5 Thus, JA confers plants the ability to confront multiple biotic stimuli such as pathogens and herbivorous insects, as well as abiotic stresses like temperature, drought and salt stress.6,7 But it is necessary to take into account that the plant defense against stresses involves an energy cost so it is often accompanied by significant growth inhibition.8,9 How plants coordinate the fluctuating growth-defense dynamics is a critical issue in plant science still not fully understood and it remains a fundamental question in agriculture.10
The recently identified res tomato mutant (restored cell structure by salinity) is the first JA-overexpressing mutant identified in tomato, and the increased expression of genes from the hormone biosynthetic and signaling pathways observed in the mutant plant is associated to high levels of endogenous JA in roots, especially under non-stressful conditions.11 Therefore, res mutant seems to be always in a state of ‘alert’ to confront stress, and the remarkable growth inhibition it suffers represents the energy cost this constitutive activated defense state entails. In this regard, res could be more tolerant to different stressful conditions, of abiotic and biotic nature, precisely because it seems to have permanently activated the genes from the JA-biosynthesis/signaling pathways involved in defense against stress.12 Currently, strategies of positional cloning combined with whole-genome sequencing are being pursued for the localization within the tomato genome of the mutation responsible for the res phenotype.
Significant progress toward a better understanding of stress tolerance has been made in model and crop plants grown normally in controlled environments, but crop plants are exposed to complex environmental challenges in the field, in real agronomic conditions of cultivation, and phenotyping in natural conditions is essential for potential agricultural applications.13 In the recent publication describing res mutant its characterization was carried out under controlled conditions. In these conditions, we observed that res mutant plants exhibited important morphological alterations and cellular disorganization when plants were grown in absence of stress, with leaves showing chlorosis, disorganization of palisade/spongy parenchyma and epidermis tissues and changes in the chloroplasts ultrastructure. Moreover, the res phenotype persisted in the long-term, when plants were grown in greenhouse conditions during winter, as it was shown in the previous paper. However, res plants were able to restore the characteristic WT phenotype and leaf cell structure when subjected to salt stress.11
Here we show that when res mutant plants are grown in summer natural conditions of the Mediterranean area, in greenhouse as it is fulfilled in agriculture systems of the region, where temperatures exceed 40°C and relative humidity may be lower than 25% during daylight, the res mutant is also able to restore its phenotype. WT (cv Moneymaker) and res mutant plants were grown in greenhouse from May to July, using fertirrigation without addition of salt, that is to say, using the optimum culture conditions regarding plant nutrition.11 As showed in Fig. 1A-C, leaf chlorosis disappears and leaves from res mutant became dark green over time, with a similar aspect than that of WT plants. The time-course evolution of chlorophyll content and fluorescence (Fv/Fm) levels showed that WT and mutant plants achieve similar levels in these parameters after 30 d from transplant to greenhouse (Fig. 1D, E). Moreover, it is interesting to point out that the res plants showed a healthy aspect during the whole culture cycle, as observed in res plants after 75 d from transplant to greenhouse, while the WT leaves showed a high degree of senescence and presented diverse symptoms of biotic stresses at the end of the life cycle (Fig. 2). In sum, the phenotype of the res mutant observed in summer conditions typical of the semi-arid areas supports the idea that res mutant can be expected to be more tolerant to diverse stressful conditions, so it is not only limited to tolerance toward salt stress, and it is able to show such features because of its constitutive activation of subsets of defense genes. Furthermore, the research on res could allow very significant advances in the knowledge of JA function in the trade-off plant growth vs defense, a central question today in plant science research and a critical one from the agronomic point of view.
Figure 1.
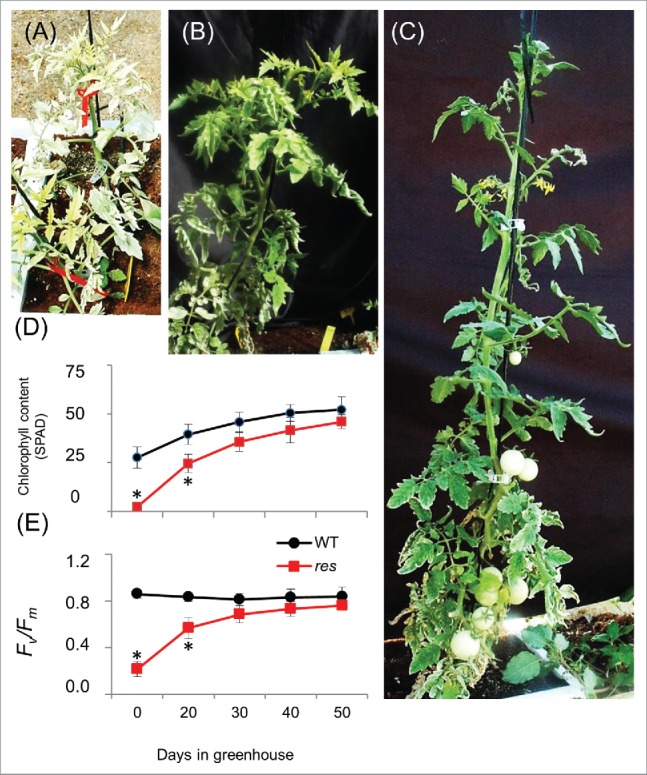
The res tomato mutant phenotype reverts to WT phenotype when the plants are grown to long-term under summer natural conditions. Representative pictures of res mutant when the plants are transplanted to greenhouse (A), and after 20 (B) and 50 (C) days. (D, E) Evolution of leaf chlorophyll content (SPAD) and fluorescence (Fv/Fm) to long-term. Data are expressed as mean values ± SE of 6 individual plants per line. Asterisks indicate significant differences by Student t-test between WT and mutant plants (P < 0.05).
Figure 2.

The res tomato plants show a healthy aspect at the end of the culture cycle under summer natural conditions. Representative pictures of WT and res mutant plants after 75 d from transplant to greenhouse. On the right, detail of the WT and res leaves in the upper part of the plant (white marked areas on the left), where a high degree of senescence is evident in the WT leaves but not in the res ones.
Material and methods
Plant material and culture conditions
The res mutant comes from tomato (Solanum lycopersicum L.) cv Moneymaker. To monitor growth development during greenhouse culture of mutant plants non-mutated wild-type (WT) Moneymaker tomato plants were also grown. The culture conditions of greenhouse are described in Garcia-Abellan et al.14 The greenhouse is located in the South-East region of Murcia (Spain), in the municipality of Santomera.
Chlorophylls analysis
Chlorophylls content and fluorescence analyses were fulfilled according to Garcia-Abellan et al.11
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant AGL2015-64991-C3-2-R from the Spanish ‘Ministerio de Economía’.
References
- 1.Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M. A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant Cell Environ. 2014;37:2024–35; PMID:24417440; doi: 10.1111/pce.12274. [DOI] [PubMed] [Google Scholar]
- 2.Shaik R, Ramakrishna W. Machine learning approaches distinguish multiple stress conditions using stress-responsive genes and identify candidate genes for broad resistance in rice. Plant Physiol. 2014;164:481–95; PMID:24235132; doi: 10.1104/pp.113.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: From genes to the field. J Exp Bot. 2014;63:3523–44; PMID:22467407; doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 4.Prasch CM, Sonnewald U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013;162:1849–66; PMID:23753177; doi: 10.1104/pp.113.221044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol. 2013;161:2159–70; PMID:23444343; doi: 10.1104/pp.113.214544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015;20:219–29; PMID:25731753; doi: 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann Bot. 2013;111:1021–58; PMID:23558912; doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui H. Killing two birds with one Stone. Plant Signal Behav. 2012;7:701–3; PMID:22580500; doi: 10.4161/psb.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huot B, Yao J, Montgomery BL, He SY. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant. 2014;7:1267–87; PMID:24777989; doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shyu C, Brutnell TP. Growth–defence balance in grass biomass production: the role of jasmonates. J Exp Bot. 2015;66:4165–76; PMID:25711704; doi: 10.1093/jxb/erv011. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Abellan JO, Fernandez-Garcia N, Lopez-Berenguer C, Egea I, Flores FB, Angosto T, Capel J, Lozano R, Pineda B, Moreno V, et al.. The tomato res mutant which accumulates JA in roots in non-stressed conditions restores cell structure alterations under salinity. Physiol Plant. 2015;155:296–314. 2015; PMID:25582191; doi: 10.1111/ppl.12320. [DOI] [PubMed] [Google Scholar]
- 12.Wasternack C. Action of jasmonates in plant stress responses and development — Applied aspects. Biotech Adv. 2014;32:31–9; PMID:24095665; doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Araus JL, Cairns JE. Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci. 2014;19:52–61; PMID:24139902; doi: 10.1016/j.tplants.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Abellan JO, Egea I, Pineda B, Sanchez-Bel P, Belver A, Garcia-Sogo B, Flores FB, Atares A, Moreno V, Bolarin MC. Heterologous expression of the yeast HAL5 gene in tomato enhances salt tolerance by reducing shoot Na+ accumulation in the long term. Physiol Plant. 2014;152: 700–13; PMID:24773242; doi: 10.1111/ppl.12217. [DOI] [PubMed] [Google Scholar]


