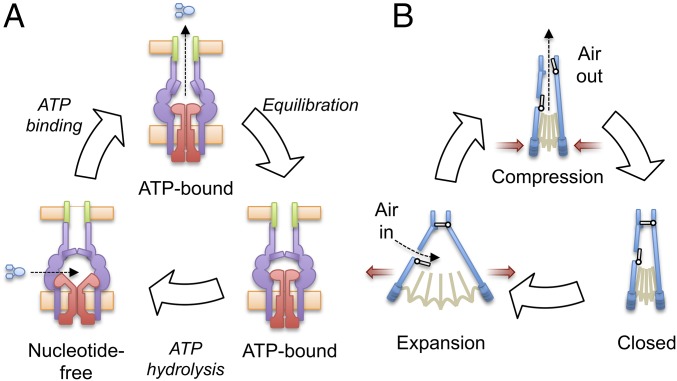
Significance
Bacterial ABC transporters typically mediate transport of substrates across the cytoplasmic membrane, using either alternating access or toppling-based mechanisms. The noncanonical ABC transporter MacB does not behave in this manner, but instead couples cytoplasmic ATP hydrolysis with periplasmic conformational changes that drive substrates from the periplasm to the extracellular space via the TolC exit duct. Here we describe the mechanotransmission mechanism of MacB in molecular detail by comparing ATP-bound and nucleotide-free structures. We further show that MacB shares its structural architecture with an entire superfamily of ABC transporters responsible for fundamental bacterial processes, including cell division and outer membrane biogenesis, suggesting a common mode of operation, and raise the possibility of targeting such proteins for the development of new antibiotics.
Keywords: ABC transporter, X-ray crystallography, tripartite efflux pump
Abstract
MacB is an ABC transporter that collaborates with the MacA adaptor protein and TolC exit duct to drive efflux of antibiotics and enterotoxin STII out of the bacterial cell. Here we present the structure of ATP-bound MacB and reveal precise molecular details of its mechanism. The MacB transmembrane domain lacks a central cavity through which substrates could be passed, but instead conveys conformational changes from one side of the membrane to the other, a process we term mechanotransmission. Comparison of ATP-bound and nucleotide-free states reveals how reversible dimerization of the nucleotide binding domains drives opening and closing of the MacB periplasmic domains via concerted movements of the second transmembrane segment and major coupling helix. We propose that the assembled tripartite pump acts as a molecular bellows to propel substrates through the TolC exit duct, driven by MacB mechanotransmission. Homologs of MacB that do not form tripartite pumps, but share structural features underpinning mechanotransmission, include the LolCDE lipoprotein trafficking complex and FtsEX cell division signaling protein. The MacB architecture serves as the blueprint for understanding the structure and mechanism of an entire ABC transporter superfamily and the many diverse functions it supports.
Tripartite efflux pumps (TEPs) span the inner and outer membranes of Gram-negative bacteria to mediate export of protein toxins, antibiotics, and virulence factors (1). In Escherichia coli, the inner membrane ABC transporter MacB (2), adaptor protein MacA, and TolC exit duct (3) form a TEP that confers resistance to macrolide antibiotics (2, 4, 5) and exports heat-stable enterotoxin STII (6, 7). ABC transporters are composed of nucleotide binding domains (NBDs) required for ATP hydrolysis and transmembrane domains that typically facilitate passage of the substrate through the membrane in which they reside, using an alternating access or toppling mechanism (8, 9). MacB is unlikely to use such mechanisms for enterotoxin transport, as it resides in the inner membrane and transports enterotoxin STII from the periplasm to the extracellular space (6).
MacB contains four transmembrane helices, an N-terminal NBD, and an extensive periplasmic domain located between TM1 and TM2 (10, 11). MacB homologs operate either within or independent of TEPs to perform a multitude of roles (12, 13). TEP-independent MacB homologs include key proteins involved in lipoprotein trafficking (LolCDE) and cell division (FtsEX) (12). LolCDE extracts lipoproteins from the inner membrane to a periplasmic chaperone (14), and FtsEX is thought to alter conformation of its periplasmic domain to recruit and activate periplasmic peptidoglycan hydrolases during bacterial cell division (15). Similar to MacB, LolCDE and FtsEX do not transport substrates across the inner membrane, but instead use cytoplasmic ATP hydrolysis to perform work in the periplasm.
Here, we present the crystal structure of ATP-bound MacB at 3.35 Å that, in combination with a nucleotide-free form derived from a cryoEM structure of the MacAB-TolC assembly (11), reveals a mechanotransmission mechanism. We demonstrate that MacB is representative of a wider family of ABC transporters that use the same architecture and mechanism to perform diverse biological functions.
Results
The Structure of MacB Defines the Type VII ABC Transporter Fold.
We determined structures of ATP-bound MacB, using X-ray crystallography. After screening multiple MacB homologs, crystals of Aggregatibacter actinomycetemcomitans (hereafter Aa) MacB were obtained in two space groups, P6522 and P21, and their structures solved at 3.90 and 3.35 Å resolution, respectively. Structures of the E. coli MacB periplasmic domain (1.95 Å) and cytoplasmic NBD (2.40 Å) were also determined. Structure determination for full-length AaMacB was achieved using a combination of molecular replacement with the individual domains and selenomethionine-based anomalous scattering approaches. X-ray data and refinement statistics are given in SI Appendix, Tables S1 and S2, and representative electron density for the full-length MacB structure is shown in Movie S1.
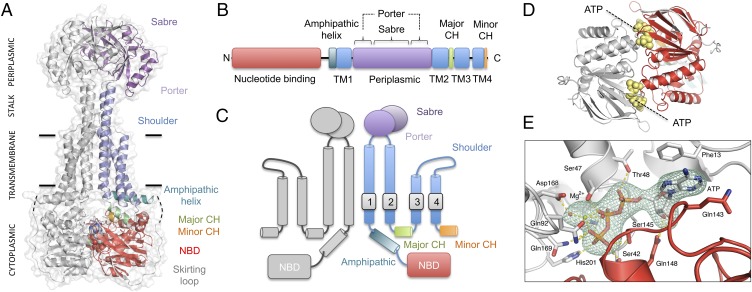
The structure of AaMacB is shown in Fig. 1A, colored consistently with its linear domain arrangement (Fig. 1B) and transmembrane topology (Fig. 1C). Each MacB monomer within the dimer contains four transmembrane helices (TM1–TM4, blue), an N-terminal nucleotide-binding domain (NBD, red), and a 198-residue periplasmic domain (purple) located between TM1 and TM2. The periplasmic domain is itself made up of two subdomains; the Sabre (Small alpha/beta rich extracytoplasmic) subdomain is composed of a single contiguous region of the MacB polypeptide (residues 347–465), and the Porter subdomain is formed from two β-α-β motifs (306–346 and 466–503) located on either side of the Sabre-encoding region. The entire periplasmic domain is elevated above the cytoplasmic membrane by a four-helix bundle (Fig. 1A, stalk) comprising extensions of TM1 and TM2 from both monomers that interact along the dimer’s symmetry axis. TM3 and TM4 are both shorter than TM1 and TM2 and are not involved in dimerization, packing on the outside of the four-helix bundle. An eight-residue loop connects TM3 and TM4 on the periplasmic side straddling the interface between the outer leaflet of the cytoplasmic membrane and the periplasm (Fig. 1A, shoulder). On the cytoplasmic side of TM1, an 18-amino acid amphipathic helix (teal, 246–263) runs parallel with the plane of the membrane and is connected to the NBD by a 41-residue skirting loop (gray, residues 224–264) that lacks secondary structure and is highly flexible. Residues 242–246 are too disordered to model. A short helix located between TM2 and TM3 forms the major coupling helix (green, residues 548–556), and an additional interaction with the NBD is made by the C terminus (minor coupling helix, orange, residues 637–646).
Fig. 1.
Crystal structure of AaMacB with bound ATP. (A) Overall structure of the MacB dimer. (B) Linear domain organization of the MacB polypeptide. (C) Topology of MacB. (D) Location of the two ATP molecules (yellow) clamped between the NBDs. (E) Detailed view of bound ATP with omit map density contoured at 3.5 sigma (teal). Residues interacting with ATP are labeled and potential hydrogen bonds (dashed yellow lines) are indicated. Coloring is consistent across the figure: one monomer is shown in gray and the second is colored according to the domain arrangement.
The fold and topology of the MacB transmembrane and periplasmic domain are strikingly different from the six currently recognized ABC transporter superfamilies (SI Appendix, Fig. S1). The key distinguishing features of MacB are its four-transmembrane helix topology, periplasmic domain, and stalk. MacB also has a rigid transmembrane dimerization interface dominated by hydrophobic interactions and lacks transmembrane cavities (SI Appendix, Fig. S2). The MacB structure supports previous bioinformatics-based analyses, suggesting it belongs to a distinct monophyletic group with an independent evolutionary origin from all other ABC transporters that have been structurally characterized (12, 13). We therefore designate MacB the type specimen for the type VII ABC transporter superfamily on the basis of its novel topology, dimerization interface, and transmembrane fold, all of which are unique to MacB.
Mode of ATP-Binding in MacB.
The structures presented here represent ATP-bound conformations of MacB in which the two cytoplasmic ATP binding sites are each occupied by a single nucleotide. The locations of the ATP-binding sites are shown in Fig. 1D, and an omit map showing unbiased electron density for one of the nucleotides appears in Fig. 1E and Movie S2. The ATP binding mode is similar to that seen in other ABC transporters (8, 9), with the nucleotides sandwiched between dimerized NBDs. The ATP phosphates are coordinated by a bound magnesium ion and Lys46 (Lys47 in E. coli). The catalytic glutamate, Glu169 (Glu170 in E. coli, here mutated to glutamine), is positioned directly adjacent to the γ-phosphate consistent with a role in ATP hydrolysis. The MacB major coupling helix interacts intramolecularly with a groove on the surface of the NBD, providing a probable means by which conformational changes associated with the nucleotide binding and hydrolysis cycle are conveyed to the transmembrane domain. The MacB NBDs may operate as a conformational switch (16).
MacB Confers Resistance to Antibiotics and Supports Enterotoxin STII Secretion.
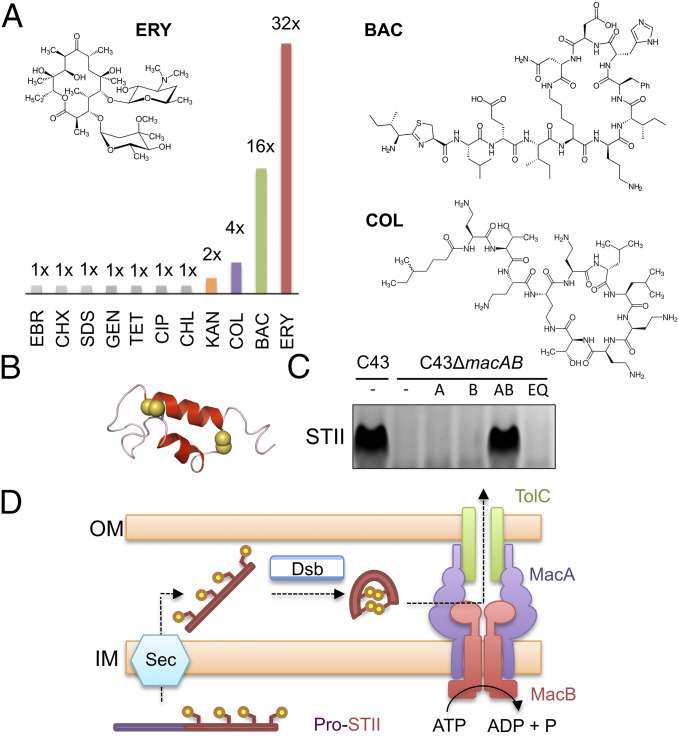
E. coli MacB was first identified and named for its role in macrolide resistance (2), but has since been shown to transport enterotoxin STII (6), and may have a role in protoporphyrin expulsion (17). To identify further MacB substrates, we determined the minimal inhibitory concentrations (MICs) of 11 distinct antimicrobial compounds against E. coli strains lacking chromosomal acrAB and macAB and expressing either plasmid-borne wild-type or catalytically inactive E. coli MacAB. The strain used lacks the broad-spectrum RND efflux pump AcrAB, facilitating detection of MacAB substrates that might otherwise be obscured. We observed a significant protective effect for MacAB against erythromycin, bacitracin, and colistin (Fig. 2A and SI Appendix, Table S3). Identification of tolerance to bacitracin and colistin, both of which are cyclic peptides, shows MacB has a broader role in antibiotic resistance than previously recognized and is not limited to macrolides such as erythromycin.
Fig. 2.
Functional analysis of E. coli MacAB-TolC in vivo. (A) Antibiotic susceptibility comparison between E. coli C43 (DE3) ΔacrABΔmacAB cells expressing E. coli MacA and either wild-type or Glu170Gln E. coli MacB variant. The ratio of the MIC for the wild-type MacB to the Glu170Gln variant is shown graphically for ethidium bromide (EBR), chlorhexidine (CHX), SDS, gentamycin (GEN), tetracycline (TET), ciprofloxacin (CIP), chloramphenicol (CHL), kanamycin (KAN), colistin (COL), bacitracin (BAC), and erythromycin (ERY). Structures of erythromycin, bacitracin, and colistin are shown (Inset). (B) Structure of enterotoxin STII with disulfide bond-forming sulfur atoms (yellow spheres; Protein Data Bank: 1EHS) (32). (C) Detection of secreted enterotoxin in E. coli culture supernatants by SDS/PAGE. Lanes from left to right: C43 wild-type, C43ΔmacAB, C43ΔmacAB expressing plasmid-borne macA (A), macB (B), macAB (AB), or macAB with an ATPase inactivating Glu170Gln substitution (EQ). All strains contain a plasmid-expressing enterotoxin STII. (D) Model for two-step secretion of enterotoxin STII across the E. coli cell envelope. Sec and Dsb represent the general protein secretion machinery and disulfide bond incorporation systems, respectively.
Having shown that MacB handles peptide-like antibiotics such as colistin and bacitracin, we further investigated its role in enterotoxin STII secretion. Enterotoxin STII is a 48-amino acid protein with two internal disulfide bonds that is produced as a virulence factor by enterotoxigenic E. coli (Fig. 2B). Expression of STII in E. coli leads to the appearance of mature toxin in the culture supernatant that is disrupted when chromosomal macAB genes are deleted. Secretion of STII is restored by complementation with wild-type MacAB, but not by expression of either MacA or MacB alone, or by coexpression of MacAB with an ATPase-inactivating mutation (Glu170Gln) in MacB (Fig. 2C). Our experiments confirm a role for the MacAB-TolC efflux pump in secretion of enterotoxin STII (6, 18) and demonstrate MacB ATPase activity is required to extract this substrate from the periplasm and transport it to the extracellular space (Fig. 2D).
Structure-Led Mutagenesis of macB Identifies Residues Involved in Antibiotic Resistance.
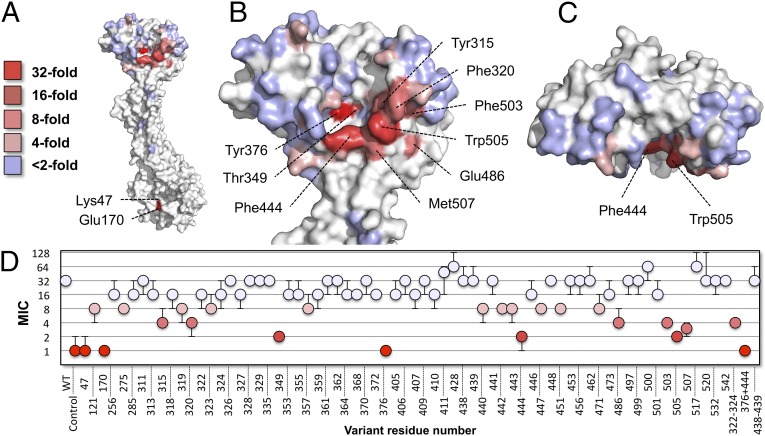
To probe the relationship between structure and function in MacB, we used site-directed mutagenesis to disrupt structural features within the NBD, transmembrane domain, stalk, and periplasmic domain and tested each variant’s ability to confer resistance to erythromycin (SI Appendix, Table S4). The MIC results were color-mapped to a homology model of E. coli MacB generated from the Aa full-length structure and E. coli-soluble domains (Fig. 3). We first targeted residues on the cytoplasmic side of the membrane, including residues shown to be mechanistically essential in MacB and other ABC systems (5, 9). As expected, mutation of residues involved in ATP binding (Lys47Ala) and hydrolysis (Glu170Gln) each reduced MIC values to levels comparable with that of the empty vector control (a 32-fold reduction; Fig. 3 A and D). Deletion of the minor coupling helix or disruption of an electrostatic interaction (Arg121-Glu256) between the amphipathic helix and NBD did not significantly affect MacB function, showing these features are not essential (SI Appendix, Table S4). Next, we focused on polar residues at the dimer interface. Alanine substitutions for Thr275, Ser285, Ser532, and Asn542 in the MacB transmembrane domain, and residues Thr517 and Thr520 in the periplasmic stalk, did not substantially affect MacB function, suggesting these residues are unlikely to interact with substrates. Finally, through extensive mutagenesis of the periplasmic domain, we revealed an obvious hotspot of residues on the interior surface, indicating a plausible substrate interaction site (Fig. 3 B–D). Key positions associated with the periplasmic hotspot are Tyr376, Phe444, Trp505, and Thr349, for which alanine substitution yields at least a 16-fold reduction in MIC, and Tyr315, Phe320, Phe503, and Met507, which each give eightfold reductions. Further MIC determinations for a subset of MacB variants were performed using bacitracin and colistin, revealing a similar pattern of MIC reduction to that of erythromycin, suggesting the same periplasmic site is important for resistance to all these molecules (SI Appendix, Fig. S3 and Table S5).
Fig. 3.
Structure-based mutational analysis of E. coli MacB. Erythromycin resistance of C43 (DE3) ΔacrAB ΔmacAB cells expressing E. coli MacA and variant MacB mapped to a model of one monomer of E. coli MacB. Key indicates the fold decrease in MIC relative to cells expressing wild-type MacAB. (A) The MacB monomer presenting the dimer interface. (B) Close-up view of the periplasmic domain. (C) Top-down view of the MacB periplasmic domain. (D) MICs (µg/mL) for the 70 MacB variants tested. Homology model shown is based on our full-length AaMacB crystal structure and high-resolution structure of the E. coli MacB periplasmic domain.
We further tested our 70-strong cohort of MacB variants for their capacity to secrete enterotoxin STII. With the exception of Lys47Ala and Glu170Gln, all MacB variants were capable of toxin secretion (SI Appendix, Table S4). The reason for differential effects of periplasmic domain mutations on antibiotic resistance and enterotoxin secretion is not yet clear. One possibility is that the structure of enterotoxin presents multiple opportunities for productive interaction with MacB that render individual point mutations insufficient to disrupt transport. Alternatively, the site of enterotoxin interaction may be distinct from the site identified here relating to antibiotic resistance; a second site could plausibly be located, either in whole or in part, elsewhere in MacB, or within another component of the tripartite assembly (MacA or TolC). We conclude that individual amino acids lining the interior face of the MacB periplasmic domain are vital for antibiotic resistance but dispensable for enterotoxin secretion, whereas residues associated with ATP binding and hydrolysis are essential for both.
ATP-Bound and Nucleotide-Free States of MacB Reveal the Molecular Basis of Mechanotransmission.
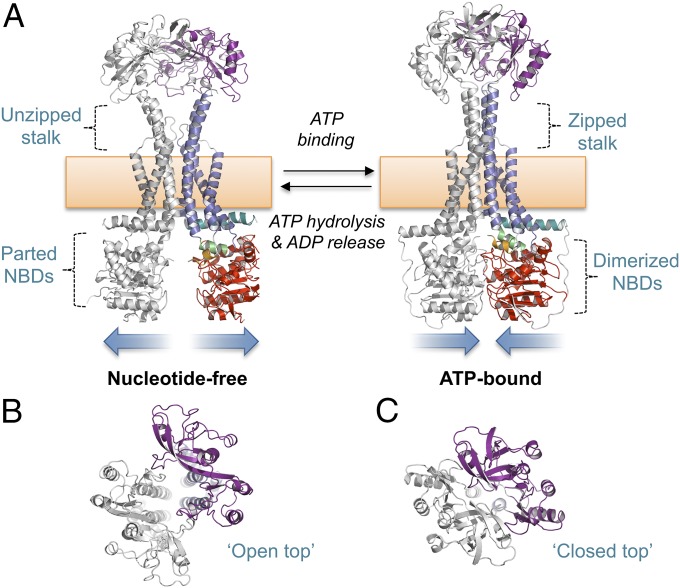
We assessed the conformational changes associated with ATP binding and hydrolysis by comparing our ATP-bound MacB with a nucleotide-free form extracted from a MacAB-TolC assembly structure determined by cryoEM (11) (Fig. 4). After superposition of the two MacB forms, we generated a molecular morph revealing the probable motions that link these states (Movie S3). Our comparison reveals distinctive long-range conformational changes in the transmembrane and periplasmic domains linked to the nucleotide status of the NBD we define here as mechanotransmission. In the nucleotide-free form, the MacB NBDs are separated and a prominent V-shaped split is apparent in the periplasmic stalk (Fig. 4A, Left). The periplasmic head of MacB adopts an open form with an obvious cavity between the two monomers (Fig. 4B). In the ATP-bound state, the cytoplasmic domains are dimerized and the two halves of the periplasmic stalk stand straight and perpendicular to the plane of the membrane (Fig. 4A, Right). In this form, the cavity within the periplasmic domain is absent and the entire structure is much more compact (Fig. 4C). Conformational changes apparent on both sides of the membrane are coordinated by the transmembrane domain, stalk, and coupling helices. Specifically, on ATP binding, NBD dimerization acts through the major coupling helix to push TM2 upward through the membrane, causing a shift in the register of opposing residues on either side of the dimer interface. The newly generated interface promotes association of the transmembrane domain and stalk into a rigid four-helix bundle, bringing together the periplasmic domains. Details of the dimer interface observed for each conformation are given in SI Appendix, Fig. S4.
Fig. 4.
Mechanotransmission mechanism of MacB. (A) Nucleotide-free (Left) and ATP-bound MacB (Right). (B) Top-down view of the MacB dimer showing the open head of the nucleotide-free form. (C) Equivalent view of the ATP-bound form showing closure of the periplasmic dimer. Domains of MacB colored as in Fig. 1. Both models represent E. coli MacB; the nucleotide-free form is extracted from the cryoEM structure of the MacAB-TolC complex (5NIL), and the ATP-bound form is a homology model generated from our crystal structure of the nucleotide-bound AaMacB (5LIL). A full molecular morph between the two states is presented in Movie S3.
To validate the conformational changes observed by comparison of MacB structures, we used a disulfide cross-linking experiment testing whether restricting mechanotransmission affects activity. Guided by the structure, we produced a MacB cysteine variant (Thr517Cys) predicted to lock the stalk in its closed (zipped) conformation and tested its ability to confer erythromycin tolerance (SI Appendix, Fig. S5 and Table S6). Consistent with inhibition of MacB as a result of disulfide locking, the Thr517Cys variant has a lower-than-wild-type MIC that is further diminished by addition of CuCl2 (an oxidant favoring disulfide formation) and restored to wild-type levels by DTT (favoring disulfide reduction). Contrastingly, the activities of the wild-type, Glu170Gln, and Thr517Ala variants are unaffected by either CuCl2 or DTT. The cysteine cross-linking experiment provides in vivo support for the mechanotransmission mechanism.
Molecular Bellows Mechanism for the MacAB-TolC Tripartite Efflux Pump.
We considered how MacB mechanotransmission might operate within the context of the assembled MacAB-TolC pump to drive substrate from the periplasm to the extracellular space. The structure of the nucleotide-free MacAB-TolC complex is known (11), and superposition of our ATP-bound MacB structure suggests a model for the missing assembled state. The model suggests the MacA hexamer accommodates MacB in both of its two conformational states, and that a tight seal between these components is maintained throughout the mechanotransmission cycle. An interior cavity at the interface between MacA and MacB undergoes dramatic changes in volume on transition between nucleotide-free and ATP-bound forms (SI Appendix, Fig. S6), leading us to suggest that the assembled MacAB-TolC complex acts as a molecular bellows during efflux (Fig. 5 and SI Appendix).
Fig. 5.
A molecular bellows mechanism for substrate secretion by the MacAB-TolC tripartite efflux pump. (A) Proposed catalytic cycle of the MacAB-TolC efflux pump. MacB (red), MacA (purple), TolC (green) and substrate (blue). (B) Operation of a fireplace bellows.
In the molecular bellows mechanism (Fig. 5 and SI Appendix), substrates are proposed to enter the interior cavity via an opening in MacB located between parted periplasmic domains and stalk (see also Fig. 4A, Left) before subsequent ATP-binding causes closure of the periplasmic domain by mechanotransmission. The reduction in interior cavity volume forces the contents of the MacB periplasmic domain, under pressure, through the MacA gate ring, previously suggested to act as a one-way valve (11). Once the pressure on either side of the gate has equalized, the gate ring relaxes to its closed resting state, preventing backflow of substrates. ATP hydrolysis then resets the system with further substrates and the solvent necessary to refill the cavity entering from the periplasm as the interior cavity expands.
Coordination of ATP binding and hydrolysis with detection of a substrate in the periplasmic cavity would prevent wasteful expenditure of ATP. Whether MacB can sense periplasmic-bound substrates and communicate this to the NBDs is unclear, but MacA spans the inner membrane and stimulates MacB ATPase activity suggesting the adaptor may mediate this signal (5, 19).
MacB Represents a Superfamily of Mechanotransmissive ABC Transporters Including LolCDE and FtsEX.
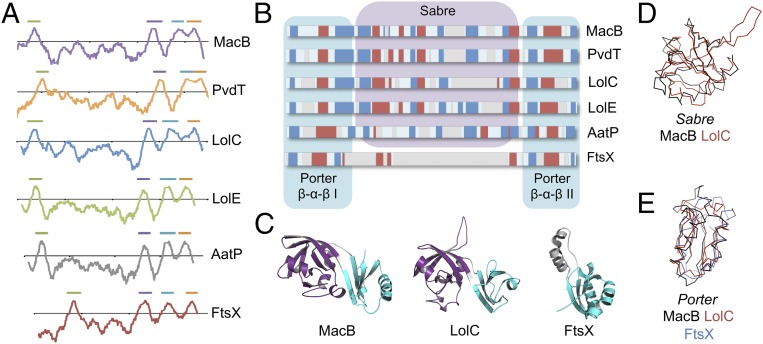
We next examined the degree to which structural features underpinning the mechanotransmission mechanism in MacB are conserved in related proteins. MacB belongs to a large and functionally diverse monophyletic group of ABC transporters (sometimes termed the ABC3 superfamily) that has been defined on the basis of sequence-level features including hydropathy profiles and amino acid similarity (12, 13). Many homologs of MacB are genetically encoded in operons alongside genes for a periplasmic adaptor and TolC-like protein, suggesting they form TEPs similar to MacAB-TolC. Two examples are PvdT of Pseudomonas aeruginosa (20) and AatP from enteroaggregative E. coli (21). Similar to MacAB-TolC, these TEPs extract their substrates from the periplasm and use cytoplasmic ATP hydrolysis to power efflux across the outer membrane. Other homologs of MacB do not form TEPs, but do couple cytoplasmic ATP hydrolysis with activities in the periplasm or extracytoplasmic space. LolC and LolE collaborate with NBDs encoded by LolD to translocate lipoproteins from the outer face of the inner membrane to a periplasmic chaperone during outer membrane biogenesis (14). Similarly, FtsE (NBD) and FtsX (transmembrane domain) form a substrateless ABC transporter involved in transmembrane signaling between cytoplasmic and periplasmic components of the cell division machinery (15, 22). Hydropathy plots show that PvdT, AatP, LolC, LolE, and FtsX all have the same transmembrane topology as MacB, with a periplasmic domain located between the first and second membrane-spanning helices (Fig. 6A). Secondary structure analysis predicts that all these proteins except FtsX contain both the Porter and Sabre subdomains present in the MacB crystal structure (Fig. 6B). For FtsX, the presence of the Porter and absence of the Sabre is confirmed by its crystal structure from Mycobacterium tuberculosis. However, our prediction for LolCDE conflicts with previous suggestions that the periplasmic domain may have a β-barrel fold similar to LolA and LolB (23). Assigning the correct fold to LolC is essential because the previously proposed mouth-to-mouth transfer of lipoprotein substrates from LolCDE to LolA is not compatible with a MacB-like fold. We experimentally tested our prediction by solving the crystal structure of the E. coli LolC periplasmic domain. Data collection and refinement statistics are provided in SI Appendix, Table S7, and the LolC periplasmic domain is shown in comparison with those of MacB and FtsX in Fig. 6C. The 1.88-Å structure of LolC confirms a MacB-like fold with both Sabre (Fig. 6D) and Porter (Fig. 6E) subdomains (Movie S4). We conclude that features of the MacB architecture underpinning its distinctive mechanotransmission mechanism, including transmembrane topology and core aspects of its extracytoplasmic domain structure, are conserved throughout this ABC superfamily.
Fig. 6.
MacB is the archetypal member of a topological unique ABC superfamily. (A) Hydropathy profile of MacB and five key homologs demonstrating a common four-transmembrane helix topology. Horizontal bars above the plots indicate TM helices. (B) Comparison of secondary structure predictions for the periplasmic domain located between TM1 and TM2 for MacB and homologs. Beta sheets (blue), alpha helices (red), and alignment gaps (gray) are shown. (C) Periplasmic domain structures of MacB, LolC, and FtsX. Sabre subdomain shown in purple, Porter subdomain cyan. (D) Superposition of Sabre subdomains from MacB and LolC. (E) Superposition of Porter subdomains from MacB, LolC, and FtsX.
Discussion
We determined the crystal structure of MacB in an ATP-bound conformation revealing a noncanonical ABC transporter fold (Fig. 1) and discovered that MacB confers resistance to cyclic peptides such as bacitracin and colistin, in addition to expelling the known substrates erythromycin and enterotoxin STII (Fig. 2). Structure-guided mutagenesis of MacB led to identification of residues in the periplasmic domain essential for erythromycin tolerance (Fig. 3), and comparison of ATP-bound and nucleotide-free structures of MacB revealed allosteric coupling of periplasmic conformational change with reversible dimerization of the cytoplasmic NBDs (Fig. 4). We term the distinctive transmembrane conformational coupling mechanism of MacB mechanotransmission and propose that it is harnessed within the context of the assembled tripartite efflux pump to drive substrate efflux from the periplasm to the extracellular space by acting as a molecular bellows (Fig. 5). Finally, we demonstrate that the structural architecture underlying mechanotransmission is present in homologous systems such as the LolCDE lipoprotein trafficking complex and FtsEX cell division machinery (Fig. 6), suggesting a unified mechanism for the entire superfamily.
The key feature of MacB that distinguishes it from other ABC transporters is its mechanotransmission mechanism (Fig. 4 and Movie S3). Neither ATP-bound nor nucleotide-free structures of MacB reveal evidence of a central pore through which substrates might be passed, nor does either exhibit the characteristic inward- and outward-facing cavities that are the hallmark of the alternating-access mechanism. MacB operates solely through extracytoplasmic conformational change driven by cytoplasmic ATP hydrolysis. In collaboration with the MacA adaptor and TolC exit duct, MacB powers the expulsion of enterotoxin STII from periplasm to extracellular space, and it seems likely that antibiotic substrates of MacB (erythromycin, colistin, and bacitracin) take the same route, rather than being driven across the inner membrane as previously thought. Alternatively, it is possible that MacB does not interact directly with antibiotics, but instead transports another, as-yet-unidentified factor affecting the cell’s susceptibility. Outer membrane glycolipids have been implicated as potential substrates for MacAB-TolC (24), and such substrates could conceivably drive resistance indirectly by modifying permeability of the cell envelope.
MacB’s role in erythromycin resistance partially overlaps that of the RND family tripartite efflux pump AcrAB-TolC. RND family TEPs, including AcrAB-TolC, have been described as periplasmic vacuum cleaners (25) on account of their broad substrate specificity. Although AcrAB-TolC is highly proficient in secretion of small molecules, including erythromycin, enterotoxin STII is solely exported via MacAB-TolC. Systems homologous to MacAB-TolC secrete pyoverdine [a peptide-like siderophore from P. aeruginosa (20)] and dispersin [a 10-kDa enteroaggregative E. coli signaling protein (21)], which suggests a division of labor in which RND transporters tackle small molecule efflux and MacAB-TolC-based systems drive export of larger peptides and small proteins.
The structural architecture of MacB is conserved in both FtsEX and LolCDE (Fig. 6), suggesting the capacity for mechanotransmission is also preserved. FtsEX regulates periplasmic peptidoglycan hydrolases during cell division (15, 22, 26), whereas LolCDE extracts lipoproteins from the outer leaflet of the bacterial inner membrane and presents them to the periplasmic chaperone LolA for trafficking to the outer membrane (14, 27). Roles for mechanotransmission in these processes remain to be fully elucidated; however, for LolCDE, the upward force generated by zipping together the periplasmic stalk could aid lipoprotein extraction from the bacterial membrane. Similarly, for FtsEX, the two conformational states could form a molecular switch, stimulating recruitment and/or activation of extracytoplasmic enzymes in one conformation and frustrating this process in the other. The MacB structures constitute a valuable tool for guiding future research on FtsEX and LolCDE, both of which are key antibiotic targets (28, 29).
Intriguingly, the LptBFG complex has a similar function as LolCDE, but an entirely different transmembrane topology (30). The use of several distinct structural folds to direct similar in vivo functions is a recurring feature of ABC transporter evolution, as evidenced by the three different ABC superfamilies linked to substrate import (SI Appendix, Fig. S1, types I, II, and III) and a further two associated with substrate export (SI Appendix, Fig. S1, types IV and V). It will be interesting to compare the mechanism of MacB/LolCDE (type VII) with that of LptBFG (type VI) once further conformational states of the latter are known.
In summary, the MacB structure defines an atypical ABC transporter superfamily and reveals the mechanotransmission mechanism it uses. MacB superfamily members do not transport substrates across the inner membrane in which they reside but, instead, couple cytoplasmic ATP hydrolysis with extracytoplasmic conformational change. Whether mechanotransmission is used to gate access to an outer membrane exit duct (as for MacB), pass a substrate from the membrane to a chaperone (LolCDE), or else regulate the activity of periplasmic enzymes (FtsEX), the same underlying four-transmembrane helix architecture provides the means of transmitting conformational change across the membrane. The MacB architecture provides a fresh structural and mechanistic perspective on a plethora of homologous mechanotransmissive ABC transporters that mediate diverse biological functions in the bacterial envelope and extracellular space.
Methods
Detailed SI Appendix, Methods are available online. Briefly, AaMacB was expressed with an N-terminal His-tag in E. coli C43 (DE3) before purification in lauryl maltose neopentyl glycol as a stabilizing detergent. MacB was crystallized in two distinct crystal forms in the presence of either ATPγS or ATP and the structures solved by a combination of molecular replacement and selenomethionine phasing methods. Structures of the E. coli MacB periplasmic domain (residues 309–508) and cytoplasmic NBD (residues 1–223) were determined to aid this phasing strategy. Minimum inhibitory concentrations were assessed by serial dilution method in E. coli C43ΔacrABΔmacAB with either wild-type or variant MacB coexpressed with MacA from a plasmid. Enterotoxin STII secretion was assessed by trichloroacetic acid precipitation of culture supernatants and analyzed by SDS/PAGE. Molecular morphs used a homology model of E. coli MacB based on the Aa ATP-bound state and the nucleotide-free state extracted from 5NIL (11) as start and end points. Bioinformatic analysis used TMPred for hydropathy plots and Promals3D (31) for secondary structure prediction and sequence alignment. The E. coli LolC periplasmic domain (residues 48–266) was expressed with a C-terminal His-tag in E. coli BL21 (DE3) and the structure solved by molecular replacement using the AaMacB periplasmic domain.
Supplementary Material
Acknowledgments
We thank staff at Soleil (France) and Diamond (UK) synchrotrons for beam line facilities and Professor Yamanaka for providing the pET11-STII plasmid. This work was supported by grants from the UK Medical Research Council (MR/N000994/1) and the Wellcome Trust (101828/Z/13/Z).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 5LIL, 5LJ6, 5LJ7, 5LJ8, 5LJ9, 5LJA, and 5NAA).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712153114/-/DCSupplemental.
References
- 1.Hinchliffe P, Symmons MF, Hughes C, Koronakis V. Structure and operation of bacterial tripartite pumps. Annu Rev Microbiol. 2013;67:221–242. doi: 10.1146/annurev-micro-092412-155718. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi N, Nishino K, Yamaguchi A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J Bacteriol. 2001;183:5639–5644. doi: 10.1128/JB.183.19.5639-5644.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 4.Lu S, Zgurskaya HI. Role of ATP binding and hydrolysis in assembly of MacAB-TolC macrolide transporter. Mol Microbiol. 2012;86:1132–1143. doi: 10.1111/mmi.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tikhonova EB, Devroy VK, Lau SY, Zgurskaya HI. Reconstitution of the Escherichia coli macrolide transporter: The periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol Microbiol. 2007;63:895–910. doi: 10.1111/j.1365-2958.2006.05549.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka H, Kobayashi H, Takahashi E, Okamoto K. MacAB is involved in the secretion of Escherichia coli heat-stable enterotoxin II. J Bacteriol. 2008;190:7693–7698. doi: 10.1128/JB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyfus LA, et al. Calcium influx mediated by the Escherichia coli heat-stable enterotoxin B (STB) Proc Natl Acad Sci USA. 1993;90:3202–3206. doi: 10.1073/pnas.90.8.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 9.ter Beek J, Guskov A, Slotboom DJ. Structural diversity of ABC transporters. J Gen Physiol. 2014;143:419–435. doi: 10.1085/jgp.201411164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi N, Nishino K, Hirata T, Yamaguchi A. Membrane topology of ABC-type macrolide antibiotic exporter MacB in Escherichia coli. FEBS Lett. 2003;546:241–246. doi: 10.1016/s0014-5793(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick AWP, et al. Structure of the MacAB-TolC ABC-type tripartite multidrug efflux pump. Nat Microbiol. 2017;2:17070. doi: 10.1038/nmicrobiol.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khwaja M, Ma Q, Saier MH., Jr Topological analysis of integral membrane constituents of prokaryotic ABC efflux systems. Res Microbiol. 2005;156:270–277. doi: 10.1016/j.resmic.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Dukarevich M, Sun EI, Yen MR, Saier MH., Jr Membrane porters of ATP-binding cassette transport systems are polyphyletic. J Membr Biol. 2009;231:1–10. doi: 10.1007/s00232-009-9200-6. [DOI] [PubMed] [Google Scholar]
- 14.Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat Cell Biol. 2000;2:212–218. doi: 10.1038/35008635. [DOI] [PubMed] [Google Scholar]
- 15.Yang DC, et al. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Natl Acad Sci USA. 2011;108:E1052–E1060. doi: 10.1073/pnas.1107780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. doi: 10.1038/nsmb836. [DOI] [PubMed] [Google Scholar]
- 17.Turlin E, et al. Protoporphyrin (PPIX) efflux by the MacAB-TolC pump in Escherichia coli. MicrobiologyOpen. 2014;3:849–859. doi: 10.1002/mbo3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foreman DT, Martinez Y, Coombs G, Torres A, Kupersztoch YM. TolC and DsbA are needed for the secretion of STB, a heat-stable enterotoxin of Escherichia coli. Mol Microbiol. 1995;18:237–245. doi: 10.1111/j.1365-2958.1995.mmi_18020237.x. [DOI] [PubMed] [Google Scholar]
- 19.Modali SD, Zgurskaya HI. The periplasmic membrane proximal domain of MacA acts as a switch in stimulation of ATP hydrolysis by MacB transporter. Mol Microbiol. 2011;81:937–951. doi: 10.1111/j.1365-2958.2011.07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imperi F, Tiburzi F, Visca P. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2009;106:20440–20445. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi J, et al. The export of coat protein from enteroaggregative Escherichia coli by a specific ATP-binding cassette transporter system. J Biol Chem. 2003;278:45680–45689. doi: 10.1074/jbc.M306413200. [DOI] [PubMed] [Google Scholar]
- 22.Du S, Pichoff S, Lutkenhaus J. FtsEX acts on FtsA to regulate divisome assembly and activity. Proc Natl Acad Sci USA. 2016;113:E5052–E5061. doi: 10.1073/pnas.1606656113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda S, Tokuda H. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc Natl Acad Sci USA. 2009;106:5877–5882. doi: 10.1073/pnas.0900896106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S, Zgurskaya HI. MacA, a periplasmic membrane fusion protein of the macrolide transporter MacAB-TolC, binds lipopolysaccharide core specifically and with high affinity. J Bacteriol. 2013;195:4865–4872. doi: 10.1128/JB.00756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aires JR, Nikaido H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J Bacteriol. 2005;187:1923–1929. doi: 10.1128/JB.187.6.1923-1929.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavrici D, et al. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc Natl Acad Sci USA. 2014;111:8037–8042. doi: 10.1073/pnas.1321812111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narita S, Tokuda H. Bacterial lipoproteins; biogenesis, sorting and quality control. Biochim Biophys Acta. 2017;1862:1414–1423. doi: 10.1016/j.bbalip.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 28.McLeod SM, et al. Small-molecule inhibitors of gram-negative lipoprotein trafficking discovered by phenotypic screening. J Bacteriol. 2015;197:1075–1082. doi: 10.1128/JB.02352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lock RL, Harry EJ. Cell-division inhibitors: New insights for future antibiotics. Nat Rev Drug Discov. 2008;7:324–338. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 30.Luo Q, et al. Structural basis for lipopolysaccharide extraction by ABC transporter LptB2FG. Nat Struct Mol Biol. 2017;24:469–474. doi: 10.1038/nsmb.3399. [DOI] [PubMed] [Google Scholar]
- 31.Pei J, Kim B-H, Grishin NV. PROMALS3D: A tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008;36:2295–2300. doi: 10.1093/nar/gkn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukumar M, et al. The structure of Escherichia coli heat-stable enterotoxin b by nuclear magnetic resonance and circular dichroism. Protein Sci. 1995;4:1718–1729. doi: 10.1002/pro.5560040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.