ABSTRACT
Mildew resistance locus O (MLO) proteins are transmembrane proteins that mediate cell-cell communication in plants. We recently demonstrated the importance of subcellular localization to MLO function during pollen tube reception. NORTIA (NTA), the MLO protein involved in this process, localizes to the Golgi of the synergid cell before interaction with the pollen tube. MLO proteins that can substitute for NTA's function in this pathway all partially localize with the same Golgi marker in the synergid cell. In this study, we report that MLO subcellular localization is cell type-dependent, with different distributions of some MLOs observed when ectopically expressed in the epidermal cells of tobacco and Arabidopsis compared to synergids. This dependency may be due to co-factors that influence MLO function within a given cell type, providing an intriguing new target for understanding MLO distribution and subsequent function in their respective processes.
KEYWORDS: Cell biology, Golgi, golgi apparatus, endomembrane system, MLO, plant sexual reproduction, secretory system, secretory pathway, subcellular localization, transient expression
Mildew resistance locus O (MLO) proteins are a plant-specific family of seven-spanning transmembrane proteins that mediates cell-cell communication in response to various stimuli.1 Most notably MLOs were first identified as susceptibility factors involved in powdery mildew infection as part of a conserved signaling pathway found in both monocots and dicots.2,3 In Arabidopsis thaliana, MLOs have since been found to regulate root thigmomorphogenesis and the process of pollen tube (PT) reception.4,5 PT reception occurs in the final stages of sexual reproduction in flowering plants as the tip-elongating PT interacts with the synergid cells of the female gametophyte initiating a signaling cascade that results in one of two synergids degenerating and PT rupture, releasing two sperm cells into the embryo sac.6 NORTIA (NTA) is an MLO protein that mediates this communication from within the synergid cell. In nortia homozygous mutants, PT-synergid communication is disrupted resulting in an overall reduction in fertilization.5
As with other MLOs, the molecular function of NTA within its pathway has remained elusive. NTA localizes within Golgi-associated compartments prior to interaction with the PT but polarly redistributes toward a region of invaginated plasma membrane, called the filiform apparatus, sometime during PT reception.5,7 This redistribution is dependent on the activity of the receptor-like kinase FERONIA, an upstream component regulating PT reception, and may be important for NTA's role in regulating PT-synergid communication.5 The barley MLO protein also redistributes in response to fungal penetration to become polarly localized to the site of infection.8 In both PT reception and powdery mildew infection contact between a plant cell and a tip-growing cell triggers MLO redistribution, presumably following the perception of some signal. This signal-dependent polarization may be a conserved trait amongst MLOs, thus understanding the regulation of MLO subcellular trafficking may provide insight into MLO function. In a recent study, we found a divergence in the ability of related Arabidopsis MLO proteins to function in PT reception when ectopically expressed in the synergid cells of the nortia (nta-1) mutant.7 MLO function in this pathway correlated with their subcellular localization prior to PT arrival. The MLOs tested that could rescue nta-1 also co-localized with a fluorescent Golgi marker in the synergid cell (MLO10 and MLO2), whereas those that couldn't rescue nta-1 did not overlap with the same marker (MLO8 and MLO1). This implies that MLO localization within Golgi-associated compartments of synergids is important for their function in PT reception.
Here we report that MLO localization may be dependent on the cell type in which the proteins are expressed. Co-localization experiments in tobacco epidermal cells reveal that some MLOs show common subcellular distributions in synergid cells and epidermal cells, while others exhibit different subcellular localizations when ectopically expressed in the two cell types. NTA, MLO10, and MLO8 all displayed similar localization patterns in synergids and tobacco epidermal cells. NTA-GFP partially co-localized with a Golgi marker and had no overlap with markers for either the endoplasmic reticulum or peroxisomes in both cell types (Fig. 1).7 MLO10 and MLO8 also showed no appreciable difference in localization between the two cell types, with MLO10 co-localizing with the Golgi marker and MLO8 accumulating in punctate compartments similar to those seen in synergid cells (Fig. 2A, B).7 However, both MLO2 and MLO1 had unique distribution patterns in tobacco, localizing to the periphery of the epidermal cells with no detectable accumulation in punctate compartments (Fig. 2C, D). Whereas MLO2 partially co-localized with the Golgi marker in synergid cells, MLO2 accumulated only within specific subdomains (potentially in the plasma membrane) associated with epidermal cell lobes (Fig. 2C). In contrast to MLO1's polar distribution at the filiform apparatus in synergid cells, MLO1-GFP was evenly distributed around the periphery of tobacco epidermal cells (Fig. 2D). In order to determine if the observed cell type-dependent localization was due to our transient assay being done in a heterologous system, we also generated stable overexpression lines of MLO1 and NTA in the Columbia ecotype (Col-0) of A. thaliana (Fig. 3). As in our transient assay, MLO1-YFP localized predominantly to the periphery of epidermal pavement cells in a pattern similar to that seen in tobacco (Fig. 3A, B vs. Fig. 2D), while NTA-YFP accumulated in punctate compartments consistent with the endomembrane system (Fig. 3C, D).
Figure 1.
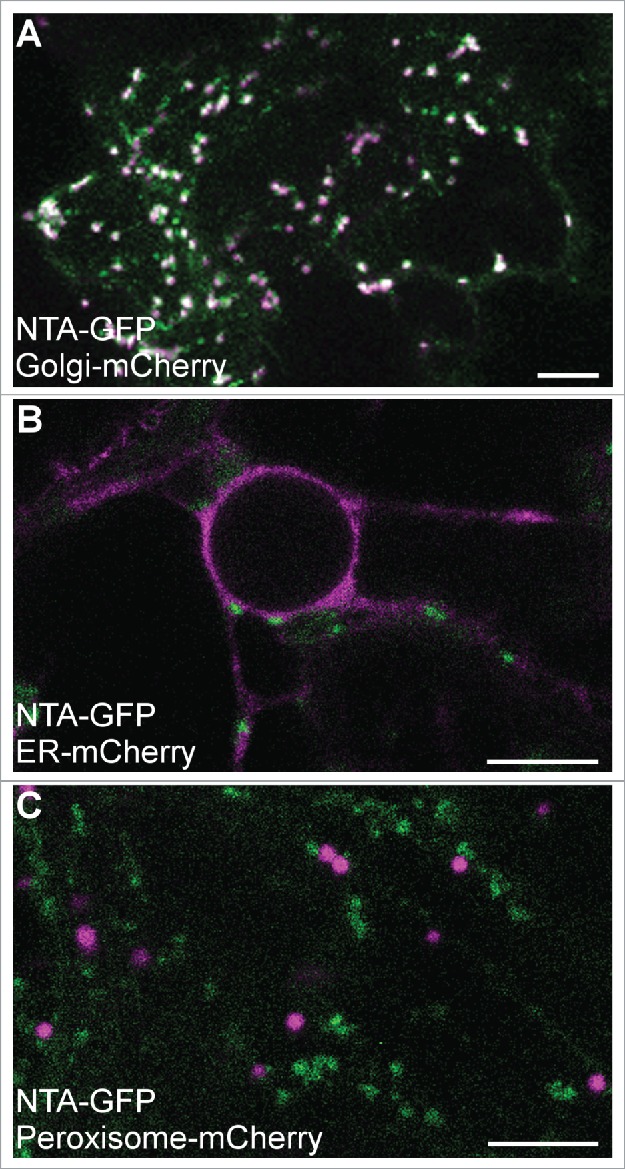
Transient co-expression of 35Spro:NTA-GFP (green signal) with mCherry-labeled secretory markers (magenta signal) in in tobacco epidermal cells.13 (A) NTA-GFP (green) colocalizes with Golgi (Man49-mCherry) (white indicates overlapping GFP and mCherry signals). (B) NTA-GFP does not colocalize with an Endoplasmic Reticulum marker (SP-mCherry-HDEL). (C) NTA-GFP does not colocalize with peroxisomes (mCherry-PTS1). Four- to Six-week-old Nicotiana benthamiana plants were used in all transient co-expression assays. Agrobacterium tumefaciens (GV3101) harboring the NTA-GFP (or other MLO) and respective marker constructs were co-infiltrated into mature leaves. Imaging was done using a Leica SP8 confocal microscope three days after infiltration similar to our previous study.7 Bars = 10 μm.
Figure 2.
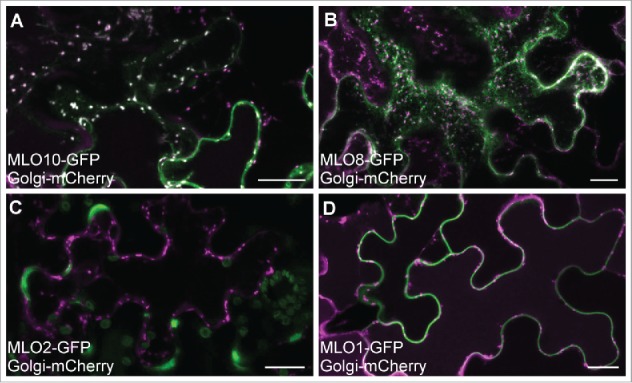
MLO co-localization with a Golgi marker in tobacco epidermal cells. A-D) 35Spro:MLO-GFP (green) transient co-expression with the Man49-mCherry Golgi marker (magenta). (A) MLO10-GFP colocalizes with the Golgi marker (white spots indicate co-localization). (B) MLO8-GFP is distributed in punctate pattern that does not overlap with Golgi. (C) MLO2-GFP localizes to the periphery of epidermal lobes and does not overlap with Golgi. (D) MLO1-GFP localizes with the periphery of the cells and does not overlap with Golgi. Bars = 20 μm.
Figure 3.
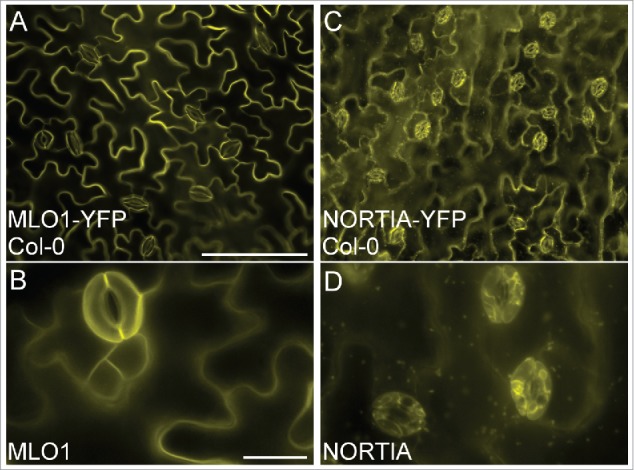
Distribution of ectopically expressed MLO1 and NTA in the epidermal cells of Arabidopsis thaliana. (A-B) 35Spro:MLO1-YFP localizes to the periphery of epidermal pavement cells (yellow signal). (C-D) 35Spro:NTA-YFP localizes in a punctate pattern consistent with the endomembrane system in epidermal pavement cells (yellow signal). Stable lines were generated via floral dip transformation14 of Col-0 using the GV3101 strain of A. tumefaciens harboring each construct. 35Spro:NTA-YFP cloning was described previously7 and the 35Spro:MLO1-YFP construct was cloned using the MLO1 cDNA Gateway compatible vector previously reported7 in a manner similar to NTA's construction. T1 plants were grown on soil and lines were selected following 4 days of BASTA herbicide application. Rosette leaves were imaged from 15–20 d old plants after screening for YFP, on a Nikon Eclipse Ni-U compound epifluorescent microscope equipped with an X-Cite LED fluorescent lamp and YFP dichroic filter. Bars = A, C) 100 μm; B, D) 20 μm.
In summary, NTA, MLO10, and MLO8 were incorporated into endomembrane-associated compartments within both synergid cells and tobacco epidermal cells, while MLO2 and MLO1 had cell type-dependent localization patterns. We also demonstrated that NTA and MLO1 maintained their respective epidermal cell distributions when stably transformed into A. thaliana. NTA, MLO10, and MLO8 are closely related and are all a part of Clade III within the MLO family.9 We recently reported a difference between these three MLOs and the rest of the family in that NTA, MLO10 and MLO8 all have predicted N-terminal signal peptides.7 Upon removal of NTA's signal peptide (NTA Δsp), there was no difference in its function in PT reception or its localization within the synergid cell. However, when expressed in tobacco epidermal cells, NTA Δsp's distribution resembled that of MLO2's, accumulating in subdomains of epidermal cell lobes instead of partially co-localizing with the Golgi marker.7 Since MLO2 has no predicted N-terminal signal peptide, the differences in MLO localization may represent the influence of additional co-factors involved in PT reception (in the synergid cell) or involved in an unknown process occurring within the epidermal cell lobe. This observation fits well with the finding that both MLO2 and NTA Δsp rescue nta-1 and thus can function in PT reception.7 Co-factors influencing localization may be of functional significance within these pathways/processes and remain a target of interest for understanding the role of MLO localization (as well as polar redistribution). As MLO proteins have a conserved calmodulin-binding domain in their C-terminal intracellular tail, and interaction with calmodulin is required for MLO function in powdery mildew susceptibility10, an obvious candidate for the co-factor influencing MLO distribution is calmodulin. In barley, interactions between MLO and calmodulin differ along the cell periphery in epidermal cells, with positive interactions occurring within subdomains of this cell type.8 This provides some evidence for differential processes involving MLOs occurring within subdomains of the epidermal cell; however, the functional significance of these spatial limitations remains unknown.
Overall the observed cell type-dependent localization patterns of MLOs indicate a reliance on additional components within the cell for their distribution. With the recent finding that the ability of MLOs to function in PT reception correlates with their protein localization to a Golgi-associated compartment, we propose that regulation of subcellular distribution of MLO proteins within a cell may be a mechanism for controlling MLO function in response to various external stimuli. Synergid cells are very unique among plant cells. Before PT arrival they act as secretory factories that pump out LURE peptides from the filiform apparatus to attract PTs.11 After attraction, the PT halts its growth and communicates with the receptive synergid cell, inducing subcellular changes in the synergid involving calcium oscillations and NTA redistribution to the filiform apparatus.5,12 While the molecular mechanism and functional relevance behind the redistribution of NTA during PT reception is still under investigation, our results suggest that the ability to regulate MLO trafficking may provide plasticity within the MLO pathway in a given cell type and provides evidence for a more complex regulation of MLO-mediated intercellular communication.
References
- 1.Acevedo-Garcia J, Kusch S, Panstruga R. Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytol. 2014;204:273–81. [DOI] [PubMed] [Google Scholar]
- 2.Buschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, et al.. The barley Mlo gene: a novel control element of plant pathogen resistance. Cell. 1997;88:695–705. doi: 10.1016/S0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- 3.Consonni C, Humphry ME, Hartmann HA, Livaja M, Durner J, Westphal L, Vogel J, Lipka V, Kemmerling B, Schulze-Lefert P, et al.. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat Genet. 2006;38:716–20. doi: 10.1038/ng1806. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Noir S, Kwaaitaal M, Hartmann HA, Wu MJ, Mudgil Y, Sukumar P, Muday G, Panstruga R, Jones AM. Two seven-transmembrane domain MILDEW RESISTANCE LOCUS O proteins cofunction in Arabidopsis root thigmomorphogenesis. The Plant cell. 2009;21:1972–91. doi: 10.1105/tpc.108.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–71. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 6.Beale KM, Johnson MA. Speed dating, rejection, and finding the perfect mate: advice from flowering plants. Curr Opin Plant Biol. 2013;16:590–7. doi: 10.1016/j.pbi.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Jones DS, Yuan J, Smith B, Willoughby A, Kumimoto EL, Kessler SA. Mildew Resistance Locus o function in pollen tube reception is linked to its oligomerization and subcellular distribution. Plant Physiol. 2017;175(1):172–185. doi: 10.1104/pp.17.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat RA, Miklis M, Schmelzer E, Schulze-Lefert P, Panstruga R. Recruitment and interaction dynamics of plant penetration resistance components in a plasma membrane microdomain. Proc Natl Acad Sci U S A. 2005;102:3135–40. doi: 10.1073/pnas.0500012102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusch S, Pesch L, Panstruga R. Comprehensive Phylogenetic Analysis Sheds Light on the Diversity and Origin of the MLO Family of Integral Membrane Proteins. Genome Biol Evol. 2016;8:878–95. doi: 10.1093/gbe/evw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MC, Panstruga R, Elliott C, Muller J, Devoto A, Yoon HW, Park HC, Cho MJ, Schulze-Lefert P. Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nature. 2002;416:447–51. doi: 10.1038/416447a. [DOI] [PubMed] [Google Scholar]
- 11.Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol. 2015;66:393–413. doi: 10.1146/annurev-arplant-043014-115635. [DOI] [PubMed] [Google Scholar]
- 12.Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Nelson BK, Cai X, Nebenfuhr A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J: for cell and molecular biology. 2007;51:1126–36. doi: 10.1111/j.1365-313X.2007.03212.x. [DOI] [PubMed] [Google Scholar]
- 14.Bent A. Arabidopsis thaliana Floral Dip Transformation Method. In: Wang K, ed. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2006. [DOI] [PubMed] [Google Scholar]


