Significance
Sensory experience, even at prenatal periods, can shape brain connectivity. Thus, the emergence of sensory responses is a key step in cortical development. Sensory cortical responses are thought to emerge in cortical layer 4, which is the adult target of thalamic projections. However, in developing animals, thalamic fibers do not target layer 4 but instead target subplate neurons in the white matter. We show that subplate neurons respond to sounds before layer 4 is activated by thalamic axons. Moreover, early local field potential (LFP) responses demonstrate nascent topographic organization. Together we find that sound-evoked cortical activity and topographic organization emerge in a different layer than thought. Since subplate circuits are disrupted in autism spectrum disorder (ASD) models, disrupted emergence of sensory activity could be utilized for diagnosis and intervention.
Keywords: cortex, development, fetal, hearing, sensory
Abstract
In utero experience, such as maternal speech in humans, can shape later perception, although the underlying cortical substrate is unknown. In adult mammals, ascending thalamocortical projections target layer 4, and the onset of sensory responses in the cortex is thought to be dependent on the onset of thalamocortical transmission to layer 4 as well as the ear and eye opening. In developing animals, thalamic fibers do not target layer 4 but instead target subplate neurons deep in the developing white matter. We investigated if subplate neurons respond to sensory stimuli. Using electrophysiological recordings in young ferrets, we show that auditory cortex neurons respond to sound at very young ages, even before the opening of the ears. Single unit recordings showed that auditory responses emerged first in cortical subplate neurons. Subsequently, responses appeared in the future thalamocortical input layer 4, and sound-evoked spike latencies were longer in layer 4 than in subplate, consistent with the known relay of thalamic information to layer 4 by subplate neurons. Electrode array recordings show that early auditory responses demonstrate a nascent topographic organization, suggesting that topographic maps emerge before the onset of spiking responses in layer 4. Together our results show that sound-evoked activity and topographic organization of the cortex emerge earlier and in a different layer than previously thought. Thus, early sound experience can activate and potentially sculpt subplate circuits before permanent thalamocortical circuits to layer 4 are present, and disruption of this early sensory activity could be utilized for early diagnosis of developmental disorders.
Sensory experience can shape brain connectivity and later perception. Such sensory experience can occur during the prenatal period, as evidenced by the newborn humans’ preference for maternal speech (1–3), which likely is due to in utero experience of the mother’s voice. The preference for maternal speech and species-specific voices is disrupted in neurodevelopmental disorders such as autism spectrum disorders (ASDs) (4–8), and disruption in early sensory-evoked activity might predict the emergence of developmental disorders (9). This raises the question of when sensory-evoked activity arises in the cerebral cortex and which specific neural circuits are responsive to sensory stimuli at the earliest ages.
Sensory stimuli are processed in primary sensory cortices, and these cortices contain large-scale maps of sensory stimulus properties—for example, tonotopic maps in the auditory cortex (ACX) or ocular dominance columns in the visual cortex (VCX). These maps are formed by patterned projections from the thalamus to layer 4 (L4) and can be sculpted by sensory experience during the postnatal critical period (10–16). In adults, L4 neurons are the target of thalamocortical (TC) axons and are thought to be the locus of the emergence of sensory responses and functional organization; thus, studies of the emergence and influence of experience have focused on L4 and beyond (10–16). However, during early development, the ingrowing TC axons do not innervate L4 directly. Instead of growing into L4, TC axons grow into and synapse within the future white matter and excite the earlier-born subplate neurons (SPNs), which in turn project to L4 (17–21) (Fig. 1A). Thus, there is a period during which thalamic information is relayed to L4 via SPNs, and this period lasts from days in rodents to several weeks in humans (17), raising the possibility that sensory responses and topographic organization might emerge earlier and in a different layer, SP, than thought. Such an early emergence of sensory responses might provide a substrate for early experience-dependent plasticity, for example, that could underlie the preference of newborn humans for maternal speech.
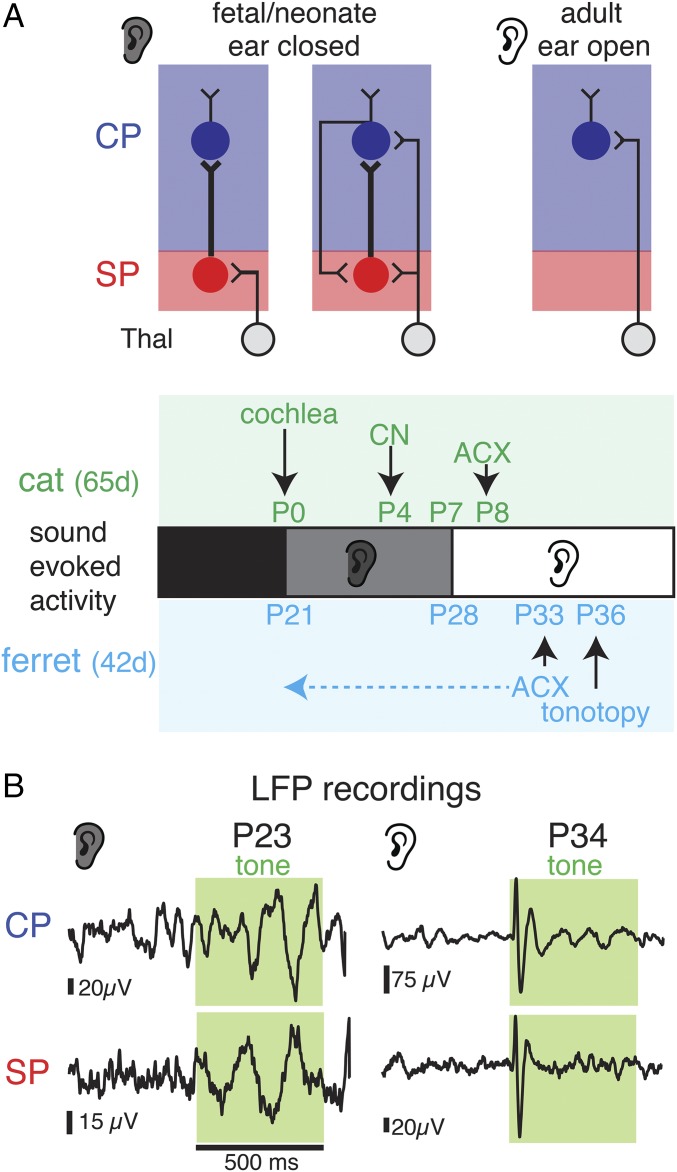
Fig. 1.
Sound-evoked field potentials in the developing ferret ACX. (A) Schematic of changing TC and SP circuits. At young ages, SP receives thalamic input and projects to the CP, specifically L4 (17, 18, 24). Ears are closed at this early stage (gray shading). Later in development, TC afferents innervate CP and SPNs receive inputs from the CP (17, 23, 24). Ears are open at this stage (white ear). Developmental timeline below shows events and published responses in ferret and cat. Horizontal dashed arrow shows that our data show cortical responses close to the onset of cochlear function. (B) LFP traces in response to sounds (green shading) at P22 and P34 in SP and CP.
SPNs are a key part of the developing TC and intracortical circuits during this early developmental period, which lasts at least to around birth in cat VCX and during the first 10 postnatal (P) days in mouse ACX (17, 22–25). Removal of SPNs prevents development of both TC and intracortical circuits, indicating that SPNs are essential for cortical development (17, 22, 23, 26) and SPN malfunction has been implicated in neurodevelopmental disorders (21, 27). Sensory experience during the critical period, when thalamic afferents innervate L4, can sculpt L4 circuits and beyond (13–16, 24), but the existence of thalamic inputs to SPNs raises the possibility that sensory stimuli can activate SPNs before L4 becomes active.
In altricial animals (animals born in an inchoate state), the onset of thalamic transmission to L4 coincides with ear opening (24); thus, sound experience before the ears open might activate and influence cortical circuits at early ages. Indeed, early sound-evoked activity is present at lower stages of auditory processing. Ear canals in domestic cats open at ∼1 wk of age, and cortical responses have been shown in the middle layers of ACX from P8 onwards (28). However, sound-evoked responses are present in the cochlea around birth (29), in cochlear nucleus by P4 (30–32), and in the inferior colliculus from P6 (33) (Fig. 1A), raising the potential that immature ACX can respond to sound as well. In the visual system, recordings in young ferrets revealed responses in the lateral geniculate nucleus (LGN) and VCX to visual stimulation through the closed eyelids at ∼P20 [eyes open at ∼P32 ∼ P11 cat (34)] (Fig. 1A) (35, 36). Thus, sensory information can be accessible to the brain at early ages, but the laminar emergence of sensory responses is unknown. Since SPNs are the first target of thalamic axons, we investigated in ferrets if early sound exposure can activate SPNs in ACX.
Results
To test the possibility that SPN neurons in ACX respond to sounds at young ages, we used in vivo electrophysiological recordings in anesthetized neonatal ferrets (n = 48 animals; P21–P37) (Fig. 1A). Ferrets are similar in cortical organization to cats but are much more immature at birth [P8 cat ∼ P29 ferret (34); Fig. 1A]. Sound-evoked responses in ferrets have been shown to emerge in the middle and superficial ACX as early as P33, and tonotopic maps can be delineated by P36 (37). Multielectrode array (MEA) recordings (16 channels, 2 × 8 electrode linear array, 250 μm spacing) were sequentially obtained from the midcortical plate (CP) (459 ± 102 μm, putative L4, n = 1,360 recordings in 85 tracks) and deep cortical locations (958 ± 208 μm, putative subplate, SP, n = 1,376 recordings in 86 tracks) (Fig. S1). We detected sound-evoked local field potentials (LFPs) in 2,519/2,736 recordings across all ages. Varying the frequency and level of the presented sounds to map the frequency response area (FRA) showed that LFPs could show frequency selectivity (Fig. S1). LFPs were oscillatory and longer lasting in young animals than in older animals (Fig. 1B and Fig. S1). Thus, while prior work in cats demonstrated neuronal responses in ACX at P8 (∼P29 in ferret) (28), our results show that sound-evoked responses in ACX are present at much earlier ages, suggesting that ascending pathways to the cortex are present at these ages. In particular, our recordings show responses in both the deep and midcortical layers.
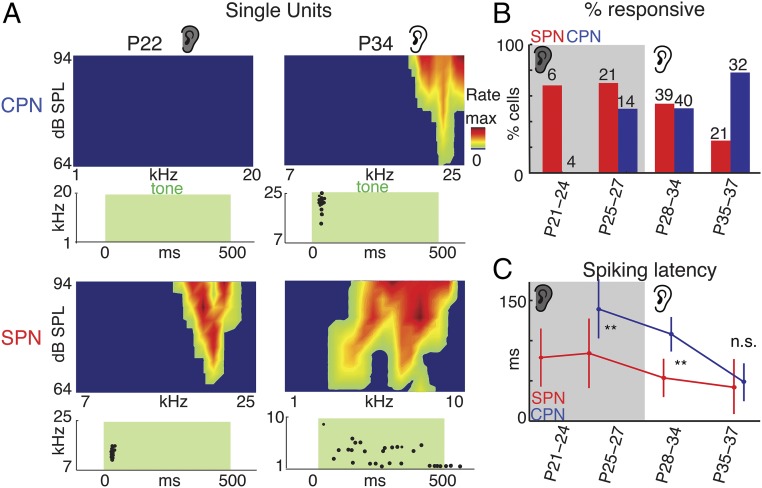
To identify which neurons contributed to the sound-evoked LFP, we isolated single units from both deep and superficial recording sites (n = 177 cells) (Fig. 2A and Fig. S2). Sound-responsive single units were present in SP as early as P21 and in CP by P25 (Fig. 2B and Fig. S2). Together these recordings show that SPNs are the first neurons responsive to sound in the ACX.
Fig. 2.
SP and L4 neurons respond to sound. (A) Spike raster plot and FRAs of exemplar neurons to pure tones (green shading) presented at 64–94 dB sound pressure level (SPL). Max rates are as follows: CP, 0.0 Hz, 3.4 Hz; SP, 2.8 Hz, 1.2 Hz. (B) Fraction of responsive and unresponsive CPNs and SPNs. Gray shading indicates when ears were closed. Numbers indicate recorded cells at each age and group. (C) First spike latencies (mean ± SD) to sounds for SPNs and CPNs. CPN latencies were adult-like by P35 (79). **P < 0.001. Since no CPNs responded at P21–P24, no latency could be measured.
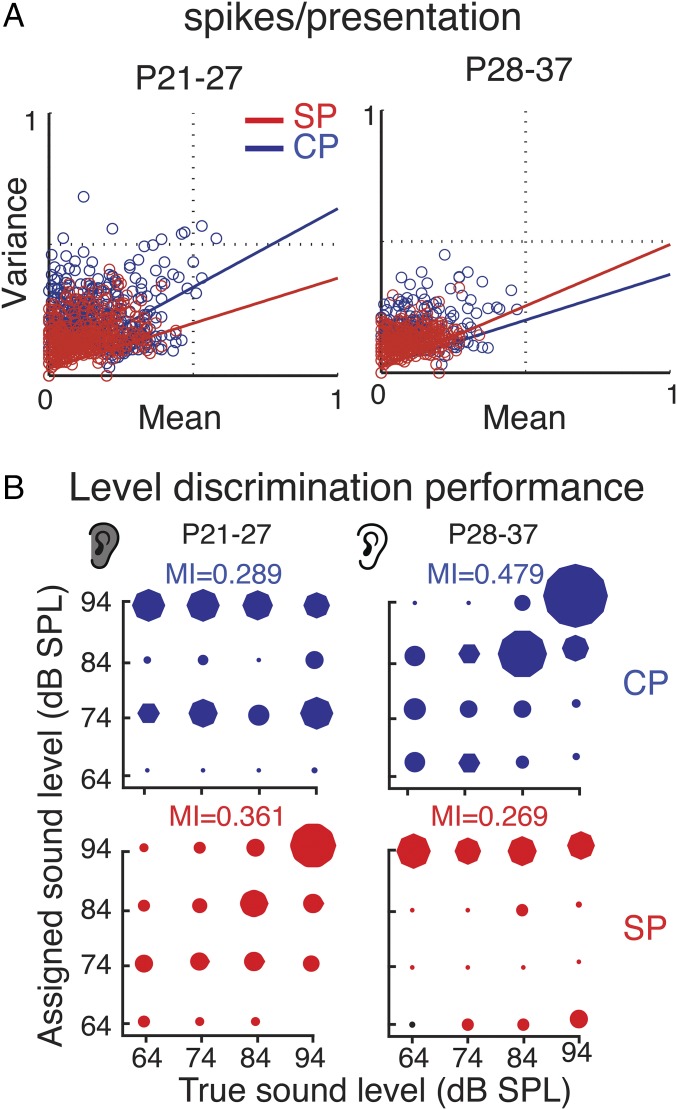
Having established the existence of sound-driven spiking neurons in both layers, we next compared the properties of sound-evoked responses. White matter stimulation in slices shows a sequential activation of SPN and L4 due to the relay of thalamic activity from SPN to L4 before thalamic axons drive L4 (17, 18, 24). We thus compared sound-evoked spiking latencies in SPN and CP neurons. Spiking latencies are shorter in SPNs than in CP before P35 (Fig. 2C), consistent with a role of SPNs relaying sensory information to CP at young ages (17, 18, 24) (Fig. 1A). To compare the fidelity of the sound-evoked response, we analyzed the sound-evoked spike trains from SPNs and CP neurons. Before ear opening, SPNs show lower variability in their spike trains (Fig. 3A) and encode sound-level changes with higher fidelity than CP neurons (Fig. 3B). Thus, at early ages, sound stimuli are first and best encoded by cortical SPNs and not L4 neurons. Together our data show that SPNs are the first neurons in ACX to respond to sound. While SPN responses are largely transient, the evoked oscillations (Fig. 1B and Fig. S1) persist for a time period, suggesting that either a population of SPNs are activated at varying latencies or that spiking SPNs drive an intrinsic oscillatory circuit. Nevertheless, we likely underestimated the number of responsive cells due to anesthesia (38) and limitations of electrophysiological recordings in young animals due to lack of myelination, which makes cell isolation more difficult. Therefore, it is possible that SPNs respond to sounds at even earlier ages. In addition, the lack of sound-driven spiking responses in CP before P25 while LFPs were present in CP at these ages suggests that sound-driven LFPs in CP reflect synaptic inputs from SPNs.
Fig. 3.
SP neurons encode sounds better than CP neurons at young ages. (A) Graphs show reliability (variance over mean) of spiking over repeated sound presentations for SP and CP neurons. Colored lines indicate linear regression. At young ages (Left), SP neurons show less variability, while at older ages CP neurons show lower variability. (B) Confusion matrices for sound level-based multiple discriminate analysis (MDA) classification. MDA was used to assign neural responses to individual stimuli according to intensity based on the spike count vectors for every stimulus repetition. The mutual information (MI) was calculated to quantify the relationship between the predicted and predictor variable.
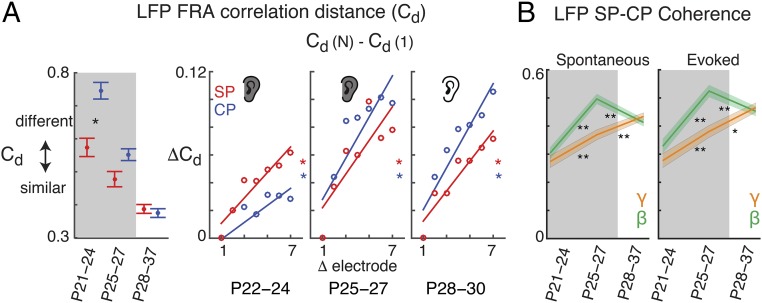
The existence of early sound-evoked responses in ACX raised the question of whether a nascent topographic organization of sound features exists. Adult ACX shows a tonotopic organization of frequency preference due to topographic TC projections to L4 that establish local similarity of frequency tuning preference (39, 40). We therefore measured the similarity of simultaneously recorded FRAs measured from LFPs (Fig. S1E) by computing the correlation distance (Cd) within each layer. Cd measures the dissimilarity of the FRAs. The average Cd was lower across electrodes within SP than electrodes within CP; thus, FRAs on different electrodes were more similar within SP than CP (Fig. 4A, Left and Fig. S3). Moreover, plotting Cd as a function of electrode position, FRAs recorded from neighboring electrodes (250 μm apart) within each layer were more similar than those recorded from electrodes farther (e.g., up to 1.75 mm) apart (Fig. 4A, Right and Fig. S3). Thus, neurons at neighboring electrodes prefer similar stimuli, and this similarity is largest in SP. Over development, Cd decreased in the CP, indicating that the local FRA similarity in CP increased. In addition, Cd between distant electrodes on the MEA increased, indicating that distant FRAs became different (Fig. 4A, Right and Fig. S3). These changes are consistent with the onset of responsiveness and emergence of tonotopy in L4 (37). The higher local tuning similarity and larger distance dependence in SP suggests that the nascent tonotopy was stronger in the SP.
Fig. 4.
Coarse topographic organization is present in SP. (A) LFP–FRA Cd measured between different electrodes within SP or CP in the MEA. A Cd of 0 indicates maximum positive correlation of the FRA, while a Cd of 1 indicates maximum dissimilarity (no correlation) of the FRA. A Cd of 2 indicates maximum anticorrelation. (Left) Average Cd across all electrodes pairs (P < 0.05). (Right) Cd normalized to Cd at the first electrode pair (data from Fig. S3A and normalized to Cd at an electrode distance of 1; error bars omitted for clarity). Line shows regression fit (for slopes: P22–P24 CP, SP: P = 0.015, P = 0.0018; P25–P27 CP, SP: P = 0.012, P = 0.022; P28–P30 CP, SP: P = 0.0018, P = 0.0014). (B) Mean CP/SP coherence in beta (14–30 Hz) and gamma band (30–110 Hz) for stimulus-driven and spontaneous activity (*P < 0.05; **P < 0.001; Welch’s t test).
Since after P24 both SPNs and CP neurons respond to sound and since SPNs excite L4 neurons (17, 18), we investigated if we could detect functional interactions between SP and CP. We performed simultaneous recordings from SP and CP using staggered electrode arrays (fixed depth separation, 600 μm, n = 384 recordings in 24 tracks) and calculated the spectral coherence between SP and CP (Fig. S4). Significant coherences between SP and CP activity are both present at P21, indicating a potential functional interaction between these layers by this age (Fig. 4B and Fig. S4). Coherence increased with age, paralleling the onset of CP spiking responses. This finding appears to be consistent with the sequential thalamic innervation of SPNs and L4 neurons and the excitatory projections from SPNs to L4 (17, 18, 24). We observed significant coherence between SP and CP not only during stimulus presentation but also during ongoing activity. This suggests that while peripheral spontaneous and evoked activity can drive SP circuits, other sources might be present. Immature cortical circuits can generate spontaneous activity in isolation (41), and this activity might originate or might drive activity in SPNs.
Together our in vivo recordings in ferret show that sound stimuli at early ages through the closed ears trigger neural activity in ACX and that this activity as well as its topographic organization occurs first in SP and not in L4.
Discussion
Our results show that acoustic stimuli activate young cortical neurons, especially SPNs, before responses in L4 can be detected. Moreover, we find that a nascent topographic organization is present from an early age. These results suggest that sensory-evoked cortical activity and organization emerge in a different layer than previously appreciated. While both circuit maturation (e.g., lack of direct TC inputs to L4 at young ages) and cellular maturation (e.g., numbers of ion channels) can contribute to later laminar differences in response to strength and reliability, refinement of connectivity likely underlies the differences in tonotopy.
In adults, besides the dominant projection to L4, thalamic fibers innervate deep cortical neurons, setting up parallel pathways (42). Since some SPNs remain into adulthood and become integrated into layer 6 (17, 20), the responding neurons we find here could be “future” thalamorecipient layer 6 neurons consistent with morphological similarities between SPNs and layer 6b neurons (43). Thus, our results suggest that topographic maps in deep cortical layers precede those in the middle and superficial layers. Deep cortical layers in adults are also targets of TC inputs and might form a parallel processing stream to the L4-initiated superficial stream (42). Our results suggest that this potential parallel path emerges earlier in development. Since SPNs project to L4 (18, 20), a remnant of this projection might ensure the synchrony of these two streams.
In early development, peripheral spontaneous activity is crucial for the establishment of ascending circuits (44–51), while at later ages, specifically after opening of the eyes and ears, sensory experience refines this nascent architecture (16, 52). The earlier sound-evoked activity we describe possibly interacts with spontaneously generated activity, and manipulations of early spontaneous activity may also alter processing of early auditory stimuli.
The onset of the classic critical period in L4 coincides with the maturation of TC connections to L4. Therefore, we delineate an intermediate developmental phase during which the thalamic innervation to L4 is immature but when sensory stimuli (e.g., sound deprivation or dark rearing) might influence cortical SPNs. Thus, we speculate that there exists an earlier period to the classic critical period in which sensory stimuli can influence SPN circuits and which can set the stage for plasticity during the classic critical period.
Early visual-evoked oscillatory LFP responses exist in the VCX before eye opening (38). Since SPN removal prevents sensory-evoked cortical oscillations (26), these early oscillations are likely mediated by SPNs.
While our results show that early sensory stimuli activate cortical circuits in a primary sensory area, it is unclear if higher order areas respond as well. While our studies did not address this issue, the delayed maturation of intracortical circuits (53) suggests that this is unlikely.
Although closed ear canals in altricial animals muffle sounds, we show that sounds can activate the cortex. Similarly, auditory experience in a human fetus is attenuated by the womb but can lead to a preference for maternal voice in newborns (1–3). In prenatal humans, auropalpebral reflexes to sound emerge in the 20th gestational week, indicating that the cochlea and reflex circuits are functioning (54). However, auditory thresholds are also higher because sounds are attenuated by the uterus (55). Additionally, both external sounds and maternal heartbeats can elicit fetal magnetoencephalographic (MEG) responses (56–65), but due to the nature of MEG, a laminar source could not be identified. Moreover, because MEG is a population measure, it is likely that individual neural responses emerge earlier.
Due to the attenuation by the uterus or closed ears, only certain stimuli will activate cortical circuits. While rhythmic sounds (e.g., heartbeats) might lead to attenuated responses due to synaptic depression, nonrhythmic sounds such as maternal or pup vocalization might elicit SPN responses and could therefore drive network plasticity. Indeed, our results show that SPNs could have single-peaked or multipeaked FRAs. Multipeaked FRAs are seen in adult A1 of many species and might underlie processing of harmonic stimuli—for example, vocalizations (66–69). Our results reveal that the sensory activity emerges in a sequential fashion and that SPNs are the earliest responsive neurons. SPNs are present in all sensory systems. While the maturational time courses differ between sensory systems, it is likely that early sensory experiences of other modalities also activate SPNs in the respective cortical areas. SPNs are also present in other neocortical areas, but the function of SPNs in these areas has not been explored.
Since SPN malfunction has been implicated in neurodevelopmental disorders, such as ASDs (21, 27), changes in the early sensory-evoked activity might underlie the functional deficits in speech preference and perception in ASDs (4–6, 8) and could be utilized for early diagnosis and intervention (7, 9, 70).
Materials and Methods
Detailed methods can be found in SI Materials and Methods.
All procedures were approved by the University of Maryland College Park Institutional Animal Care and Use Committee (IACUC). All ferrets were obtained from Marshall Farms. Ferret kits (n = 48) of both sexes were anesthetized, and a small craniotomy (>2 mm) was made above the ACX. Extracellular electrode arrays (MEAs) (6 MΩ impedance; Microprobe) were inserted into A1, orthogonally to the cortical surface, using a motorized manipulator (MP285; Sutter Instruments). Recording locations were categorized as being in the CP (L4) if they were recorded in midcortical locations (∼300–700 µm from pia) and putative SPNs if they were recorded >700 to >1,000 µm from pia based on histology (71–73) (Fig. S1).
Electrode signals were amplified and digitized using a 32-channel recording system (Neuralynx Cheetah). All stimuli were generated using a computer-controlled digital signal processor (RX6; Tucker-Davis Technologies, TDT), attenuated (PA5; TDT), and presented using a power amplifier (Crown) and high-output speakers (Fostex). Spike sorting was carried out using a standard model of unsupervised clustering, and significant neuronal responses were identified using a binless algorithm, following which standard metrics were calculated (74). Cells were classified as driven if their firing rate was at least 2 SDs above the mean of the distribution of spontaneous rates for the population of cells. A multiple discriminant analysis (MDA) was used to assign neural responses to individual stimuli according to intensity (74).
For simultaneous laminar recordings in CP and SP, custom electrodes (2–6 MΩ impedance; Microprobe) consisting of two rows of either eight or two electrodes per row with the rows staggered in depth by 600 µm and rows spaced by 250 µm were used to record LFP signals. Coherence was calculated using a multitaper method (75–77) essentially as the normalized zero-lag cross-correlation in frequency domain. Mean coherence was calculated across all stimuli, all electrodes (grouped by deep or shallow), and across penetrations.
LFP-driven activity was measured by z scoring stimulus interval activity relative to LFP variance measured during the 5-s prestimulus interval, which allowed for the inclusion of both long latency slow oscillation responses and more adult-like LFP responses (78). The similarity of simultaneously recorded LFP FRAs was measured as the Cd between z-scored LFP responses at each FRA stimulus (frequency and sound level):
where xs and xt are the z-scored LFP FRAs obtained by the same sound frequency and level combinations for the pairs of electrodes s and t. Cd can vary between 0 and 2, with 1 indicating no correlation, 0 indicating perfect correlation, and 2 indicating anticorrelation.
Results are plotted as means ± SD unless otherwise indicated. Populations are compared with a rank sum or Student’s t test (based on Lilliefors test for normality) unless indicated otherwise.
Supplementary Material
Acknowledgments
We thank Drs. Didier Depireux and Barak Shechter for help with preliminary studies; Ji Liu for help with in vivo analysis; and Aminah Sheikh, Jacob Calvert, and Emily Zhang for histological help. This work was supported by NIH Grant R01DC009607 (to P.O.K.) and the Alfred P. Sloan Foundation (P.O.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710793114/-/DCSupplemental.
References
- 1.DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 2.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 3.Voegtline KM, Costigan KA, Pater HA, DiPietro JA. Near-term fetal response to maternal spoken voice. Infant Behav Dev. 2013;36:526–533. doi: 10.1016/j.infbeh.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klin A. Young autistic children’s listening preferences in regard to speech: A possible characterization of the symptom of social withdrawal. J Autism Dev Disord. 1991;21:29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- 5.Paul R, Chawarska K, Fowler C, Cicchetti D, Volkmar F. “Listen my children and you shall hear”: Auditory preferences in toddlers with autism spectrum disorders. J Speech Lang Hear Res. 2007;50:1350–1364. doi: 10.1044/1092-4388(2007/094). [DOI] [PubMed] [Google Scholar]
- 6.Whitehouse AJ, Bishop DV. Do children with autism ‘switch off’ to speech sounds? An investigation using event-related potentials. Dev Sci. 2008;11:516–524. doi: 10.1111/j.1467-7687.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtin S, Vouloumanos A. Speech preference is associated with autistic-like behavior in 18-months-olds at risk for autism spectrum disorder. J Autism Dev Disord. 2013;43:2114–2120. doi: 10.1007/s10803-013-1759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klin A. Listening preferences in regard to speech in four children with developmental disabilities. J Child Psychol Psychiatry. 1992;33:763–769. doi: 10.1111/j.1469-7610.1992.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 9.Bacon EC, et al. Rethinking the idea of late autism spectrum disorder onset. Dev Psychopathol. 2017:1–17. doi: 10.1017/S0954579417001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 11.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 12.Schreiner CE, Polley DB. Auditory map plasticity: Diversity in causes and consequences. Curr Opin Neurobiol. 2014;24:143–156. doi: 10.1016/j.conb.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 17.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Kao JP, Kanold PO. Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. J Neurosci. 2009;29:15479–15488. doi: 10.1523/JNEUROSCI.4471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng R, Kao JPY, Kanold PO. Distinct translaminar glutamatergic circuits to GABAergic interneurons in the neonatal auditory cortex. Cell Rep. 2017;19:1141–1150. doi: 10.1016/j.celrep.2017.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan S, Sheikh A, Looger LL, Kanold PO. Molecularly defined subplate neurons project both to thalamocortical recipient layers and thalamus. Cereb Cortex. 2016;27:4759–4768. doi: 10.1093/cercor/bhw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoerder-Suabedissen A, Molnár Z. Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci. 2015;16:133–146. doi: 10.1038/nrn3915. [DOI] [PubMed] [Google Scholar]
- 22.Meng X, Kao JP, Kanold PO. Differential signaling to subplate neurons by spatially specific silent synapses in developing auditory cortex. J Neurosci. 2014;34:8855–8864. doi: 10.1523/JNEUROSCI.0233-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32:1589–1601. doi: 10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friauf E, Shatz CJ. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol. 1991;66:2059–2071. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- 26.Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci. 2012;32:692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagode DA, et al. Abnormal development of the earliest cortical circuits in a mouse model of autism spectrum disorder. Cell Rep. 2017;18:1100–1108. doi: 10.1016/j.celrep.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugge JF, Reale RA, Wilson GF. Sensitivity of auditory cortical neurons of kittens to monaural and binaural high frequency sound. Hear Res. 1988;34:127–140. doi: 10.1016/0378-5955(88)90100-1. [DOI] [PubMed] [Google Scholar]
- 29.Pujol R, Hilding D. Anatomy and physiology of the onset of auditory function. Acta Otolaryngol. 1973;76:1–10. doi: 10.3109/00016487309121476. [DOI] [PubMed] [Google Scholar]
- 30.Brugge JF, Kitzes LM, Javel E. Postnatal development of frequency and intensity sensitivity of neurons in the anteroventral cochlear nucleus of kittens. Hear Res. 1981;5:217–229. doi: 10.1016/0378-5955(81)90047-2. [DOI] [PubMed] [Google Scholar]
- 31.Brugge JF, Javel E, Kitzes LM. Signs of functional maturation of peripheral auditory system in discharge patterns of neurons in anteroventral cochlear nucleus of kitten. J Neurophysiol. 1978;41:1557–1559. doi: 10.1152/jn.1978.41.6.1557. [DOI] [PubMed] [Google Scholar]
- 32.Brugge J. Development of the lower brainstem auditory nuclei. In: Romand R, editor. Development of the Auditory and Vestibular System. Academic; New York: 1983. pp. 89–120. [Google Scholar]
- 33.Blatchley BJ, Brugge JF. Sensitivity to binaural intensity and phase difference cues in kitten inferior colliculus. J Neurophysiol. 1990;64:582–597. doi: 10.1152/jn.1990.64.2.582. [DOI] [PubMed] [Google Scholar]
- 34.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 35.Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/s0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- 36.Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed lids. J Neurophysiol. 2001;85:1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- 37.Mrsic-Flogel TD, Versnel H, King AJ. Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. Eur J Neurosci. 2006;23:780–792. doi: 10.1111/j.1460-9568.2006.04609.x. [DOI] [PubMed] [Google Scholar]
- 38.Colonnese MT, et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackett TA, Barkat TR, O’Brien BM, Hensch TK, Polley DB. Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J Neurosci. 2011;31:2983–2995. doi: 10.1523/JNEUROSCI.5333-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: Structure and function. Trends Neurosci. 2005;28:255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- 42.Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marx M, et al. Neocortical layer 6B as a remnant of the subplate–A morphological comparison. Cereb Cortex. 2015;27:1011–1026. doi: 10.1093/cercor/bhv279. [DOI] [PubMed] [Google Scholar]
- 44.Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- 45.Cang J, et al. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 47.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 48.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clause A, et al. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron. 2014;82:822–835. doi: 10.1016/j.neuron.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- 54.Birnholz JC, Benacerraf BR. The development of human fetal hearing. Science. 1983;222:516–518. doi: 10.1126/science.6623091. [DOI] [PubMed] [Google Scholar]
- 55.Werner LA. Issues in human auditory development. J Commun Disord. 2007;40:275–283. doi: 10.1016/j.jcomdis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porcaro C, et al. Fetal auditory responses to external sounds and mother’s heart beat: Detection improved by independent component analysis. Brain Res. 2006;1101:51–58. doi: 10.1016/j.brainres.2006.04.134. [DOI] [PubMed] [Google Scholar]
- 57.Blum T, Saling E, Bauer R. First magnetoencephalographic recordings of the brain activity of a human fetus. Br J Obstet Gynaecol. 1985;92:1224–1229. doi: 10.1111/j.1471-0528.1985.tb04866.x. [DOI] [PubMed] [Google Scholar]
- 58.Wakai RT, Leuthold AC, Martin CB. Fetal auditory evoked responses detected by magnetoencephalography. Am J Obstet Gynecol. 1996;174:1484–1486. doi: 10.1016/s0002-9378(96)70592-6. [DOI] [PubMed] [Google Scholar]
- 59.Eswaran H, et al. Challenges of recording human fetal auditory-evoked response using magnetoencephalography. J Matern Fetal Med. 2000;9:303–307. doi: 10.1002/1520-6661(200009/10)9:5<303::AID-MFM10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 60.Zappasodi F, et al. Detection of fetal auditory evoked responses by means of magnetoencephalography. Brain Res. 2001;917:167–173. doi: 10.1016/s0006-8993(01)02901-8. [DOI] [PubMed] [Google Scholar]
- 61.Schleussner E, et al. Fetal magnetoencephalography: A non-invasive method for the assessment of fetal neuronal maturation. BJOG. 2001;108:1291–1294. doi: 10.1111/j.1471-0528.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 62.Schneider U, Schleussner E, Haueisen J, Nowak H, Seewald HJ. Signal analysis of auditory evoked cortical fields in fetal magnetoencephalography. Brain Topogr. 2001;14:69–80. doi: 10.1023/a:1012519923583. [DOI] [PubMed] [Google Scholar]
- 63.Lengle JM, Chen M, Wakai RT. Improved neuromagnetic detection of fetal and neonatal auditory evoked responses. Clin Neurophysiol. 2001;112:785–792. doi: 10.1016/s1388-2457(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 64.Eswaran H, et al. Short-term serial magnetoencephalography recordings of fetal auditory evoked responses. Neurosci Lett. 2002;331:128–132. doi: 10.1016/s0304-3940(02)00859-5. [DOI] [PubMed] [Google Scholar]
- 65.Draganova R, et al. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28:354–361. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Sutter ML, Schreiner CE. Physiology and topography of neurons with multipeaked tuning curves in cat primary auditory cortex. J Neurophysiol. 1991;65:1207–1226. doi: 10.1152/jn.1991.65.5.1207. [DOI] [PubMed] [Google Scholar]
- 67.Noreña AJ, Gourévitch B, Pienkowski M, Shaw G, Eggermont JJ. Increasing spectrotemporal sound density reveals an octave-based organization in cat primary auditory cortex. J Neurosci. 2008;28:8885–8896. doi: 10.1523/JNEUROSCI.2693-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadia SC, Wang X. Spectral integration in A1 of awake primates: Neurons with single- and multipeaked tuning characteristics. J Neurophysiol. 2003;89:1603–1622. doi: 10.1152/jn.00271.2001. [DOI] [PubMed] [Google Scholar]
- 69.Winkowski DE, Kanold PO. Laminar transformation of frequency organization in auditory cortex. J Neurosci. 2013;33:1498–1508. doi: 10.1523/JNEUROSCI.3101-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vouloumanos A, Curtin S. Foundational tuning: How infants’ attention to speech predicts language development. Cogn Sci. 2014;38:1675–1686. doi: 10.1111/cogs.12128. [DOI] [PubMed] [Google Scholar]
- 71.Gao WJ, Wormington AB, Newman DE, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: Immunocytochemical localization of calbindin- and parvalbumin-containing neurons. J Comp Neurol. 2000;422:140–157. doi: 10.1002/(sici)1096-9861(20000619)422:1<140::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 72.Gao WJ, Newman DE, Wormington AB, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: Immunocytochemical localization of GABAergic neurons. J Comp Neurol. 1999;409:261–273. doi: 10.1002/(sici)1096-9861(19990628)409:2<261::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 73.Smith AL, Thompson ID. Spatiotemporal patterning of glutamate receptors in developing ferret striate cortex. Eur J Neurosci. 1999;11:923–934. doi: 10.1046/j.1460-9568.1999.00501.x. [DOI] [PubMed] [Google Scholar]
- 74.Petrus E, et al. Crossmodal induction of thalamocortical potentiation leads to enhanced information processing in the auditory cortex. Neuron. 2014;81:664–673. doi: 10.1016/j.neuron.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- 76.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 77.Womelsdorf T, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 78.Eggermont JJ, Munguia R, Pienkowski M, Shaw G. Comparison of LFP-based and spike-based spectro-temporal receptive fields and cross-correlation in cat primary auditory cortex. PLoS One. 2011;6:e20046. doi: 10.1371/journal.pone.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.