ABSTRACT
Aldehyde molecules were shown to induce gene expression but because of their toxicity, the cell expresses ALDEHYDE DEHYDROGENASES (ALDH, EC 1.2.1.3) that oxidize them to carboxylic acids. To understand how the aldehydes may be both toxic and gene activators, we expressed the ALDH7B4 gene promoter fused to the β-glucuronidase reporter gene in independent transgenic lines and found that pentanal and trans-2-hexenal activated the promoter whereas trans-2-hexenal induced the ALDH7B4 protein. Paraquat led to higher amounts of malondialdehyde compared to trans-2-hexenal and H2O2, and only the treatment by Paraquat activated the ALDH7B4 promoter, indicating that a threshold level of aldehydes is required for gene activation. These findings suggest that ALDH activity may also serve to fine-tune gene activation by the aldehydes.
KEYWORDS: Aldehyde Dehydrogenase; carbonylation; lipid peroxidation; reactive carbonyl species; signalling; α,β-unsaturated aldehydes
Lipid peroxidation-derived aldehydes are potentially toxic due to their extreme reactivity with the nucleophilic compounds (nucleic acids, proteins, membrane lipids) present in different cell compartments,1 and they are cleared from the cell by ALDEHYDE DEHYDROGENASES, NAD(P)+ dependent enzymes (ALDH, EC 1.2.1.3) that are widely distributed in all organisms including human and plant genomes.2,3 We previously demonstrated that significant amounts of toxic aldehydes are produced during dehydration, salt, heat, and oxidative stresses and are followed by an increased ALDH expression. Gain-of-function and loss-of-function of selected stress-responsive ALDHs were more tolerant and more sensitive to these stresses than the wild type, respectively.1,4,5 Most recently, we found that ALDH mutants were more sensitive to heat and to the combination of heat with drought, salt, or wounding stresses than the wild type.6 All these previous findings indicate that ALDHs are most likely induced by intermediate signaling molecules such reactive oxygen species (ROS) and lipid peroxidation-derived aldehydes (reactive carbonyl species) common to dehydration, salt, wounding, and heat stresses. Indeed, some aldehyde molecules including malondialdehyde (MDA), trans-2-hexenal and 4-hydroxynonenal were proved to function as powerful gene activators despite their potential toxicity.7–13 For the aldehydes to function as gene activator despite their potential toxicity, we hypothesized that a fine-tuning of their intracellular concentration might be necessary.
To understand how the aldehydes may induce gene activation besides their cytotoxicity, we have in a first step analyzed the responsiveness of the ALDH gene promoter to ROS and aldehydes. For that, we used the promoter of the ALDH7B4 gene that was previously shown to be activated by dehydration, salinity, heat, excessive light, heavy metals (Cu2+ and Cd2+), H2O2 and ABA treatment.1,4,14,15 Transgenic plants expressing the β-glucuronidase (GUS) reporter gene driven by the ALDH7B4 gene promoter were generated.15 As in numerous studies reporting about the bioactivity of aldehyde molecules in animals and plants,16–20 we found that pentanal and trans-2-hexenal activated the ALDH7B4 gene promoter in independent transgenic lines.21 Electrophilic aldehydes containing an α,β-unsaturated carbonyl (Michael acceptor) were shown to induce gene expression more efficiently than their aliphatic counterparts.8 Consistent with this, we found that the ALDH7B4 promoter activity measured by the GUS activity was induced more strongly by trans-2-hexenal than pentanal (27.0 ± 2.6 versus 11.1 ± 1.1 pmol 4-MU min−1 mg protein−1; test t of student, P < 0.05).21 The ALDH7B4 protein was monitored in parallel with the endogenous ALDH7B4 promoter activity by using protein-blot. The ALDH7B4 protein also accumulated in response to trans-2-hexenal,21 thus demonstrating the responsiveness of the ALDH7B4 gene to aldehydes and particularly to trans-2-hexenal. In another experiment, the ALDH7B4 promoter activity was compared to the contents of MDA, which is a Michael acceptor widely used as a marker of free-radical-catalyzed lipid peroxidation.8 Leaves were detached from soil-grown 4 week-old transgenic plants and incubated in water (as control), 300 mM NaCl, 30 mM H2O2, 5 µmol trans-2-hexenal or 50 µM Paraquat® (methyl viologen) for 8 h.21 The leaves were then divided into pools and used for the in situ detection of reductive aldehydes, the quantification of the GUS activity and the determination of the MDA content. There were more MDA in leaves treated by H2O2 than in trans 2-hexenal-treated and water-treated leaves (19.2 ± 2.5, 13.7 ± 1.0, and 33.4 ± 1.1 nmol MDA equivalents fresh weight−1 for water, trans 2-hexenal, and H2O2, respectively; test t of student, P < 0.05) but no difference was observed for the ALDH7B4 promoter activity in these samples after 8 h.21 In contrast, the Paraquat® treatment led to a significantly high amounts of aldehydes (shown by the Schiff reagent assay18) and MDA (49.8 ± 5.2 nmol MDA equivalents fresh weight−1, test t of student, P < 0.05), concomitantly with a significant increase of the ALDH7B4 promoter activity (172.4 ± 18.7, 163.1 ± 8.9, 126.1 ± 57.7, and 401.2 ± 64.1 pmol 4-MU min−1 mg protein−1, for water, H2O2, trans 2-hexenal, and Paraquat®, respectively; test t of student, P < 0.05).21 The ALDH7B4 gene thus appeared to be induced only beyond a threshold of the intracellular concentration of aldehydes and ROS. In fact, it was shown that the concentration and the localization of unbound MDA would be key factors directing its biological activities in vivo, and that about 75% of total MDA content in expanded leaves was shown to originate from trienoic fatty acids while the source of the second pool is so far unknown.8 Moreover, by using triple fatty acid desaturase (fad3-2fad7-2fad8) mutants deficient in the accumulation of the trienoic fatty acid, it was found that a basal MDA level is kept in plant tissues in physiological conditions, and that the biological activity of MDA mostly derives from changes in the free MDA pool that is dynamic and increases upon stress.10,22,23 Our observation that Paraquat® led to a significant accumulation of MDA in parallel with a strong ALDH7B4 promoter activity is consistent with these previous works and underlines the correlation between the intracellular ROS level, bioactive aldehydes, and gene expression. Moreover, the induction of ALDH7B4 by direct application of aldehydes clearly indicates that ALDHs can sense and fine-tune the levels of aldehydes by reacting with the reactive carbonyls for proper gene activation. The findings that the ALDH7B4 protein was carbonylated in salt-stressed Arabidopsis leaves support this hypothesis.24 Moreover, ALDHs have been co-purified in signaling complexes,25–27 and ALDH3H1 was recently shown to interact with the Arabidopsis extra-large G proteins XLG1 and XLG3 on the plasma membrane; the interaction was confirmed in both yeast-two-hybrid and bimolecular fluorescence complementation assays.28 Our data and these previous findings clearly suggest a dual role for the ALDH proteins as sensors of lipid-derived reactive carbonyl species and as modulators of gene activation by aldehydes and ROS (Fig. 1). The α,β-unsaturated aldehydes possess a thiol-reactivity that allows them to covalently modify proteins in vivo,13,29 and it is unlikely that lipid peroxidation-derived aldehydes directly interact with the target gene promoters in the nucleus. The most obvious signal relay mechanism by aldehydes must be a covalent modification of trans-acting factors. The ALDHs would exert their role of sensors of oxidative stress or modulators of gene activation by aldehydes either by terminating the activity of aldehydes when oxidizing them into carboxylic acids, or after being themselves carbonylated, which may lead to the release of a downstream positive or negative regulator of the target genes as shown before.30
Figure 1.
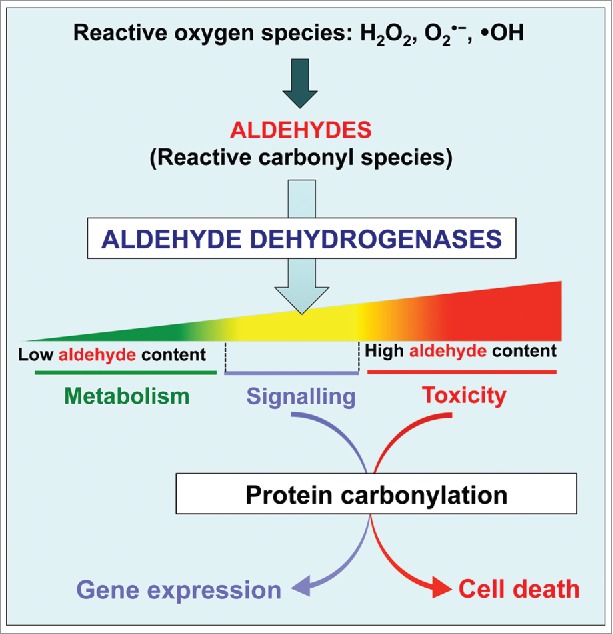
Modulation of the aldehyde bioactivity by ALDEHYDE DEHYDROGENASES (ALDHs). Reactive carbonyl species including aldehydes are derived from the metabolism and the peroxidation of membrane lipids by reactive oxygen species. As the aldehyde levels increase at the onset of stress or developmental cues, protein carbonylation occurs and may serve for signaling or trigger cell death if carbonylated proteins accumulate in the cells. ALDH expression is induced to control the levels of bioactive aldehydes by oxidizing them to their corresponding carboxylic acids.
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
TDM is grateful for DAAD financial support for the completion of Ph.D. thesis. TDM would like to thank Dr. Dorothea Bartels (University of Bonn, Germany) for her generosity and Ph.D. mentorship. TDM is grateful for the technical support of Dr. Kotchoni, SO (Rutgers-Camden University).
References
- 1.Stiti N, Missihoun TD, Kotchoni SO, Kirch H-H, Bartels D. Aldehyde dehydrogenases in Arabidopsis thaliana: Aiochemical requirements, metabolic pathways, and functional analysis. Front Plant Sci. 2011;2:65. doi: 10.3389/fpls.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocker C, Vasiliou M, Carpenter S, Carpenter C, Zhang Y, Wang X, Kotchoni SO, Wood AJ, Kirch H-H, Kopečný D, et al.. Aldehyde dehydrogenase (ALDH) superfamily in plants: gene nomenclature and comparative genomics. Planta. 2013;237:189–210. doi: 10.1007/s00425-012-1749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotchoni SO, Kuhns C, Ditzer A, Kirch H-H, Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell Environ. 2006;29:1033–48. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 5.Missihoun TD, Willée E, Guegan J-P, Berardocco S, Shafiq MR, Bouchereau A, Bartels D. Overexpression of ALDH10A8 and ALDH10A9 genes providesinsight into their role in glycine betaine synthesis and affects primary metabolism in Arabidopsis thaliana. Plant Cell Physiol. 2015;56:1798–807. doi: 10.1093/pcp/pcv105. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Missihoun TD, Bartels D. The role of Arabidopsis aldehyde dehydrogenase genes in response to high temperature and stress combinations. J Exp Bot. 2017;171:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita M, Hossain MZ. Modulation of pumpkin glutathione S-transferases by aldehydes and related compounds. Plant Cell Physiol . 2003;44:481–90. doi: 10.1093/pcp/pcg060. [DOI] [PubMed] [Google Scholar]
- 8.Weber H, Chételat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004;37:877–88. doi: 10.1111/j.1365-313X.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- 9.Scott W; Sattler E, Mè Ne-Saffrané L, Farmer EE, Krischke M, Mueller MJ, Dellapenna D. Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell. 2006;18:3706–20. doi: 10.1105/tpc.106.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mène-Saffrané L, Davoine C, Stolz S, Majcherczyk P, Farmer EE. Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant: Whole-body mapping of malondialdehyde pools in a complex eukaryote. J Biol Chem. 2007;282:35749–56. doi: 10.1074/jbc.M706838200. [DOI] [PubMed] [Google Scholar]
- 11.Mirabella R, Rauwerda H, Struys EA, Jakobs C, Triantaphylidès C, Haring MA, Schuurink RC. The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J. 2008;53:197–213. doi: 10.1111/j.1365-313X.2007.03323.x. [DOI] [PubMed] [Google Scholar]
- 12.Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, Berger S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell. 2008;20:768–85. doi: 10.1105/tpc.107.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller MJ, Berger S. Reactive electrophilic oxylipins: pattern recognition and signalling. Phytochemistry. 2009;70:1511–21. doi: 10.1016/j.phytochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Kirch H-H, Schlingensiepen S, Kotchoni S, Sunkar R, Bartels D. Detailed expression analysis of selected genes of the aldehyde dehydrogenase (ALDH) gene superfamily in Arabidopsis thaliana. Plant Mol Biol. 2005;57:315–32. doi: 10.1007/s11103-004-7796-6. [DOI] [PubMed] [Google Scholar]
- 15.Missihoun TD, Hou Q, Mertens D, Bartels D. Sequence and functional analyses of the aldehyde dehydrogenase 7B4 gene promoter in Arabidopsis thaliana and selected Brassicaceae: regulation patterns in response to wounding and osmotic stress. Planta. 2014;239:1281–98. doi: 10.1007/s00425-014-2051-0. [DOI] [PubMed] [Google Scholar]
- 16.Ullery JC, Marnett LJ. Protein modification by oxidized phospholipids and hydrolytically released lipid electrophiles: Investigating cellular responses. Biochim Biophys Acta. 2012;1818:2424–35. doi: 10.1016/j.bbamem.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weismann D, Binder CJ. The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta. 2012;1818:2465–75. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas MS, Mano J. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 2015;168:885–98. doi: 10.1104/pp.115.256834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, et al.. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SS, Song ZB, Sun Z, Zhang J, Mei Y, Nian HJ, Li KZ, Chen LM. Physiological and transcriptional analysis of the effects of formaldehyde exposure on Arabidopsis thaliana. Acta Physiol Plant. 2012;34:923–36. doi: 10.1007/s11738-011-0889-3. [DOI] [Google Scholar]
- 21.Missihoun TD. Characterisation of selected Arabidopsis aldehyde dehydrogenase genes: Role in plant stress physiology and regulation of gene expression. 2011; https://bonnus.ulb.uni-bonn.de/SummonRecord/FETCH-bonn_catalog_30988862/SISISHoldings#tabnav
- 22.Schmid-Siegert E, Loscos J, Farmer EE. Inducible malondialdehyde pools in zones of cell proliferation and developing tissues in Arabidopsis. J Biol Chem. 2012;287:8954–62. doi: 10.1074/jbc.M111.322842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid-Siegert E, Stepushenko O, Glauser G, Farmer EE. Membranes as structural antioxidants: Recycling of malondialdehyde to its source in oxidation-sensitive chloroplast fatty acids. J Biol Chem. 2016;291:13005–13. doi: 10.1074/jbc.M116.729921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mano J, Nagata M, Okamura S, Shiraya T, Mitsui T. Identification of oxidatively modified proteins in salt-stressed arabidopsis: A carbonyl-targeted proteomics approach. Plant Cell Physiol. 2014;55:1233–44. doi: 10.1093/pcp/pcu072. [DOI] [PubMed] [Google Scholar]
- 25.Knuesting J, Riondet C, Maria C, Kruse I, Bécuwe N, König N, Berndt C, Tourrette S, Guilleminot-Montoya J, Herrero E, et al.. Arabidopsis glutaredoxin S17 and its partner, the nuclear factor Y subunit C11/negative cofactor 2α, contribute to maintenance of the shoot apical meristem under long-day photoperiod. Plant Physiol. 2015;167:1643–58. doi: 10.1104/pp.15.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Wang Y, Zheng X, Jia Q, Zhao J, Bai F, Hong Y, Liu Y. Cytoplastic Glyceraldehyde-3-phosphate dehydrogenases interact with ATG3 to negatively regulate autophagy and Immunity in Nicotiana benthamiana. Plant Cell. 2015;27:1316–31. doi: 10.1105/tpc.114.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, et al.. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–74. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Gao Y, Jones AM. Extra large G-protein interactome reveals multiple stress response function and partner-dependent XLG subcellular localization. Front Plant Sci. 2017;8:1015. doi: 10.3389/fpls.2017.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farmer EE, Davoine C. Reactive electrophile species. Curr Opin Plant Biol. 2007;10:380–6. doi: 10.1016/j.pbi.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442(3):453–64. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]


