Significance
Homeostasis of Pdpn+ fibroblastic reticular cells (FRCs) is thought to be regulated by hematopoietic cells. However, the cellular and molecular mechanisms of such homeostasis have been poorly understood, especially under the steady-state condition in adults. We show that dendritic cells, particularly CD4+ conventional dendritic cells (cDCs), are crucial for the homeostasis of FRCs in the adult spleen under the steady-state condition. The production of TNF receptor ligands by CD4+ cDCs regulates such homeostasis of FRCs, with SIRPα and CD47 likely being indispensable for the production of TNFR ligands by CD4+ cDCs. The CD47–SIRPα interaction on CD4+ cDCs may thus control the homeostasis of FRCs, which in turn maintain homeostasis of T cells in the white pulp of the spleen.
Keywords: dendritic cell, fibroblastic reticular cell, signal regulatory protein α, CD47, tumor necrosis factor-α
Abstract
In secondary lymphoid organs, development and homeostasis of stromal cells such as podoplanin (Pdpn)–positive fibroblastic reticular cells (FRCs) are regulated by hematopoietic cells, but the cellular and molecular mechanisms of such regulation have remained unclear. Here we show that ablation of either signal regulatory protein α (SIRPα), an Ig superfamily protein, or its ligand CD47 in conventional dendritic cells (cDCs) markedly reduced the number of CD4+ cDCs as well as that of Pdpn+ FRCs and T cells in the adult mouse spleen. Such ablation also impaired the survival of FRCs as well as the production by CD4+ cDCs of tumor necrosis factor receptor (TNFR) ligands, including TNF-α, which was shown to promote the proliferation and survival of Pdpn+ FRCs. CD4+ cDCs thus regulate the steady-state homeostasis of FRCs in the adult spleen via the production of TNFR ligands, with the CD47–SIRPα interaction in cDCs likely being indispensable for such regulation.
Secondary lymphoid organs (SLOs) such as lymph nodes (LNs) and the spleen serve as sites for the interaction of a variety of hematopoietic cells in mammals. Dendritic cells (DCs) thus migrate from peripheral tissues and present antigens to T cells in SLOs, and SLOs are essential for the interaction of T cells with B cells. Such important functions of SLOs are supported by their stromal cell (SC) components (1–3). SCs contribute to the structural organization of SLOs by producing chemokines, which regulate the positioning and segregation of incoming hematopoietic cells. SCs in the white pulp of the spleen or paracortex of LNs are termed “fibroblastic reticular cells” (FRCs) and produce the chemokines CCL19 and CCL21, both of which attract naive T cells and activated DCs expressing the chemokine receptor CCR7 (4). In addition, FRCs produce IL-7, which is thought to support the survival of T cells and DCs (5). Moreover, FRCs produce extracellular matrix proteins such as collagens and laminins, and they form a tubular system, designated a “conduit system,” by which small molecules and fluid are transported from the marginal zone to the T cell area (6, 7). In contrast, follicular dendritic cells (FDCs), another subset of SCs, produce CXCL13 to attract B cells (1, 2).
Generation of SLO SCs is thought to require both hematopoietic cells and mesenchymal cells (2, 8). During fetal development of SLOs, mesenchymal precursors interact with lymphoid tissue-inducer (LTi) cells, which are derived from lymphoid precursors and belong to the family of innate lymphoid cells (9). The interaction of lymphotoxin α1β2 (LTα1β2), a membrane-anchored heterotrimeric protein expressed on the surface of LTi cells, with lymphotoxin β-receptor (LTβR) on mesenchymal precursors induces differentiation of the latter cells into stromal organizer cells (or lymphoid tissue organizer cells), which in turn are thought to give rise to various types of SCs through further interaction with hematopoietic cells. Differentiation of mesenchymal cells toward FDCs in SLOs is thought to require the presence of B cells, which express TNF-α as well as LTα1β2 (2). In addition, differentiation of FRCs requires LTα1β2—in particular, that expressed on B cells—in the spleen, but not in LNs (10). Systemic ablation of TNF-α was also shown to reduce the number of FRCs in the spleen (11, 12). However, which types of hematopoietic cells deliver specific signals important for the induction and homeostasis of FRCs in SLOs, especially under the steady-state condition in adults, has remained poorly understood.
Signal regulatory protein α (SIRPα) is an Ig superfamily protein that is highly expressed in CD11c+ conventional DCs (cDCs)—in particular, CD4+CD8α− cDCs (CD4+ cDCs)—as well as in macrophages (13, 14). SIRPα comprises three Ig-like domains in its extracellular region and an immunoreceptor tyrosine-based inhibition motif (ITIM) that binds the protein tyrosine phosphatases Shp1 and Shp2 in its intracellular region (15, 16). The extracellular region of SIRPα binds the ligand CD47, another membrane-bound protein of the Ig superfamily that is expressed ubiquitously. We have previously shown that both SIRPα-deficient mice and mice that express a mutant form of SIRPα lacking the cytoplasmic region manifest a marked reduction in the number of CD4+ cDCs in the spleen and LNs (14, 17). Such SIRPα-mutant mice also manifested a reduction in the number of T cells and podoplanin (Pdpn)+ FRCs in the spleen (18), suggesting that SIRPα is important for homeostasis of CD4+ cDC subsets as well as of T cells and FRCs in SLOs. The cellular and molecular basis for such regulation by SIRPα has remained largely unknown, however. To characterize such regulation, we have generated and analyzed DC-specific SIRPα or CD47 conditional-knockout mice.
Results
Importance of SIRPα on DCs for Homeostasis of CD4+ cDCs, T Cells, and FRCs in the Spleen.
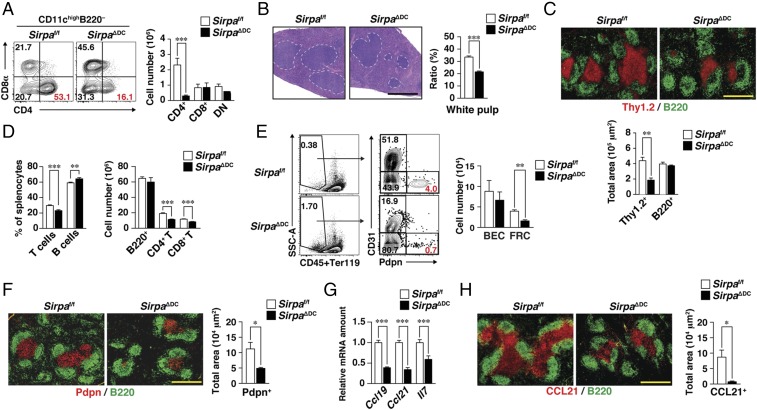
We recently generated mice in which SIRPα is specifically ablated in CD11c+ DCs (SirpaΔDC mice) (17). As we previously reported, we confirmed that SirpaΔDC mice (at 8–12 wk of age) manifested a marked reduction specifically in the population of CD4+ cDCs in the spleen (Fig. 1A) and in peripheral LNs (17) compared with control Sirpaf/f mice. We also found that SIRPα expression was partially down-regulated in both CD11c−CD11b+Ly6Chi monocytes and CD11c−CD11b+Ly6G+granulocytes in the spleens of SirpaΔDC mice (Fig. S1A) (19). In contrast to CD4+ cDCs, the number of Ly6Chi monocytes was increased in the spleens of SirpaΔDC mice compared with control mice (Fig. S1B). In addition, H&E staining revealed that the white pulp of the spleen was significantly smaller in SirpaΔDC mice than in Sirpaf/f mice (Fig. 1B). Quantitative immunohistofluorescence analysis of T cells (Thy1.2+) and B cells (B220+) in the spleens of adult SirpaΔDC mice revealed that the area of the T cell zone, but not that of the B cell zone, was indeed reduced (Fig. 1C). Consistent with this finding, the frequency of T cells and the absolute numbers of both CD4+ and CD8+ T cells were markedly reduced in the spleens of SirpaΔDC mice compared with control mice (Fig. 1D). By contrast, the frequency and absolute numbers of B cells were not reduced in the spleens of SirpaΔDC mice (Fig. 1D). Furthermore, the absolute numbers of T and B cells in (Fig. S2 A–C) peripheral LNs, mesenteric LNs, or Peyer’s patch did not differ between SirpaΔDC and Sirpaf/f mice. These results thus suggested that SIRPα on DCs is an important determinant of the abundance of CD4+ cDCs and T cells in the spleen.
Fig. 1.
Importance of SIRPα on DCs for homeostasis of CD4+ cDCs, T cells, and FRCs in the spleen. (A) Splenocytes isolated from Sirpaf/f or SirpaΔDC mice at 8–12 wk of age were analyzed for cDC subsets by flow cytometry. Representative plots for CD4+ (CD4+CD8α−), CD8+ (CD4−CD8α+), and DN (CD4−CD8α−) subsets among CD11chighB220− cDCs (Left) and the absolute numbers of these cells (Right) are shown. (B, Left) Paraffin-embedded sections of the spleen from Sirpaf/f or SirpaΔDC mice were stained with H&E. Dashed lines demarcate white pulp area. (Right) The size of the white pulp area was determined as the percentage of total spleen area with the use of ImageJ software. (C, Upper) Frozen sections of the spleen from Sirpaf/f or SirpaΔDC mice were stained with antibodies to Thy1.2 (red) and B220 (green). (Scale bar, 500 μm.) (Lower) The Thy1.2+ or B220+ area in each image was measured with the use of ImageJ software. (D) Splenocytes isolated from Sirpaf/f or SirpaΔDC mice were stained for flow cytometric determination of the frequency of T cells (CD3ε+) and B cells (B220+) among total splenocytes (Left) or of the absolute numbers of B cells, CD4+ T cells (CD3ε+CD4+), and CD8+ T cells (CD3ε+CD8α+) (Right). (E) Splenocytes isolated from Sirpaf/f or SirpaΔDC mice were analyzed for SC subsets by flow cytometry. Representative plots for FRCs (CD31−Pdpn+) and BECs (CD31+Pdpn−) among Ter119−CD45− nonhematopoietic cells (Left) and the absolute numbers of these cells (Right) are shown. SSC, side scatter. (F) Frozen sections of the spleen from Sirpaf/f or SirpaΔDC mice were stained with antibodies to Pdpn (red) and to B220 (green) (Left), and the Pdpn+ area was measured in each image (Right). (G) Relative Ccl19, Ccl21, and Il7 mRNA abundance in the spleens of Sirpaf/f or SirpaΔDC mice. The amount of each target mRNA was normalized by that of Gapdh mRNA and expressed relative to the value for Sirpaf/f mice. (H) Frozen sections of the spleen from Sirpaf/f or SirpaΔDC mice were stained for CCL21 (red) and B220 (green) (Left), and the CCL21+ area was quantified in each image (Right). (Scale bar, 500 μm.) All quantitative data are pooled from three independent experiments and are expressed as means ± SE for three (A and E), five (B, C, F, and H), six (G), or eight (D) mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test).
We next examined the population of FRCs in the spleens of SirpaΔDC mice. With the use of flow cytometry, CD45−Ter119− splenic SCs were separated into three subsets on the basis of surface expression of Pdpn and CD31 (20): Pdpn+CD31− (FRCs), Pdpn−CD31+ (blood endothelial cells, or BECs), and Pdpn−CD31− cells (Fig. 1E). The absolute number of FRCs was significantly reduced in the spleens of SirpaΔDC mice compared with that of Sirpaf/f mice (Fig. 1E). Consistent with this finding, immunohistofluorescence analysis showed that staining for Pdpn, which identifies FRCs in the T cell zone (21, 22), was also markedly reduced in the spleens of SirpaΔDC mice (Fig. 1F). By contrast, the absolute number of FRCs in peripheral LNs, mesenteric LNs, or Peyer’s patch did not differ between SirpaΔDC and Sirpaf/f mice (Fig. S2 D–F).
The homing and survival of T cells in SLOs are thought to be supported by CCL19, CCL21, and IL-7, all of which are produced by FRCs (4, 5), whereas the homing of B cells is supported by CXCL13 produced by FDCs (1). The abundance of Ccl19, Ccl21, and Il7 mRNAs in the spleen was significantly reduced in SirpaΔDC mice compared with Sirpaf/f mice (Fig. 1G). Moreover, immunohistofluorescence analysis revealed a markedly reduced level of CCL21 staining in the T cell zone of SirpaΔDC mice (Fig. 1H), whereas CXCL13 staining in B cell follicles did not differ between SirpaΔDC and Sirpaf/f mice (Fig. S2G).
Increased Turnover and Apoptosis of FRCs in the Spleens of SirpaΔDC Mice.
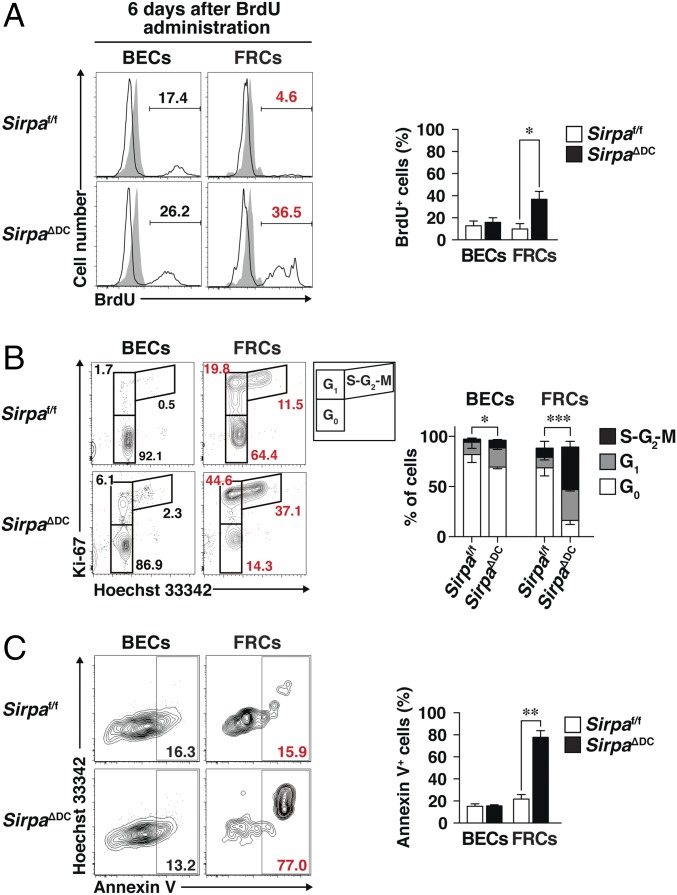
We next examined the turnover of splenic SC subpopulations by monitoring the kinetics of cell labeling with BrdU in the continuous presence of this agent (14). Whereas 9.7 ± 5.0% (mean ± SE) of FRCs in the spleens of Sirpaf/f mice were labeled with BrdU at 6 d after exposure to this agent, 33.3 ± 7.0% of those in the spleens of SirpaΔDC mice were so labeled (Fig. 2A). Analysis of cell-cycle status for SCs by staining with antibodies to Ki-67 and Hoechst 33342 similarly revealed that the proportion of quiescent FRCs in G0 phase was markedly reduced, whereas that of interphase FRCs in G1 or S-G2-M was increased in the spleens of SirpaΔDC mice (G0, 16.2 ± 4.0%; G1, 32.8 ± 1.5%; S-G2-M, 42.4 ± 5.7%) compared with the proportions in Sirpaf/f mice (G0, 68.8 ± 8.0%; G1, 10.5 ± 2.6%; S-G2-M, 9.2 ± 6.7%) (Fig. 2B). These data suggested that the turnover rate of FRCs was markedly increased in the spleens of SirpaΔDC mice. We further examined the frequency of apoptotic cells by staining with Annexin V. The frequency of Annexin V+ cells among total FRCs in the spleen was greatly increased in SirpaΔDC mice compared with Sirpaf/f mice (77.8 ± 6.0% versus 16.8 ± 1.9%) (Fig. 2C), suggesting that the deficiency of FRCs in the spleens of SirpaΔDC mice is attributable, at least in part, to reduced survival of these cells.
Fig. 2.
Increased turnover and apoptosis of FRCs in the spleens of SirpaΔDC mice. (A, Left) Sirpaf/f and Sirpa∆DC mice were injected i.p. with 1 mg of BrdU and then were continuously supplied with BrdU (0.8 mg/mL) in drinking water for 6 d. Then staining for BrdU among BECs and FRCs in the spleens of Sirpaf/f or SirpaΔDC mice treated (open traces) or untreated (filled traces) with BrdU was determined by flow cytometry. (Right) The percentage of BrdU+ cells among BECs and FRCs was also determined. Data are pooled from three independent experiments and are expressed as means ± SE for six mice per group. *P < 0.05 (Student’s t test). (B) Representative flow cytometric profiles for G0 (Ki-67−Hoechst 33342lo), G1 (Ki-67+ Hoechst 33342lo), and S-G2-M (Ki-67+Hoechst 33342hi) cells (Left) and the proportion of cells in G0, G1, or S-G2-M phases (Right) among BECs and FRCs in the spleens of Sirpaf/f or SirpaΔDC mice. Data are pooled from three independent experiments and are expressed as means ± SE for three (Sirpaf/f) or five (SirpaΔDC) mice. *P < 0.05, ***P < 0.001 (two-way ANOVA and Sidak’s test). (C) Representative flow cytometric profiles for Annexin V+ cells (Left) and the percentage of Annexin V+ cells (Right), among BECs and FRCs in the spleens of Sirpaf/f or SirpaΔDC mice. Data are pooled from three independent experiments and are expressed as means ± SE for four mice per group. **P < 0.01 (Student’s t test).
Importance of SIRPα on DCs for Homeostasis of FRCs in the Adult Spleen.
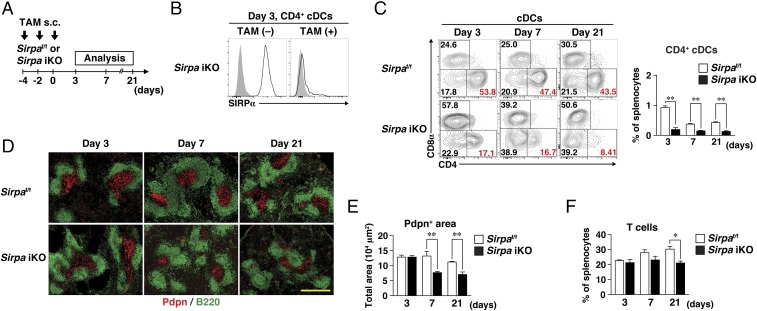
We next examined whether the reduction in the number of FRCs in the spleens of SirpaΔDC mice was also apparent during the early stages of postnatal development. Whereas a significant difference in the Pdpn+ area in the spleens of SirpaΔDC mice and Sirpaf/f mice could not be demonstrated at 1 and 2 wk after birth, the area was markedly diminished in SirpaΔDC mice at 3 wk (Fig. S3 A and B). To investigate further the importance of SIRPα in DCs for homeostasis of cDCs and FRCs in the adult spleen under the steady-state condition, we crossed Sirpaf/f mice with ROSA26-CreERT2 mice (23) to generate tamoxifen (TAM)-inducible SIRPα-knockout (Sirpa iKO) mice. Sirpa iKO and control Sirpaf/f mice were injected s.c. with TAM on days −4, −2, and 0 and were analyzed on days 3, 7, and 21 (Fig. 3A). On day 3, both the expression of SIRPα on splenic CD4+ cDCs (Fig. 3B) and the frequency of CD4+ cDCs in the spleen (Fig. 3C) were markedly reduced in Sirpa iKO mice compared with control mice. By contrast, the size of the Pdpn+ area in the spleens of Sirpa iKO mice was similar to that in Sirpaf/f mice at this time (Fig. 3 D and E). At day 7, however, the size of the Pdpn+ area was significantly reduced in Sirpa iKO mice (Fig. 3 D and E). Furthermore, although a reduction in T cell frequency was not apparent in the spleens of Sirpa iKO mice at day 7, it was evident at day 21 (Fig. 3F). The area occupied by T cells was also reduced at day 21, whereas that occupied by B cells was not altered (Fig. S3C). These results suggested that SIRPα on DCs is important for homeostatic regulation of CD4+ cDCs as well as T cells and FRCs in the spleen in adulthood. They also indicated that the reduction in the number of CD4+ cDCs induced by SIRPα ablation in DCs precedes the reduction in FRCs, which in turn precedes the reduction in T cells.
Fig. 3.
Importance of SIRPα on DCs for homeostasis of splenic FRCs in adulthood. (A) Sirpaf/f and Sirpa iKO mice were injected s.c. with TAM on days −4, −2, and 0. The spleen was isolated on days 3, 7, or 21 for immunohistofluorescence or flow cytometric analysis. (B) Staining for SIRPα (open traces) or with an isotype control antibody (filled traces) on CD4+ cDCs isolated from the spleens of Sirpa iKO mice 3 d after the last TAM [TAM(+)] or vehicle [TAM(−)] injection. Data are representative of three mice. (C) Representative flow cytometric profiles for CD8+, CD4+, and DN cDC subsets (Left) and the percentage of CD4+ cDCs (Right) in the spleens of Sirpaf/f or Sirpa iKO mice at 3, 7, and 21 d after the last TAM injection. (D) Frozen sections of spleens from Sirpaf/f and Sirpa iKO mice at 3, 7, and 21 d after the last TAM injection were stained with antibodies to Pdpn (red) and to B220 (green). (Scale bar, 500 μm.) (E) The Pdpn+ area in the spleens of Sirpaf/f and Sirpa iKO mice at 3, 7, and 21 d after the last TAM injection was measured in images similar to those in D with the use of ImageJ software. (F) The percentage of T cells among total splenocytes of Sirpaf/f and Sirpa iKO mice at 3, 7, and 21 d after the last TAM injection was determined by flow cytometry. All quantitative data (C, E, and F) are pooled from three independent experiments and represent the means ± SE for three mice per group. *P < 0.05, **P < 0.01 (Student’s t test).
Loss of FRCs in the Spleens of SirpaΔDC Mice Is Likely Due to a Functional Defect of CD4+ cDCs.
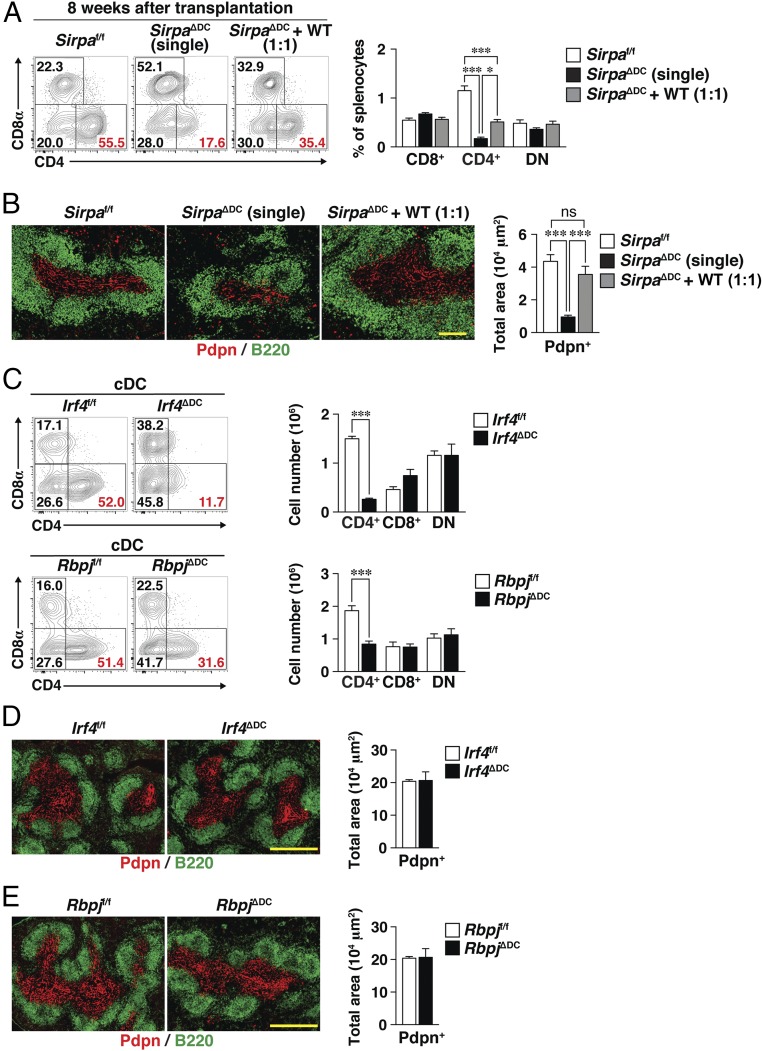
The deficiency of FRCs and T cells in the spleens of SirpaΔDC mice was likely attributable to the ablation of SIRPα specifically in DCs. We therefore next examined whether the loss of these cells is due to the reduction in the number of CD4+ cDCs or to a functional defect of cDCs. We first generated bone marrow (BM) chimeras by transferring individual or mixed (1:1 ratio) BM from SirpaΔDC (CD45.2+) and WT (CD45.1+) mice into lethally irradiated WT (CD45.1+CD45.2+) recipients. Eight weeks after reconstitution, the resulting WT:SirpaΔDC (1:1) mixed BM → WT chimeras manifested significant recovery of CD4+ cDCs in the spleen compared with the SirpaΔDC BM (single) → WT chimeras (Fig. 4A), whereas the number of CD4+ cDCs in the former chimeras was much smaller than in the control Sirpaf/f BM → WT chimeras. Moreover, 81.7 ± 1.9% (mean ± SE) of the CD4+ cDCs in WT:SirpaΔDC (1:1) mixed BM → WT chimeras were derived from CD45.1+ WT mice (Fig. S4A). These results were thus consistent with the notion that SIRPα in cDCs is intrinsically important for homeostasis of CD4+ cDCs in the spleen. In contrast, the Pdpn+ area and the T cell area in the spleens of WT:SirpaΔDC (1:1) mixed BM → WT chimeras was much greater than in SirpaΔDC BM (single) → WT chimeras and was almost equivalent to control Sirpaf/f BM → WT chimeras (Fig. 4B and Fig. S4B), suggesting that the reduction in the number of FRCs, as well as the reduction in the number of T cells, in the spleens of SirpaΔDC mice is attributable to a functional defect of, rather than to a reduced abundance of, CD4+ cDCs.
Fig. 4.
Loss of FRCs in the spleens of SirpaΔDC mice is likely due to a functional defect in CD4+ cDCs. (A) Lethally irradiated WT (CD45.1+CD45.2+) mice were reconstituted with BM cells from Sirpaf/f or Sirpa∆DC mice (CD45.2+) or with an equal mixture of BM cells from Sirpa∆DC and WT (CD45.1+) mice to generate Sirpaf/f, Sirpa∆DC (single), or Sirpa∆DC + WT (1:1) BM chimeras, respectively. Eight weeks after cell transplantation, the spleens from the BM chimeras were isolated for analysis. Representative flow cytometric profiles for CD8+, CD4+, or DN cDC subsets (Left) and the percentages of these cells among total splenocytes (Right) are shown. (B, Left) Frozen sections of the spleens of BM chimeras were stained with antibodies to Pdpn (red) and to B220 (green). (Scale bar, 200 μm.) (Right) The Pdpn+ area was measured in each image with the use of ImageJ software. (C) Representative flow cytometric profiles for CD4+, CD8+, and DN cDCs in the spleens of Irf4f/f and Irf4ΔDC mice (Upper Left) or Rbpjf/f and RbpjΔDC mice (Lower Left) and the corresponding absolute numbers of these cells (Right) are shown. (D and E) Frozen sections of the spleens from Irf4f/f and Irf4∆DC mice (D, Left) or from Rbpjf/f and Rbpj∆DC mice (E, Left) were stained with antibodies to Pdpn (red) and to B220 (green). (Scale bars, 500 μm.) (D, Right and E, Right) The Pdpn+ area in each image was also measured. Data are pooled from three independent experiments and are expressed as the means ± SE for four (A and B) or three (C–E) mice per group. *P < 0.05, ***P < 0.001 by one-way ANOVA and Tukey’s test (A and B) or by Student’s t test (C); ns, not significant.
We next examined spleens from DC-specific Irf4-deficient (Irf4ΔDC) or DC-specific Rbpj-deficient (RbpjΔDC) mice, both of which were previously found to manifest a selective reduction in the number of CD4+ cDCs in the spleen (24, 25). The extent of the selective reduction in the CD4+ cDC population in Irf4ΔDC or RbpjΔDC mice (Fig. 4C) was similar to that in SirpaΔDC mice (Fig. 1A). However, the size of the Pdpn+ area and of the T cell zone in the spleen did not differ significantly between Irf4ΔDC or RbpjΔDC mice and their corresponding control mice (Fig. 4 D and E and Fig. S4 C and D). These results thus further suggested that the reduction in the number of FRCs and the reduction in the number of T cells, in the spleens of SirpaΔDC mice is attributable to a functional defect in the corresponding CD4+ cDCs.
SIRPα+ cDCs Promote Proliferation or Survival of Splenic FRCs via TNF Receptor Ligands in Vitro.
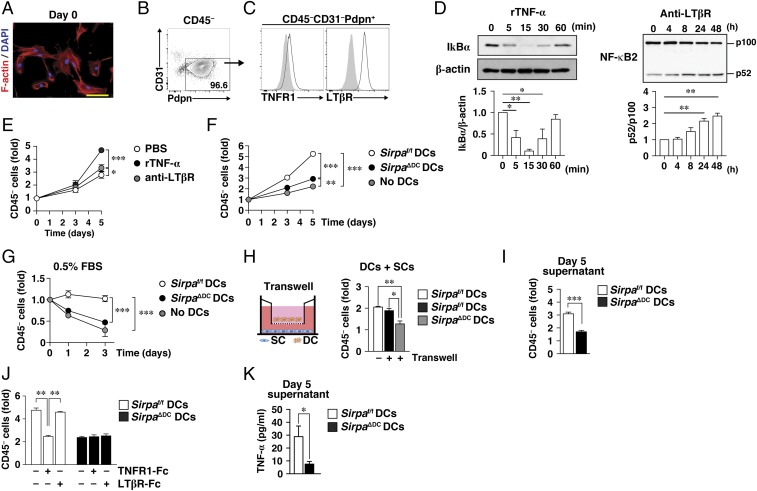
We next examined whether DCs are able to promote the proliferation or survival of SCs in vitro. To this end, we developed a coculture system for DCs and splenic SCs. The cultured SCs, isolated as CD45− cells from the spleens of WT mice (26), manifested a spindle-shaped morphology and expressed Pdpn at a high level but were negative for CD31 (Fig. 5 A and B). They also expressed both TNF receptor 1 (TNFR1) and LTβR (Fig. 5C). Stimulation of these SCs with recombinant TNF-α or an agonistic monoclonal antibody to LTβR induced activation of canonical or noncanonical NF-κB signaling pathways (27, 28), respectively, as evidenced by the degradation of either the endogenous NF-κB inhibitor IκBα or NF-κB2 (p100), respectively (Fig. 5D). Furthermore, both recombinant TNF-α and anti-LTβR significantly promoted the proliferation of these SCs (Fig. 5E), with the effect of TNF-α being more pronounced than that of anti-LTβR. The cultured splenic CD45− SCs were thus phenotypically and functionally similar to FRCs, as described previously (22, 28).
Fig. 5.
SIRPα+ cDCs promote the proliferation or survival of splenic FRCs via the production of TNFR ligands in vitro. (A) Splenic CD45− SCs isolated from C57BL/6J mice and cultured for a total of 7 d (set as day 0) were fixed with 4% paraformaldehyde and stained with DAPI (blue) and rhodamine-phalloidin (red) to visualize nuclei and F-actin, respectively. (Scale bar, 50 μm.) (B and C) Flow cytometric analysis of CD31 and Pdpn expression on splenic CD45− SCs (B) and of CD45−CD31−Pdpn+ cells stained with antibodies to TNFR1 or to LTβR (open traces) or with isotype control antibodies (filled traces) (C). (D) Splenic CD45− SCs at day 0 were exposed to recombinant TNF-α (rTNF-α, 20 ng/mL) (Left) or to anti-LTβR (5 μg/mL) (Right) in PBS for the indicated times; then cell lysates were subjected to immunoblot analysis with antibodies to IκBα or β-actin (Upper Left) or to NF-κB2 (Upper Right). Immunoblots similar to those shown in the upper panels were subjected to densitometric analysis of the relative IκBα/β-actin (Lower Left) or p52/p100 (Lower Right) band intensity ratio. (E) Splenic CD45− SCs (at day 0) were cultured in the absence or presence of rTNF-α or anti-LTβR in PBS for the indicated times; then the cells were harvested for flow cytometric determination of the number of CD45− cells. (F) Splenic CD45− SCs (at day 0) were cultured for 3 or 5 d with or without CD11c+ DCs isolated from the spleens of Sirpaf/f or Sirpa∆DC mice. Then the cells were harvested for determination of the CD45− cell yield as in E. (G) Splenic CD45− SCs (at day 0) were cultured for 1 or 3 d with or without Sirpaf/f or Sirpa∆DC DCs in medium containing 0.5% FBS; then the CD45− cell yield was determined as in E. (H) Splenic CD45− SCs (at day 0) were cultured with Sirpaf/f or Sirpa∆DC DCs either directly together (−) or separated by a Transwell filter (+) for 3 d; then the CD45− cell yield in the lower chamber was determined as in E. (I) Splenic CD45− SCs (at day 0) were cultured for 3 d in the presence of culture supernatant harvested from cocultures of SCs and Sirpaf/f or Sirpa∆DC DCs after 5 d. Then the CD45− cell yield was determined as in E. (J) Splenic CD45− SCs (at day 0) were cultured for 5 d with Sirpaf/f or Sirpa∆DC DCs in the absence or presence of TNFR1-Fc or LTβR-Fc; then the CD45− cell yield was determined as in E. (K) The concentration of TNF-α in culture supernatants of splenic CD45− SCs (at day 0) cocultured for 5 d with Sirpaf/f or Sirpa∆DC DCs. Data in A–C are representative of three independent experiments; those in D are the means ± SE from three independent experiments; those in E–J are the means ± SE of triplicate determinations and are representative of three independent experiments; and those in K are the means ± SE for five samples per group. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey’s test (D, H, and J), two-way ANOVA and Sidak’s test (E–G), or Student’s t test (I and K).
Coculture of the splenic CD45− SCs from WT mice with splenic CD11c+ DCs from Sirpaf/f mice in medium containing 10% FBS induced a threefold increase in the extent of proliferation of the former cells (Fig. 5F). By contrast, DCs from SirpaΔDC mice had a markedly smaller effect on the proliferation of SCs was markedly smaller than that of DCs from Sirpaf/f mice (Fig. 5F). In addition, we found that the number of CD45+ cells harvested from the coculture of CD11c+ cells with CD45− SCs was similar between Sirpaf/f and SirpaΔDC mice (Fig. S5A). Monitoring of the number of live CD45− splenic SCs in medium containing 0.5% FBS revealed that the survival of these cells cocultured with Sirpaf/f DCs was much greater than that of those cultured without DCs or those cocultured with SirpaΔDC DCs (Fig. 5G).
To examine whether direct contact of DCs with SCs is required for the effect of DCs on the proliferation or survival of SCs, we cultured the two cell types in separate chambers of a Transwell plate (filter pore size, 0.4 μm). The proliferation of splenic SCs placed in the lower chamber was promoted by the presence of Sirpaf/f DCs in the upper chamber to an extent similar to that observed in nonseparated cocultures (Fig. 5H). By contrast, the effect of SirpaΔDC DCs placed in the upper chamber was minimal (Fig. 5H). These results suggested that direct contact of DCs with SCs is not required for the effect of DCs on the proliferation or survival of SCs; the effect likely is mediated by a factor produced and secreted by DCs. Indeed, culture supernatants harvested from cocultures of Sirpaf/f DCs and SCs after 5 d markedly promoted the proliferation of freshly prepared SCs, whereas the effect of culture supernatants from cocultures of SirpaΔDC DCs and SCs was again minimal (Fig. 5I).
Both TNFR ligands (such as TNF-α or a soluble form of LTα3) and LTβR ligands (such as the membrane-anchored form of LTα1β2) are thought to be important for the proliferation and survival of FRCs in SLOs (22, 29). We therefore examined whether such cytokines contribute to the effect of DCs on the proliferation of SCs in the coculture system. A p55 TNFR (TNFR1)–Fc chimera, which is thought to neutralize TNFR ligands (30), markedly inhibited the stimulation of SC proliferation by cocultured Sirpaf/f DCs, whereas an LTβR-Fc chimera that neutralizes membrane-anchored LTβR ligands (30) had no such effect (Fig. 5J), suggesting that TNFR ligands are important for this stimulatory action of DCs. Neither Fc fusion protein inhibited the effect of SirpaΔDC DCs on the proliferation of cocultured SCs (Fig. 5J). We indeed detected TNF-α in culture supernatants of cocultured Sirpaf/f DCs and SCs, whereas the amount of TNF-α in the culture supernatants of cocultured SirpaΔDC DCs and SCs was much less (Fig. 5K). In contrast, we did not detect LTα in the supernatants of either type of coculture (Fig. S5B). These results thus suggested that DCs promote the proliferation or survival of SCs through the secretion of soluble TNFR ligands such as TNF-α and that SIRPα on DCs is important for this function of DCs.
Importance of TNFR Ligands for Regulation by DCs of FRC Homeostasis in the Spleen in Vivo.
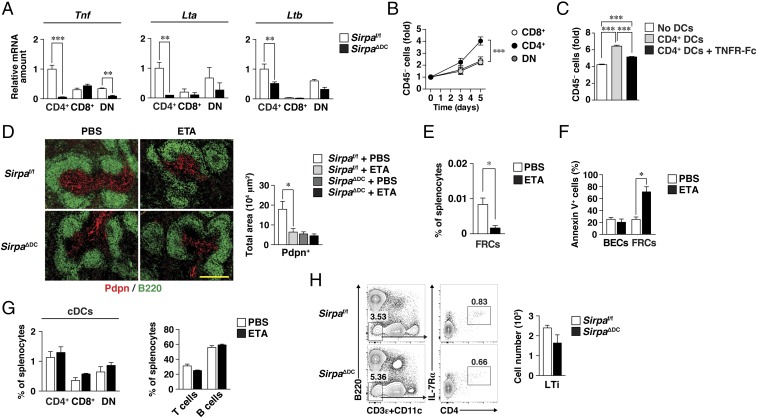
We measured the abundance of mRNAs for TNF-α and LT in cDCs of the spleen. Among the three cDC subsets, the amounts of Tnf, Lta, and Ltb mRNAs were highest in CD4+ cDCs (Fig. 6A), which also express SIRPα at the highest level (31). Indeed, the ability of CD4+ cDCs to promote the proliferation of splenic SCs in coculture was much greater than that of CD8+ or double-negative (DN) cDCs (Fig. 6B). In addition, this ability of CD4+ cDCs was inhibited by the TNFR-Fc chimera (Fig. 6C). Moreover, the expression of Tnf, Lta, and, to a lesser extent, Ltb was down-regulated in splenic CD4+ cDCs isolated from SirpaΔDC mice compared with those from Sirpaf/f mice (Fig. 6A). In addition to TNF family cytokines, the amount of Il1b was also specifically down-regulated in splenic CD4+ cDCs isolated from SirpaΔDC mice (Fig. S6A), suggesting that the defect in cytokine production from SirpaΔDC cDCs was not specific to the TNF family. To further demonstrate the impact of TNFR ligands derived from CD4+ cDCs in vivo, we isolated CD4+ cDCs derived from WT (CD45.1+) or SirpaΔDC (CD45.2+) mice in the spleens of WT:SirpaΔDC (1:1) mixed BM or single → WT chimeras (Fig. S6B). The expression of Tnf mRNA in the spleens of mixed chimeras was specifically impaired in CD4+ cDCs derived from SirpaΔDC donors, to the same extent as those derived from SirpaΔDC BM (single) → WT chimeras, compared with that from WT donors (Fig. S6C). In contrast, the expression of Tnf, Lta, and Ltb mRNAs was not reduced in Ly6Chi monocytes from SirpaΔDC mice compared with those from Sirpaf/f mice (Fig. S6D). These results thus suggested that SIRPα-deficient CD4+ cDCs are functionally defective with regard to the production of TNFR ligands.
Fig. 6.
Importance of TNFR ligands for regulation by cDCs of FRC homeostasis in the spleen in vivo. (A) Relative abundance of Tnf, Lta, and Ltb mRNAs in CD4+, CD8+, and DN cDCs sorted from the spleens of Sirpaf/f or SirpaΔDC mice. The amount of each mRNA was normalized by that of Gapdh mRNA. Data are the means ± SE for six mice per group examined in three independent experiments. **P < 0.01, ***P < 0.001 (Student’s t test). (B) Splenic CD45− SCs from C57BL/6J mice (Fig. 5) were cocultured for the indicated times with CD8+, CD4+, or DN cDCs isolated from the spleens of Sirpaf/f mice; then the cells were harvested for determination of the CD45− cell yield as in Fig. 5E. Data are the means ± SE of triplicate determinations and are representative of three independent experiments. ***P < 0.001 (two-way ANOVA and Sidak’s test). (C) Splenic CD45− SCs (at day 0) were cultured for 5 d with or without Sirpaf/f CD4+ cDCs in the absence or presence of TNFR1-Fc; then the CD45− cell yield was determined as in B. Data are the means ± SE of triplicate determinations and are representative of two independent experiments. ***P < 0.001 (one-way ANOVA with Tukey’s test). (D, Left) Sirpaf/f and SirpaΔDC mice were treated with Etanercept (ETA, 30 mg/kg) or PBS on days 0, 3, and 6, and the spleens were isolated on day 7 for preparation of frozen sections and immunohistofluorescence analysis of Pdpn (red) and B220 (green). (Scale bar, 200 μm.) (Right) The Pdpn+ area was measured in each image. (E) Splenocytes isolated from Sirpaf/f mice treated with Etanercept on day 7 were analyzed for SC subsets by flow cytometry. The frequency of FRCs (Ter119−CD45−CD31−Pdpn+) among total splenocytes is shown. (F) The percentage of Annexin V+ cells among BECs and FRCs in the spleens of Sirpaf/f mice treated with Etanercept or PBS was determined by flow cytometry on day 7. (G) The percentage of CD4+, CD8+, or DN cDCs (Left) or of T cells (Right) in the spleens of Sirpaf/f mice treated with Etanercept or PBS was determined by flow cytometry on day 7. (H, Left) Representative flow cytometric profiles for LTi-like cells (B220−CD11c−CD3ε−CD4+IL-7Rα+) in the spleens of Sirpaf/f or SirpaΔDC mice. (Right) Absolute numbers of these cells. Data in D–H are pooled from three independent experiments and are the means ± SE for four (D–G) or three (H) mice per group. *P < 0.05 by one-way ANOVA with Tukey’s test (D) or by Student’s t test (E and F).
We next examined whether TNFR ligands indeed participate in the regulation of FRC homeostasis in the spleen in vivo. To this end, we treated mice with a human p75 TNFR–Ig fusion protein (Etanercept) that is able to bind to and neutralize mouse TNF-α and LTα (32). Treatment of Sirpaf/f mice with Etanercept resulted in a marked reduction in the size of the Pdpn+ area and in the frequency of FRCs in the spleen (Fig. 6 D and E). In contrast, Etanercept did not affect the already reduced size of the Pdpn+ area in the spleens of SirpaΔDC mice (Fig. 6D). Moreover, the number of Annexin V+ FRCs was significantly increased in the spleens of Sirpaf/f mice treated with Etanercept (Fig. 6F). By contrast, treatment of Sirpaf/f mice with Etanercept had no effect on the number of CD4+ or other cDCs and resulted in a slight (but not significant) reduction of T cells in the spleen compared with PBS-treated mice (Fig. 6G). Indeed, the size of the T zone (as well as the number of T cells) was not markedly changed (32, 33), but the T cell homing was impaired (12) in the spleens of TNF-α–KO mice. Taken together, these results suggested that TNFR ligands (such as TNF-α and LTα3), which are likely derived from SIRPα-expressing CD4+ cDCs, are important for FRC homeostasis in the spleen in vivo.
LTi cells play a central role in the development of lymphoid organs during mouse embryogenesis (34) and are also thought to be present in and to be important for maintenance of the structural organization of SLOs in adulthood (35, 36). However, the frequency of CD11c−CD3−CD4+ LTi cells in the spleen was found not to differ between Sirpaf/f and SirpaΔDC mice (Fig. 6H).
Importance of CD47 on DCs for Homeostasis of CD4+ cDCs, T Cells, and FRCs in the Spleen.
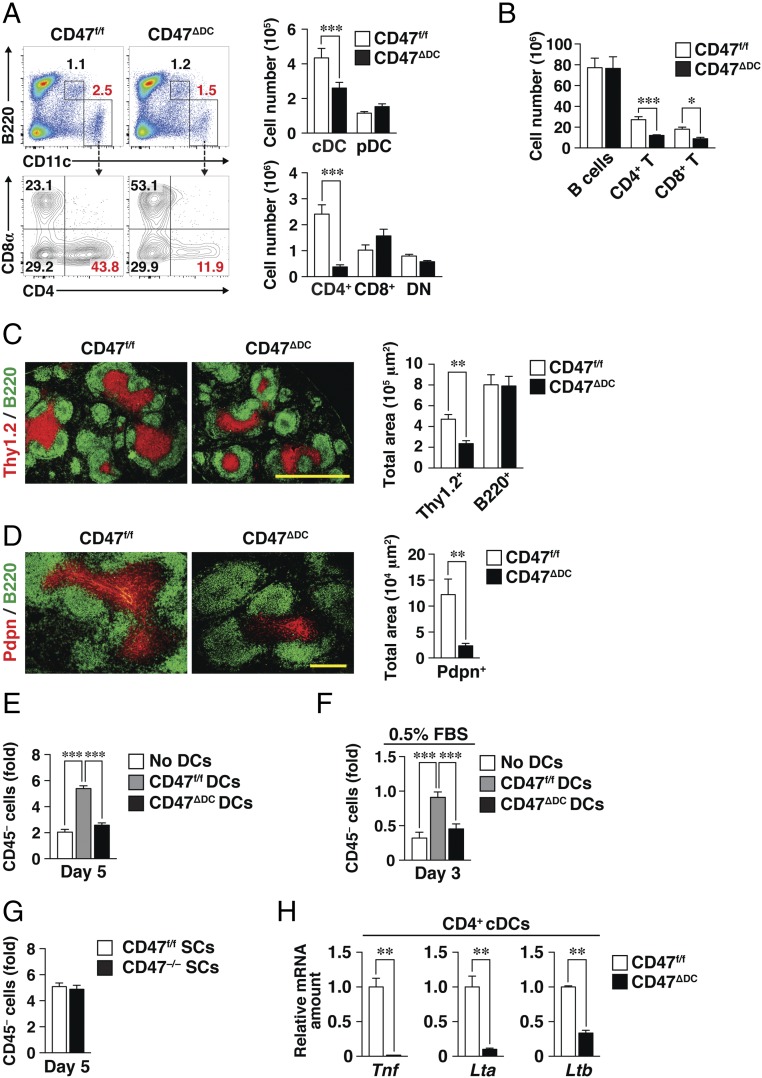
To investigate the mechanism by which SIRPα promotes the function of cDCs in regulating FRC homeostasis, we examined the possible role of the SIRPα ligand CD47 expressed on DCs in such regulation. Given that CD47 is expressed ubiquitously, we first generated CD47f/f mice in which exon 1 of the CD47 gene is flanked by two loxP sites (Fig. S7A). DC-specific CD47-deficient (CD47ΔDC) and systemic CD47-deficient (CD47−/−) mice were then generated by crossing CD47f/f mice with CD11c-Cre or E2A-Cre mice, respectively. We confirmed that CD47 expression was lost specifically in cDCs and plasmacytoid DCs (pDCs) in the spleens of CD47ΔDC mice (Fig. S7B). The number of cDCs—in particular, the number of CD4+ cDCs—was greatly reduced in the spleens of CD47ΔDC mice compared with CD47f/f mice (Fig. 7A). Furthermore, the numbers of both CD4+ and CD8+ T cells (Fig. 7B) and the size of the T cell zone (Fig. 7C) were significantly reduced in the spleens of CD47ΔDC mice. The area occupied by Pdpn+ FRCs also was reduced in the spleens of CD47ΔDC mice (Fig. 7D). CD47ΔDC mice thus appeared to be a phenocopy of SirpaΔDC mice in terms of the number of cDCs, T cells, and Pdpn+ FRCs in the spleen. The number of CD4+ cDCs also was reduced in peripheral LNs of CD47ΔDC mice, whereas the absolute number of FRCs was not reduced in these LNs of CD47ΔDC mice compared with those of CD47f/f mice (Fig. S7 C and D).
Fig. 7.
Importance of CD47 on DCs for homeostasis of CD4+ cDCs, T cells, and FRCs in the spleen. (A) Representative flow cytometric profiles for cDCs (B220−CD11chigh) and pDCs (B220+CD11cint) as well as for CD4+, CD8+, and DN cDC subsets (Left) and the absolute numbers of these cells (Right) in the spleens of CD47f/f or CD47∆DC mice. (B) Flow cytometric determination of the absolute numbers of B cells (CD3ε− B220+), CD4+ T cells (CD3ε+CD4+), and CD8+ T cells (CD3ε+CD8α+) among splenocytes isolated from CD47f/f or CD47ΔDC mice. (C and D) (Left) Frozen sections of spleens from CD47f/f and CD47∆DC mice were stained for Thy1.2 (red) and B220 (green) (C) or for Pdpn (red) and B220 (green) (D). (Scale bars: 500 μm in C; 200 μm in D.) (Right)The Thy1.2+ and B220+ areas (C) or the Pdpn+ area (D) in the images were measured with the use of ImageJ software. (E) Splenic CD45− SCs from WT mice (Fig. 5) were cocultured for 5 d with or without CD11c+ DCs isolated from the spleens of CD47f/f or CD47∆DC mice; then the CD45− cell yield was measured as in Fig. 5E. (F) Splenic CD45− SCs from WT mice were cocultured for 3 d with or without CD47f/f or CD47∆DC DCs in medium containing 0.5% FBS. Then the CD45− cell yield was measured as in E. (G) Splenic CD45− SCs isolated from CD47f/f or CD47−/− mice were cocultured for 5 d with CD47f/f DCs; then the CD45− cell yield was measured as in E. (H) Relative abundance of Tnf, Lta, and Ltb mRNAs in the splenic CD4+ cDC subset sorted from CD47f/f or CD47ΔDC mice. The amount of each mRNA was normalized by that of Gapdh mRNA. Data in A and B are pooled from three independent experiments and are the means ± SE for three mice per group; those in C and D are the means ± SE for three mice per group; those in E and G are the means ± SE of triplicate determinations and are representative of three independent experiments; and those in H are the means ± SE for a total of six mice per group examined in three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test (A–D and H) or by one-way ANOVA and Tukey’s test (E and F).
To examine further the importance of CD47 on DCs for regulation of FRC homeostasis, we cocultured CD11c+ cells isolated from the spleens of CD47ΔDC or CD47f/f mice with splenic SCs of WT mice. Coculture with CD47ΔDC DCs resulted in marked inhibition of both the proliferation (Fig. 7E) and survival (Fig. 7F) of SCs compared with those apparent on coculture with CD47f/f DCs, suggesting that CD47 on DCs is indeed important for homeostasis of DCs and FRCs in the spleen. We also examined the impact of CD47 ablation in splenic SCs on their proliferation in vitro. The ability of CD47-deficient SCs (isolated from CD47−/− mice) to proliferate on coculture with CD47f/f DCs was similar to that of CD47f/f SCs (Fig. 7G), suggesting that CD47 in SCs is dispensable for the proliferation of these cells in the presence of DCs. Furthermore, the abundance of Tnf, Lta, and Ltb mRNAs was greatly reduced in splenic CD4+ cDCs isolated from CD47ΔDC mice compared with those from CD47f/f mice (Fig. 7H).
Discussion
We have here shown that ablation of SIRPα in cDCs results in a marked reduction in the numbers of CD4+ cDCs, Pdpn+ FRCs, and T cells in the adult spleen. A similar reduction in the number of CD4+ cDCs, but not of FRCs or T cells, was also apparent in the peripheral LNs of SirpaΔDC mice. Loss of FRCs was likely responsible for the observed down-regulation of the splenic expression of CCL19, CCL21, and IL-7, all of which are produced by FRCs and attract or support T cells in white pulp, with this down-regulation in turn resulting in the loss of T cells in the spleens of SirpaΔDC mice. In addition, the reduction in the number of CD4+ cDCs induced by TAM injection in Sirpa iKO mice was followed by the consecutive depletion of FRCs and T cells in the spleen, suggesting that the loss of FRCs is attributable to either a reduced abundance of or a functional defect in CD4+ cDCs. Transfer of a 1:1 mixture of BM from WT and SirpaΔDC mice into irradiated recipient animals resulted in a substantial recovery of FRCs but not of CD4+ cDCs. In addition, both Irf4ΔDC and RbpjΔDC mice manifested a marked reduction in the number of CD4+ cDCs without any reduction in the number of FRCs or T cells in the spleen. Furthermore, DCs isolated from the spleens of RbpjΔDC mice supported the proliferation of splenic SCs to an extent similar to that observed with DCs from control mice (Fig. S8). Together, these results indicate that a functional defect in CD4+ cDCs induced by SIRPα deficiency is responsible for the reduction in the number of FRCs in the spleens of SirpaΔDC mice. In addition, other cDC subsets, such as DN cDCs, likely compensate for the lack of CD4+ cDCs of RbpjΔDC and perhaps Irf4ΔDC mice with regard to the maintenance of FRCs in the spleen.
With the use of our coculture system for DCs and splenic SCs, we found that DCs—in particular, CD4+ cDCs, which, among cDC subsets, express Tnf, Lta, and Ltb at the highest levels—promoted the proliferation or survival of CD45− SCs in vitro. Culture in a Transwell plate or in the presence of an antagonist of TNFR ligands showed that SIRPα+CD4+ cDCs likely promote the proliferation or survival of FRCs by producing TNFR ligands such as TNF-α. Indeed, the expression of Tnf and Lta was down-regulated in CD4+ cDCs from SirpaΔDC mice, whose ability to support the proliferation or survival of FRCs was greatly diminished. We further showed that treatment of mice with Etanercept, which neutralizes TNF-α or LTα3, resulted in a marked reduction in the size of the FRC area in the spleen. Together, these observations indicate that the production of TNFR ligands by CD4+ cDCs regulates homeostasis of FRCs in the adult spleen under the steady-state condition, with SIRPα likely being indispensable for the production of such ligands by CD4+ cDCs. By contrast, Etanercept had no effect on the number of CD4+ cDCs and had minimal effect on the number of T cells, although we noted a significant reduction of FRCs in the spleen. Such discrepancy between the number of T cells and the number of FRCs may be attributable to the duration of Etanercept treatment or the maintenance of T cell survival by DCs through the presentation of self-antigens (37). The expression of TNF-α in DN cDCs was not high but was down-regulated in the spleens of SirpaΔDC mice, as was the expression in CD4+ cDCs. Thus, the FRC phenotype in SirpaΔDC mice is likely attributable, in part, to a reduced production of TNF-α from DN cDCs. Consistent with this notion, Tnf−/− or Tnfr1−/− mice manifest marked down-regulation of CCL21 or CCL19 expression in the spleen (11, 12), with the former mice also manifesting poor development of Pdpn+ FRCs in the spleen but not in LNs (12). Given that DCs in peripheral LNs promote the survival of FRCs through the action of the LTβR ligands LTα1β2 or LIGHT under inflammatory conditions (29), the mechanism by which DCs regulate FRCs likely differs between the spleen and LNs or between noninflammatory and inflammatory conditions.
Generation or homeostasis of SCs is thought to require various types of hematopoietic cells in neonates as well as in adult mice. Indeed, splenic B cells were shown to regulate FRC development and maintenance in a manner dependent on LTα1β2 expression in B cells (10). LTi cells are also thought to contribute to the restoration of FRCs after lymphocytic choriomeningitis virus infection through the action of LTα1β2 expressed on the LTi cells (36). We now have shown that the reduction in the number of Pdpn+ FRCs in the spleens of SirpaΔDC mice was already pronounced by 3 wk of age. Consistent with this finding, CD4+ cDCs increase in number and become predominant among the cDC subsets in the spleen by 3 wk of age (38). We thus have shown that DCs, particularly CD4+ cDCs, play an important role in the homeostasis of FRCs in the spleen under the steady-state condition in adulthood.
We found that CD47 on DCs is also important for maintaining the abundance of CD4+ cDCs, Pdpn+ FRCs, and T cells in the spleen. In addition, CD47 was found to be indispensable for DCs’ ability to promote the proliferation of FRCs in vitro and for the expression of TNFR ligand genes in CD4+ cDCs. These results suggest that interaction of CD47 with SIRPα either in cis within individual cDCs or in trans between neighboring cDCs (39) is important for the production of TNFR ligands by CD4+ cDCs. In particular, the trans-interaction between CD47 [on nonhematopoietic cells (14) or red blood cells (40)] and SIRPα on DCs is implicated in the homeostasis of CD4+ cDCs in the spleen. Ligation of SIRPα by CD47 (likely in trans) induces the tyrosine phosphorylation and activation of the tyrosine phosphatases Shp1 and Shp2 (13). In addition, Shp2 is a positive regulator of the FcεRI-induced activation of the Fyn and Ras–MAPK signaling pathways that lead to the release of TNF-α from mast cells (41). However, further study is required to characterize the mechanism by which SIRPα through its interaction with CD47 promotes the production of TNFR ligands by cDCs in the spleen.
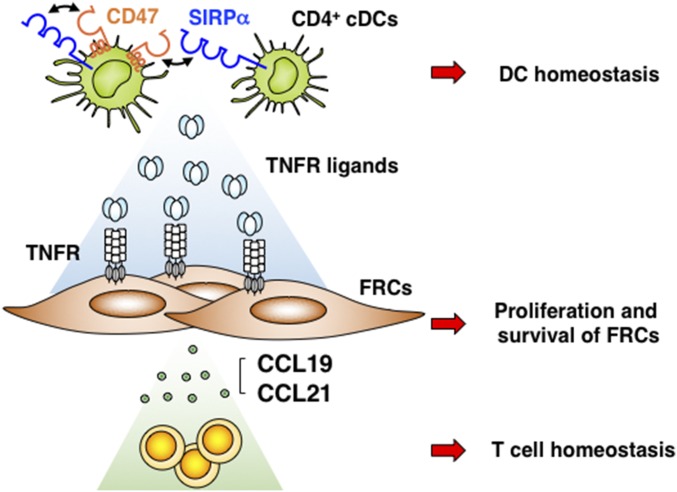
In summary, we propose a model for the role of CD47–SIRPα interaction in the regulation by cDCs of the FRC network in the adult spleen (Fig. 8). Given that the CD47-SIRPα system plays an important role in the regulation of autoimmunity and protection from pathogens (42), further study will be required to determine whether SIRPα in DCs also regulates the organization and regeneration of SLOs during inflammation. In addition, our findings may be relevant to anti-TNF therapy with Etanercept, with such treatment having been shown to repress adaptive immune responses in patients with rheumatoid arthritis. Regulation of the FRC network thus might contribute, at least in part, to the efficacy of this treatment.
Fig. 8.
Model for the role of CD47–SIRPα interaction in the regulation of the FRC network by cDCs in the adult spleen. The interaction of SIRPα with its ligand CD47 either in cis or in trans on the surface of CD4+ cDCs regulates homeostasis of CD4+ cDCs but also promotes the production by these cells of TNFR ligands such as TNF-α that are important for the proliferation and survival of Pdpn+ FRCs in the spleens of adult mice. FRCs in turn secrete CCL19 and CCL21, both of which attract T cells and maintain homeostasis of these cells in the white pulp of the spleen.
Materials and Methods
Antibodies and reagents, generation of CD47f/f mice, cell preparation and flow cytometry, isolation and culture of splenic SCs, determination of BrdU incorporation, analysis of the cell-cycle profile and apoptosis, H&E staining, immunohistofluorescence analysis, cell sorting of cDC subsets and monocytes, preparation of cDNA and RT-qPCR analysis, generation of BM chimeras, immunoblot analysis, determination of soluble LTα in culture supernatants, and statistical analysis used in this study can be found in SI Materials and Methods.
Sirpaf/f mice were generated from C57BL/6J mice at Unitech (17). CD11c-Cre (25) and ROSA26-CreERT2 (23) mice were obtained from The Jackson Laboratory and were crossed with Sirpaf/f mice, with the resulting Sirpaf/f;CD11c-Cre (SirpaΔDC) or Sirpaf/f;ROSA26-CreERT2 (Sirpa iKO) offspring being studied. For induction of Cre recombinase, Sirpa iKO mice were treated with 4 mg TAM (Sigma) dissolved in 200 μL corn oil (Wako) injected s.c. at three time points 48 h apart. Rbpjf/f;CD11c-Cre (RbpjΔDC) and Irf4f/f;CD11c-Cre (Irf4ΔDC) mice were generated previously (24, 43). CD47f/f mice were generated from C57BL/6J mice at the RIKEN Center for Developmental Biology and were crossed with CD11c-Cre or E2A-Cre mice (44), and the resulting CD47f/f;CD11c-Cre (CD47ΔDC) and CD47f/f;E2A-Cre (CD47−/−) offspring were studied. See SI Materials and Methods for the detailed procedure for the generation of CD47f/f mice. Sex- and age-matched mice were studied at 8–12 wk of age unless indicated otherwise. Mice were bred and maintained at the Institute of Experimental Animal Research of Kobe University Graduate School of Medicine under specific pathogen–free conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee of Kobe University (Permit P140508-R1, P140905, P150204) and performed according to Kobe University Animal Experimentation Regulations.
Supplementary Material
Acknowledgments
We thank Y. Takase, E. Aomatsu, and D. Tanaka for technical assistance and Dr. C. F. Ware for the gift of a mAb to mouse LTβR (4H8). This work was supported by a Grant-in-Aid for Scientific Research (B) (to T.M.), a Grant-in-Aid for Scientific Research (C) (to Y.S.), and a Grant-in-Aid for Young Scientists (B) (to T.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This work was also supported in part by Naito Foundation (T.M.) and Takeda Science Foundation (T.K).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711345114/-/DCSupplemental.
References
- 1.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: Partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 6.Nolte MA, et al. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Brendolan A, Caamaño JH. Mesenchymal cell differentiation during lymph node organogenesis. Front Immunol. 2012;3:381. doi: 10.3389/fimmu.2012.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 10.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo VN, et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, et al. Essential role of TNF-α in development of spleen fibroblastic reticular cells. Cell Immunol. 2015;293:130–136. doi: 10.1016/j.cellimm.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y, et al. Regulation by SIRPα of dendritic cell homeostasis in lymphoid tissues. Blood. 2010;116:3517–3525. doi: 10.1182/blood-2010-03-277244. [DOI] [PubMed] [Google Scholar]
- 15.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: Structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. doi: 10.1146/annurev-immunol-032713-120142. [DOI] [PubMed] [Google Scholar]
- 16.Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPα signalling system: Its physiological roles and therapeutic application. J Biochem. 2014;155:335–344. doi: 10.1093/jb/mvu017. [DOI] [PubMed] [Google Scholar]
- 17.Washio K, et al. Dendritic cell SIRPα regulates homeostasis of dendritic cells in lymphoid organs. Genes Cells. 2015;20:451–463. doi: 10.1111/gtc.12238. [DOI] [PubMed] [Google Scholar]
- 18.Sato-Hashimoto M, et al. Signal regulatory protein α regulates the homeostasis of T lymphocytes in the spleen. J Immunol. 2011;187:291–297. doi: 10.4049/jimmunol.1100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rydström A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 20.Fasnacht N, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med. 2014;211:2265–2279. doi: 10.1084/jem.20132528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farr AG, et al. Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J Exp Med. 1992;176:1477–1482. doi: 10.1084/jem.176.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 24.Akbari M, et al. IRF4 in dendritic cells inhibits IL-12 production and controls Th1 immune responses against Leishmania major. J Immunol. 2014;192:2271–2279. doi: 10.4049/jimmunol.1301914. [DOI] [PubMed] [Google Scholar]
- 25.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher AL, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weih F, Caamaño J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Dejardin E, et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, et al. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity. 2015;42:719–730. doi: 10.1016/j.immuni.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browning JL, et al. Characterization of lymphotoxin-α β complexes on the surface of mouse lymphocytes. J Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- 31.Lahoud MH, et al. Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 32.Tumanov AV, et al. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood. 2010;116:3456–3464. doi: 10.1182/blood-2009-10-249177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF α-deficient mice: A critical requirement for TNF α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kain MJW, Owens BMJ. Stromal cell regulation of homeostatic and inflammatory lymphoid organogenesis. Immunology. 2013;140:12–21. doi: 10.1111/imm.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim M-Y, et al. Function of CD4+CD3- cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–1610. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 36.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 37.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Sun C-M, Fiette L, Tanguy M, Leclerc C, Lo-Man R. Ontogeny and innate properties of neonatal dendritic cells. Blood. 2003;102:585–591. doi: 10.1182/blood-2002-09-2966. [DOI] [PubMed] [Google Scholar]
- 39.Hatherley D, et al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell. 2008;31:266–277. doi: 10.1016/j.molcel.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Yi T, et al. Splenic dendritic cells survey red blood cells for missing self-CD47 to trigger adaptive immune responses. Immunity. 2015;43:764–775. doi: 10.1016/j.immuni.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPherson VA, et al. SH2 domain-containing phosphatase-2 protein-tyrosine phosphatase promotes Fc ε RI-induced activation of Fyn and Erk pathways leading to TNF α release from bone marrow-derived mast cells. J Immunol. 2009;183:4940–4947. doi: 10.4049/jimmunol.0900702. [DOI] [PubMed] [Google Scholar]
- 42.Murata Y, et al. Autoimmune animal models in the analysis of the CD47-SIRPα signaling pathway. Methods. 2014;65:254–259. doi: 10.1016/j.ymeth.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 43.Ishifune C, et al. Differentiation of CD11c+ CX3CR1+ cells in the small intestine requires Notch signaling. Proc Natl Acad Sci USA. 2014;111:5986–5991. doi: 10.1073/pnas.1401671111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.