Significance
Exposure to hypoxia requires adaptive mechanisms for survival. During acute hypoxia, Na,K-ATPase endocytosis in alveolar epithelial cells occurs via protein kinase C zeta (PKCζ) phosphorylation of α1-Na,K-ATPase independently of the hypoxia-inducible factor (HIF). However, exaggerated Na,K-ATPase down-regulation leads to cell death. Here we report that during prolonged hypoxia plasma membrane Na,K-ATPase levels were maintained at ∼50% of normoxic values due to HIF-mediated up-regulation of HOIL-1L, which targets PKCζ for degradation. Silencing HOIL-1L in the lung epithelium prevented PKCζ degradation, causing Na,K-ATPase downregulation. Accordingly, HIF regulation of HOIL-1L targets the phosphorylated PKCζ for degradation and serves as an hypoxia-adaptive mechanism to stabilize the Na,K-ATPase, avoiding significant lung injury.
Keywords: hypoxia; Na,K-ATPase; HOIL-1L; PKCζ; alveolar epithelial cells
Abstract
Organisms have evolved adaptive mechanisms in response to stress for cellular survival. During acute hypoxic stress, cells down-regulate energy-consuming enzymes such as Na,K-ATPase. Within minutes of alveolar epithelial cell (AEC) exposure to hypoxia, protein kinase C zeta (PKCζ) phosphorylates the α1-Na,K-ATPase subunit and triggers it for endocytosis, independently of the hypoxia-inducible factor (HIF). However, the Na,K-ATPase activity is essential for cell homeostasis. HIF induces the heme-oxidized IRP2 ubiquitin ligase 1L (HOIL-1L), which leads to PKCζ degradation. Here we report a mechanism of prosurvival adaptation of AECs to prolonged hypoxia where PKCζ degradation allows plasma membrane Na,K-ATPase stabilization at ∼50% of normoxic levels, preventing its excessive down-regulation and cell death. Mice lacking HOIL-1L in lung epithelial cells (CreSPC/HOIL-1Lfl/fl) were sensitized to hypoxia because they express higher levels of PKCζ and, consequently, lower plasma membrane Na,K-ATPase levels, which increased cell death and worsened lung injury. In AECs, expression of an α1-Na,K-ATPase construct bearing an S18A (α1-S18A) mutation, which precludes PKCζ phosphorylation, stabilized the Na,K-ATPase at the plasma membrane and prevented hypoxia-induced cell death even in the absence of HOIL-1L. Adenoviral overexpression of the α1-S18A mutant Na,K-ATPase in vivo rescued the enhanced sensitivity of CreSPC/HOIL-1Lfl/fl mice to hypoxic lung injury. These data suggest that stabilization of Na,K-ATPase during severe hypoxia is a HIF-dependent process involving PKCζ degradation. Accordingly, we provide evidence of an important adaptive mechanism to severe hypoxia, whereby halting the exaggerated down-regulation of plasma membrane Na,K-ATPase prevents cell death and lung injury.
Short-term exposure to hypoxia leads to inhibition of Na,K-ATPase, a high-ATP–consuming enzyme, independently of hypoxia-inducible factor (HIF) (1). However, during prolonged hypoxia, HIF plays an important role in maintaining cell homeostasis (2–5). HIF regulates genes that increase energy generation via anaerobic glycolysis and those that decrease ATP-consuming enzymes, thereby preserving cell metabolism during hypoxia (2–4, 6). The Na,K-ATPase utilizes ∼30% of the cell’s ATP under basal conditions to maintain the Na+ and K+ concentration gradients across the cell membrane necessary for cellular homeostasis (1, 7, 8). Hypoxia occurs in individuals with normal respiratory function during ascent to high altitude and in patients with pulmonary edema due to heart failure and acute lung injury (9–11). Hypoxia has been reported to inhibit edema reabsorption from the alveolar spaces by inhibiting the sodium channels, which are responsible for the apical sodium entry, and basolateral membrane Na,K-ATPase, which is responsible for Na+ extrusion (12–14). The hypoxia-mediated down-regulation of the Na,K-ATPase at the alveolar epithelial cell (AEC) basolateral membrane is mediated by protein kinase C zeta (PKCζ) phosphorylation of the Na,K-ATPase α1 catalytic subunit at Ser-18, which in turn triggers Na,K-ATPase endocytosis (1, 15–17).
PKC isoenzymes play a role in the cellular adaptation to stress by regulating survival, proliferation, migration, and apoptosis (18–21). PKCζ is a member of the atypical class of PKC isoforms. Unlike the conventional and novel isoforms, atypical PKCs do not respond to the second messenger diacylglycerol or calcium, but they are activated by stimuli-dependent phosphorylation (22, 23). In the basal state, PKCs are auto-inhibited by their pseudosubstrates and converted into catalytically competent enzymes by a series of phosphorylations (18, 22, 24). However, the mechanisms that regulate termination of PKC signaling are incompletely understood. We reported that, in cancer cells, tumor growth is promoted via the transcription of heme-oxidized IRP2 ubiquitin ligase 1L (HOIL-1L), which acts as the E3 ubiquitin ligase for PKCζ, targeting it for proteasomal degradation (20, 25). HOIL-1L together with HOIL-1–interacting protein (HOIP) and Shank-associated RH-domain–interacting protein (SHARPIN) form the linear ubiquitination assembly complex (LUBAC) (26–29). We and others have found that, when acting independently of LUBAC, HOIL-1L adds Lys-48–linked chains and serves as an ubiquitin E3 ligase (20, 30).
Here we report that, in lung epithelial cells exposed to prolonged hypoxia in vitro, the Na,K-ATPase is stabilized at a plateau lower than levels in normoxic conditions via a HIF-mediated up-regulation of HOIL-1L. Hypoxia promotes the translocation of phosphorylated PKCζ to the plasma membrane where it interacts with HOIL-1L, which targets it for degradation. This PKCζ degradation limits Na,K-ATPase down-regulation and safeguards alveolar epithelial function. To examine this pathway in vivo, we generated mice with lung epithelial-specific deletion of HOIL-1L (CreSPC/HOIL-1Lfl/fl). Exposure of these mice to prolonged hypoxia resulted in higher levels of PKCζ, excessive depletion of plasma membrane Na,K-ATPase, and more severe lung injury. This sensitivity was rescued by overexpression of a phosphorylation-resistant mutant, Na,K-ATPase. Collectively, these data suggest that HOIL-1L–mediated ubiquitination and degradation of PKCζ results in Na,K-ATPase stabilization at the plasma membrane as an important adaptive mechanism to preserve the alveolar epithelium from significant injury.
Results
PKCζ Degradation by HOIL-1L Stabilizes Plasma Membrane Na,K-ATPase During Prolonged Hypoxia.
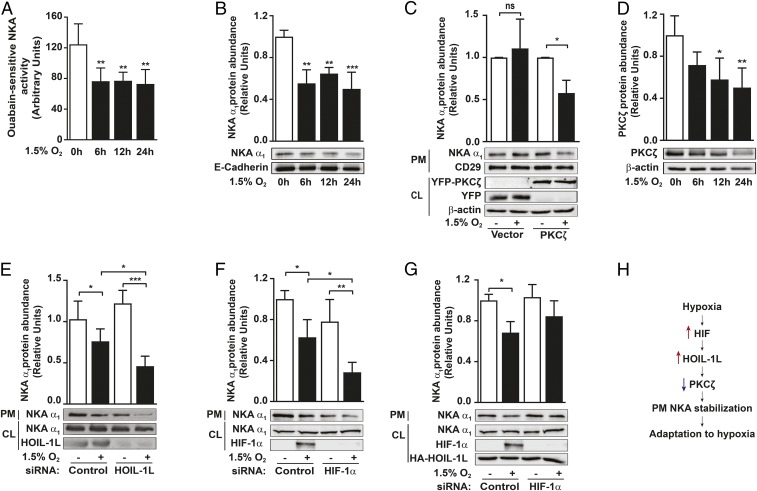
During prolonged exposure of primary rat alveolar type II (ATII) cells to 1.5% O2 (hypoxia), Na,K-ATPase activity and protein abundance at the plasma membrane decreased by ∼50%, compared with cells exposed to 21% O2 (normoxia), and then stabilized (Fig. 1 A and B). As observed in SI Appendix, Fig. S1 A–C, mitochondrial ATP production decreased after 6 h of hypoxia compared with the normoxic values. However, all hypoxia time points showed similar intracellular ATP content (SI Appendix, Fig. S1D). In pVHL-deficient renal clear carcinoma cells (RCC4), where HIF is stabilized, HOIL-1L levels increased while PKCζ levels decreased (20, 31) and hypoxia-induced α1-Na,K-ATPase endocytosis was prevented (Fig. 1C). Overexpression of YFP-PKCζ in these cells restored the Na,K-ATPase endocytosis (Fig. 1C). Hypoxia does not affect PKCζ mRNA levels or protein synthesis (20). However, hypoxia caused a time-dependent decrease of PKCζ levels in rat ATII cells (Fig. 1D). To assess the role of LUBAC during the hypoxia-induced Na,K-ATPase down-regulation, we performed loss-of-function experiments using A549 cells. Even though A549 cells are tumor cells, we and many others have reported that they have many characteristics of ATII cells (32, 33), including the regulation of Na,K-ATPase (1, 33, 34). HOIL-1L in A549 cells led to a more significant decrease of plasma membrane α1-Na,K-ATPase abundance during hypoxia compared with cells transfected with control siRNA (Fig. 1E). As with HOIL-1L silencing, HIF1α silencing resulted in fewer Na,K-ATPase molecules observed at the plasma membrane of AECs exposed to hypoxia compared with cells transfected with a control siRNA and exposed to hypoxia (Fig. 1F). Overexpression of HOIL-1L rescues the effects of HIF1α silencing, preventing the Na,K-ATPase endocytosis (Fig. 1G). However, silencing of HOIP did not further alter the α1-Na,K-ATPase abundance at the plasma membrane after 24 h of hypoxia (SI Appendix, Fig. S1E). Collectively, these data suggest that, in AECs exposed to hypoxia, HIF regulates HOIL-1L, which, by promoting PKCζ degradation, leads to plasma membrane Na,K-ATPase stabilization (Fig. 1H).
Fig. 1.
PKCζ degradation by HOIL-1L leads to the stabilization of plasma membrane Na,K-ATPase during hypoxia. (A and B) Rat ATII (r-ATII) cells were exposed to normoxia (21% O2) or hypoxia (1.5% O2) for up to 24 h. (A) Na,K-ATPase activity was measured in isolated membranes by a colorimetric assay as described in Experimental Procedures (n = 6). (B) Plasma membrane α1-Na,K-ATPase (NKA α1) was determined after cell-surface labeling with biotin in cells exposed to hypoxia or normoxia by immunoblot with a NKA α1-specific antibody (n = 3). (C) RCC4 cells were transfected with empty vector (Vector) or YFP-PKCζ (PKCζ). Forty-eight hours after transfection, cells were exposed to normoxia or hypoxia for 2 h, and then the expression of plasma membrane (PM) α1-Na,K-ATPase was determined after cell-surface labeling with biotin and determined by Western blot. Transfection efficiency was determined by performing a immunoblot of cell lysate (CL) proteins using YFP-specific antibody and β-actin antibody as a loading control (n = 6). (D) PKCζ levels were assessed in the cell lysates of r-ATII cells exposed to normoxia or hypoxia for up to 24 h by Western blot (n = 5). (E–G) A549 cells were transfected with the indicated siRNA and exposed to normoxia or hypoxia for 24 h. Expression of α1-Na,K-ATPase at the PM was determined by cell-surface biotinylation and Western blot (n = 3). (H) Schematic representation of the hypoxia-induced pathway for PM Na,K-ATPase stabilization. Representative immunoblots and bar graphs showing the densitometry quantifications of immunoblots in relation to the corresponding loading controls are shown. Data are expressed as mean ± SD. Statistical significance was calculated using one-way ANOVA and the Tukey multiple comparisons test (*P < 0.05, **P < 0.01, ***P < 0.001).
HOIL-1L Silencing Leads to Exaggerated α1-Na,K-ATPase Down-Regulation in Lung Epithelium During Hypoxia.
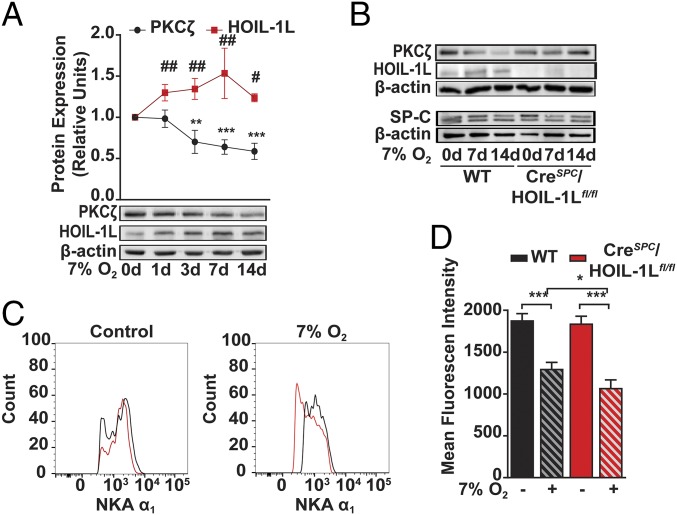
Analysis of peripheral lung tissue cell lysates from C57BL/6 (WT) mice exposed to 7% O2 (hypoxia) for up to 14 d showed a significant increase in HOIL-1L in parallel with a decrease in PKCζ protein abundance (Fig. 2A). We generated CreSPC/HOIL-1Lfl/fl mice, which bear a lung epithelial-specific deletion of the Rbck1 (HOIL-1L) gene (as described in Experimental Procedures). We analyzed, by RNA sequencing, the transcriptome of ATII cells isolated from WT and CreSPC/HOIL-1Lfl/fl mice in basal conditions. Only 20 of 13,617 detected genes were differentially expressed, suggesting that the deletion of HOIL-1L in the alveolar epithelium did not cause major changes in the epithelium (SI Appendix, Table S1). In contrast to the observed decrease in PKCζ levels in ATII cells from control mice exposed to hypoxia, PKCζ levels did not change in ATII cells isolated from CreSPC/HOIL-1Lfl/fl mice (Fig. 2B). The expression levels of neither HOIP nor SHARPIN, the other components of LUBAC, changed in lung tissue of mice exposed to hypoxia (SI Appendix, Fig. S2A). We assessed, by flow cytometry, the effects of HOIL-1L deletion on α1-Na,K-ATPase abundance at the plasma membrane of ATII cells from mice exposed to 7% O2 for 7 d (SI Appendix, Fig. S2B, for gating strategy) and found no differences between the α1-Na,K-ATPase abundance in ATII cells from WT (black) or CreSPC/HOIL-1Lfl/fl mice (red) kept in room air (control) (Fig. 2 C and D, solid bars), whereas ATII cells from CreSPC/HOIL-1Lfl/fl mice exposed to 7% O2 had lower α1-Na,K-ATPase abundance compared with cells from WT mice exposed to hypoxia (Fig. 2 C and D, striped bars). These data suggest that in AECs from mice exposed to hypoxia, the absence of HOIL-1L leads to increased PKCζ levels and decreased plasma membrane α1-Na,K-ATPase protein abundance.
Fig. 2.
Silencing of HOIL-1L in the alveolar epithelium decreases Na,K-ATPase protein abundance at the plasma membrane in vivo. (A) WT mice were kept in room air (0 d) or exposed to 7% O2 for up to 14 d, and PKCζ (black) and HOIL-1L (red) levels were analyzed by Western blot in lung peripheral tissue homogenates. Representative immunoblots and graph show the densitometry quantifications of proteins in relation to β-actin. Graph represents mean ± SD (n = 4). (B) Mouse ATII cells were isolated from WT and CreSPC/HOIL-1Lfl/fl mice treated as in A. Levels of PKCζ, HOIL-1L, SP-C, and β-actin were determined in cell lysates by Western blot. SP-C was used as a marker of ATII cells. Representative immunoblots are shown (n = 3). (C and D) The α1-Na,K-ATPase (NKA α1) levels at the plasma membrane were assessed by flow cytometry of EpCAM+ epithelial cells from lung homogenates of WT (black) and CreSPC/HOIL-1Lfl/fl (red) mice kept in room air or exposed to 7% O2 for 7 d. (C) Representative histograms from three independent experiments are shown. (Left) Room air (control). (Right) 7% O2. (D) Quantification of α1-Na,K-ATPase levels comparing α1-Na,K-ATPase mean fluorescence intensity from room air (solid bars) and 7% O2 for 7 d (striped bars; n = 3). Graph bars represent mean ± SD. Statistical significance was calculated using one-way ANOVA and the Tukey multiple comparisons test (*P < 0.05, **P < 0.01, ***P < 0.001).
Hypoxia Causes Lung Injury in Mice with HOIL-1L Deletion in the Lung Epithelium.
To assess whether deletion of HOIL-1L in the alveolar epithelium affects lung function, we exposed mice to 7% O2 for 7 d and found that the epithelial permeability to small solutes increased (Fig. 3A), as well as the total number of cells and protein concentration in the bronchoalveolar lavage fluid (BALF) (Fig. 3 B and C) in CreSPC/HOIL-1Lfl/fl mice compared with WT mice. H&E staining of lung tissue revealed that in lungs of CreSPC/HOIL-1Lfl/fl mice exposed to low O2 conditions there was increased thickening in the alveolar septa, more noticeable in the peribronchiolar area, compared with WT mice lungs exposed to hypoxia (Fig. 3 D and E). Moreover, in lungs of CreSPC/HOIL-1Lfl/fl mice exposed to hypoxia there was increased apoptosis (as measured by TUNEL assay) compared with lungs of WT mice (Fig. 3 F and G).
Fig. 3.
HOIL-1L deletion in lung epithelial cells causes lung injury in mice exposed to hypoxia. WT and CreSPC/HOIL-1Lfl/fl mice were kept in room air (21% O2) or exposed to hypoxia (7% O2) for 7 d. (A) Alveolar epithelial permeability is expressed as the ratio of the fluorescence present in BALF to the plasma (n = 3). (B) Total cell count in BALF (n = 6). (C) Total protein concentration in BALF. Graph bars represent mean ± SD (n = 6). Statistical significance was calculated for A–C using the Student’s t test (*P < 0.05). (D) Lung sections were stained with H&E, and brightfield serial images were obtained. Images show representative montages using 20× magnification images and higher magnification views of the areas indicated by squares. (Scale bar, 50 µm.) (E) Quantification of the alveolar wall area using ImageJ/FIJI 1.46 from WT (white bars) and CreSPC/HOIL-1Lfl/fl (black bars) mouse lungs. Graph bars represent mean ± SD (n = 3). (F) Lung sections were stained with TUNEL, and brightfield serial 10× magnification images were obtained. Arrows point to TUNEL-positive nuclei. (Scale bar, 50 µm.) (G) Quantification of TUNEL-positive nuclei mean intensity per lung section montage from WT (white bars) and CreSPC/HOIL-1Lfl/fl (black bars) mouse lungs. Graph bars represent mean ± SD (n = 3). Statistical significance for E and G was calculated using one-way ANOVA and the Tukey multiple comparisons test (*P < 0.05, **P < 0.01, ***P < 0.001).
It has been reported that hypoxia induces an inflammatory response with recruitment of immune cells into the alveolar spaces (35). To assess whether the increased injury observed in CreSPC/HOIL-1Lfl/fl mice in response to hypoxia was due to an exaggerated immune response, we performed flow cytometric analysis of the immune cells in the lungs. While the numbers of neutrophils and interstitial macrophages in mice exposed to hypoxia were elevated, there was no difference in immune cell composition between WT and CreSPC/HOIL-1Lfl/fl mice either in basal state or after being exposed to hypoxia (SI Appendix, Fig. S3).
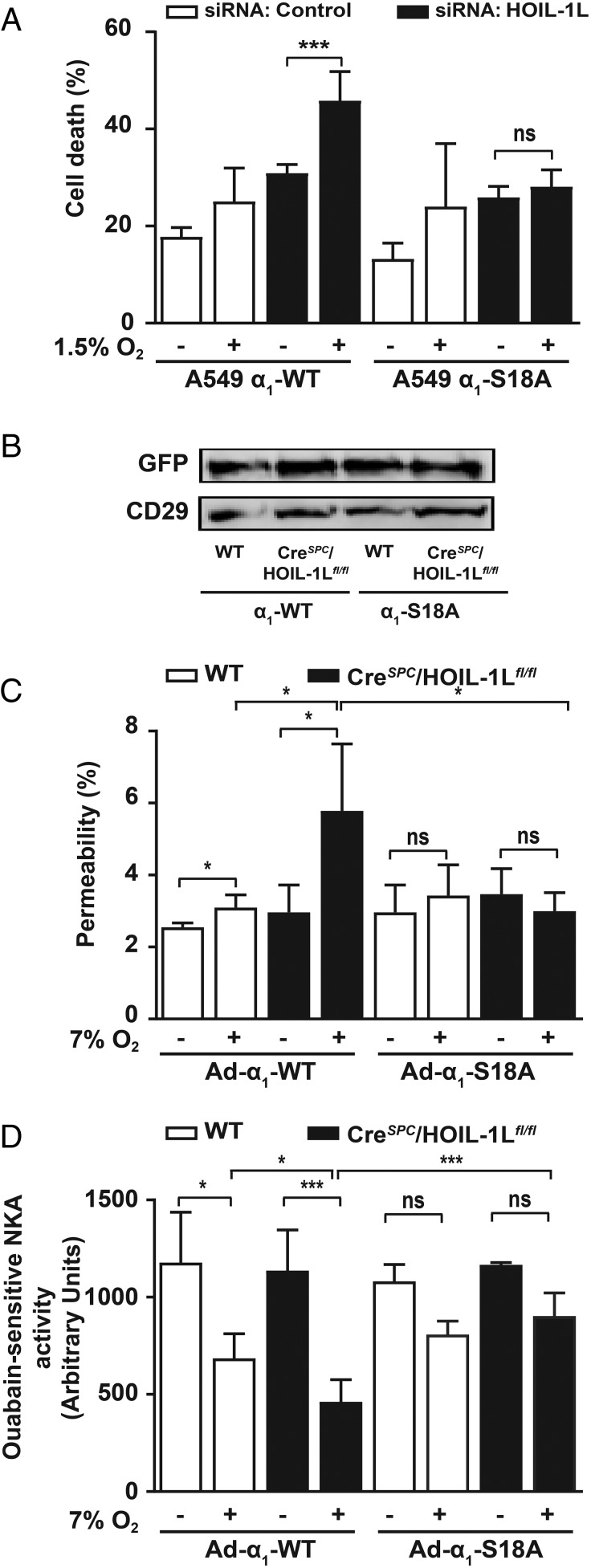
To determine whether the HOIL-1L–mediated cell adaptation to hypoxia was due to the stabilization of Na,K-ATPase at the plasma membrane, we used A549 cells expressing GFP-α1-Na,K-ATPase-WT (α1-WT) or GFP-α1-Na,K-ATPase-S18A (α1-S18A). The Na,K-ATPase bearing an S18A mutation is resistant to PKCζ phosphorylation and thus fails to internalize during hypoxia (1, 33, 36). In α1-WT cells transfected with HOIL-1L–specific siRNA, exposure to hypoxia significantly increased cell death compared with α1-WT cells transfected with a scrambled siRNA (Fig. 4A). In contrast, in α1-S18A A549 cells neither the silencing of HOIL-1L nor the exposure to hypoxia increased cell death (Fig. 4A), supporting the hypothesis that during prolonged hypoxia HOIL-1L exerts a protective effect by promoting PKCζ degradation, which stabilizes the α1-Na,K-ATPase at the AEC plasma membrane.
Fig. 4.
Alveolar epithelial function in CreSPC/HOIL-1Lfl/fl mice exposed to hypoxia is rescued by Na,K-ATPase α1-S18A overexpression. (A) Cell death was measured using a lactate dehydrogenase (LDH) assay in incubation medium from A549 cells stably expressing rat Na,K-ATPase-α1-WT (A549 α1-WT) or -α1-S18A (A549 α1-S18A) that were transfected with scrambled (control, white bars) or HOIL-1L (HOIL-1L, black bars) siRNA and exposed to normoxia or 1.5% O2 for 24 h (n = 3). (B–D) WT (white bars in C and D) and CreSPC/HOIL-1Lfl/fl (black bars in C and D) mice were infected with either adenovirus expressing GFP-NaK-ATPase α1-WT (Ad-α1-WT) or GFP-NaK-ATPase α1-S18A (Ad-α1-S18A) and kept in room air or exposed to 7% O2 for 7 d. (B) Adenovirus infection efficiency was determined by measuring GFP expression in lung peripheral tissue by Western blot. CD29 was used as a loading control. (C) Alveolar epithelial permeability is expressed as the ratio of the fluorescence present in BALF to the plasma (n = 3). (D) Na,K-ATPase activity measured in isolated plasma membranes from lung peripheral tissue by a colorimetric assay as described in Experimental Procedures. Graph bars represent mean ± SD. Statistical significance was calculated for E and G using one-way ANOVA and the Tukey multiple comparisons test (*P < 0.05, ***P < 0.001).
We also infected WT and CreSPC/HOIL-1Lfl/fl mice before exposure to 7% O2, with an adenoviral construct coding for GFP-α1-Na,K-ATPase -WT (Ad α1-WT) or GFP-α1-Na,K-ATPase-S18A (Ad α1-S18A). The infection efficiency was assessed by measuring the expression of GFP-α1-Na,K-ATPase in membranes from lung peripheral tissue by immunoblotting with an anti-GFP antibody (Fig. 4B). As observed in noninfected mice (Fig. 3A), lungs of CreSPC/HOIL-1Lfl/fl mice expressing Ad α1-WT exposed to hypoxia had increased permeability and total cell counts in the BALF compared with WT mice (Fig. 4C and SI Appendix, Fig. S4). However, CreSPC/HOIL-1Lfl/fl mice expressing α1-S18A exposed to hypoxia did not have significant changes in lung permeability or total cell counts compared with mice breathing room air (Fig. 4C and SI Appendix, Fig. S4). Moreover, the decrease in Na,K-ATPase activity observed in CreSPC/HOIL-1Lfl/fl mice exposed to hypoxia was rescued in mice infected with Ad-α1-S18A (Fig. 4D).
Phosphorylation at Thr-410 Is Required for PKCζ Ubiquitination and Degradation.
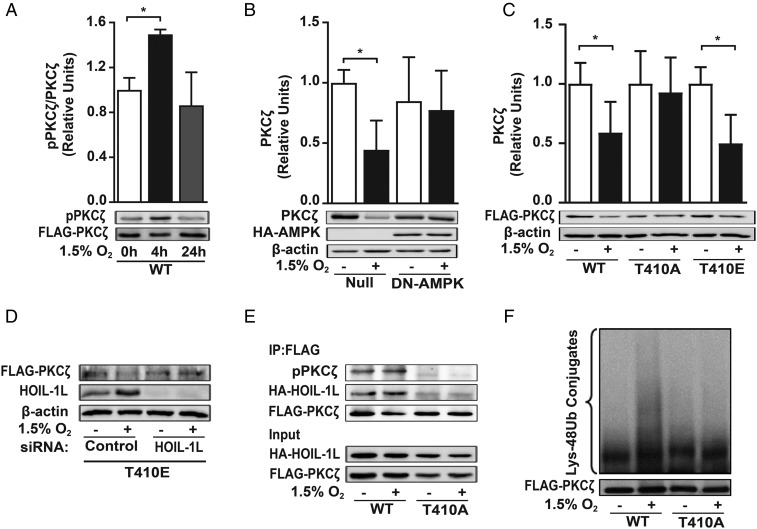
Phosphorylation in the activation loop of the PKCζ kinase domain at Thr-410 exposes it to further phosphorylation and is considered the key event in stabilizing PKCζ in the active conformation at the plasma membrane (37). In COS-7 cells transfected with FLAG-PKCζ-WT, hypoxia-induced PKCζ phosphorylation increased after 4 h and returned to basal levels after 24 h of exposure (Fig. 5A). To assess whether AMPK-induced phosphorylation is necessary for PKCζ down-regulation during hypoxia, we infected rat ATII cells with a dominant-negative AMPK α1 subunit adenovirus (DN-AMPK) or an empty adenovirus (Null). The lack of AMPK activity was sufficient to prevent the hypoxia-induced PKCζ down-regulation (Fig. 5B). To further confirm the role of phosphorylation in PKCζ degradation, we used loss- and gain-of-function analysis. Expression of PKCζ bearing a T410A mutation, FLAG-PKCζ-T410A (T410A), prevented the hypoxia-induced degradation of PKCζ, whereas a PKCζ phosphorylation mimic, FLAG-PKCζ-T410E (T410E), was degraded during hypoxia similarly to PKCζ-WT (Fig. 5C). The degradation of FLAG-PKCζ-T410E during hypoxia was prevented in HOIL-1L–silenced cells (Fig. 5D).
Fig. 5.
PKCζ phosphorylation is required for its ubiquitylation and degradation. Cells were exposed to normoxia or 1.5% O2 for up to 24 h. (A) COS-7 cells were transfected with FLAG-PKCζ-WT, and 4 h after treatment cells were lysed and proteins were immunoprecipitated with anti-FLAG antibody. Densitometry quantification of immunoblots of phospho-PKCζ (pPKCζ) in relation to FLAG is shown (n = 3). (B) Rat ATII cells were infected with null adenovirus (Null) or HA-tagged adenovirus carrying a dominant-negative, kinase-dead (K45R) variant of AMPK (DN-AMPK) after 24 h of normoxia or 1.5% O2. PKCζ expression was evaluated by Western blot; β-actin was used as a loading control (n = 3). Statistical significance was calculated for A and B using one-way ANOVA and the Tukey multiple comparisons test (*P < 0.05). (C) PKCζ degradation was assessed after 24 h of treatment in COS-7 cells that were transfected with FLAG-WT-PKCζ (WT), FLAG-T410A-PKCζ (T410A), or FLAG-T410E-PKCζ (T410E) by densitometry quantification of immunoblots of FLAG in relation to β-actin (n = 3). Statistical significance was calculated using unpaired Student’s t test (*P < 0.05). (D) COS-7 cells were transfected at the same time with FLAG-T410E-PKCζ (T410E) and scrambled (control) or HOIL-1L siRNA before 24 h of normoxia or 1.5% O2 exposure. The pPKCζ and HOIL-1L expression levels were detected by Western blot analysis; β-actin was used as a loading control. Representative immunoblots from three independent experiments are shown. (E) COS-7 cells were cotransfected with PKCζ-WT (WT) or PKCζ-T410A (T410A) and HA-HOIL-1L, exposed to normoxia or 1.5% O2 for 4 h, and lysed; cell lysate was used for coimmunoprecipitation using anti-FLAG antibody. Phospho-PKCζ and HOIL-1L were immunoblotted to assess interaction between proteins. FLAG was used as a loading control. Representative immunoblots from three independent experiments are shown. (F) PKCζ ubiquitination was evaluated in COS-7 cells transfected with PKCζ-WT (WT) or PKCζ-T410A (T410A). After 6 h, proteins in the cell lysate were immunoprecipitated using an anti-FLAG antibody and immunoblotted for Lys-48 polyubiquitination. Representative immunoblots from three independent experiments are shown.
To determine whether the interaction between PKCζ and HOIL-1L during hypoxia requires PKCζ phosphorylation, cells were cotransfected with HA-HOIL-1L and either FLAG-PKCζ-WT or FLAG-PKCζ-T410 and exposed to hypoxia, and then proteins in the cell lysate were coimmunoprecipitated by use of an anti-FLAG antibody. Exposure to hypoxia increased PKCζ-WT phosphorylation and the interaction between HOIL-1L and PKCζ-WT (Fig. 5E, lanes 1 and 2). Overexpression of PKCζ-T410A prevented not only the hypoxia-induced PKCζ phosphorylation but also the interaction between HOIL-1L and PKCζ (Fig. 5E, lanes 3 and 4). Similarly, transfection with FLAG-PKCζ-T410A prevented the hypoxia-induced PKCζ ubiquitylation (Fig. 5F).
PKCζ Degradation Is Initiated in the Plasma Membrane.
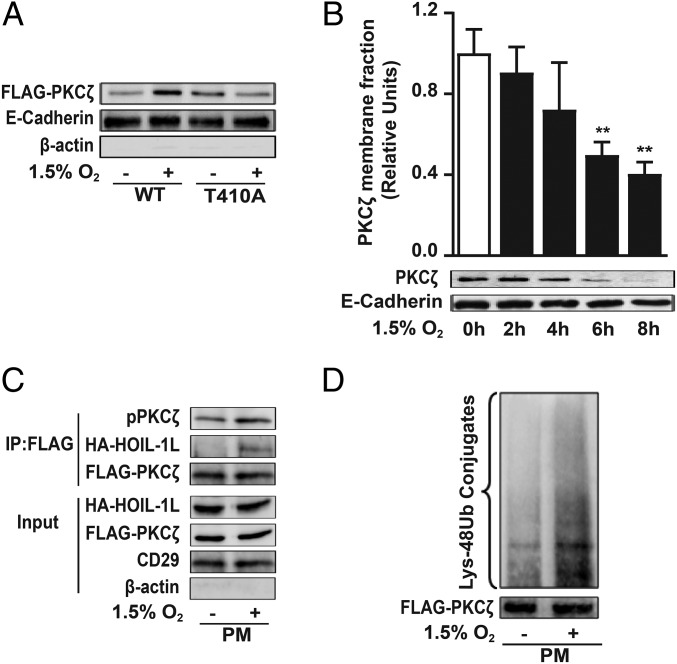
Hypoxia-induced PKCζ phosphorylation is required for its translocation to the plasma membrane, as translocation was prevented in cells overexpressing FLAG-PKCζ-T410A (Fig. 6A). PKCζ remained phosphorylated for at least 4 h (Fig. 5A), and its removal from the plasma membrane had a significant effect after 6 h of hypoxia exposure (Fig. 6B). These data suggest that the interaction between HOIL-1L and the phosphorylated PKCζ occurs at the plasma membrane, which was confirmed by transfecting COS-7 cells with FLAG-PKCζ-WT and HA-HOIL-1L, exposing the cells to hypoxia for 4 h, isolating the membranes, and coimmunoprecipitating the solubilized proteins with an anti-FLAG antibody (Fig. 6C). Moreover, the hypoxia-induced PKCζ ubiquitylation occurs at the plasma membrane (Fig. 6D).
Fig. 6.
PKCζ degradation is initiated at the cell plasma membrane. (A) COS-7 cells were transfected with FLAG-WT-PKCζ (WT) or FLAG-T410A-PKCζ (T410A) and exposed to 1.5% O2 for 10 min, and pPKCζ levels in the isolated plasma membrane fraction were determined by Western blot to assess PKCζ translocation to the plasma membrane as described in SI Appendix, SI Experimental Procedures. E-cadherin was used as loading control for membrane fraction, and β-actin was used as a marker of cytosolic proteins. Representative immunoblots from four independent experiments are shown. (B) Isolated plasma membrane proteins from rat ATII cells exposed to normoxia or 1.5% O2 were immunoblotted using an anti-PKCζ antibody. E-cadherin was used as loading control (n = 3). Statistical significance was calculated using one-way ANOVA and the Dunnett’s multiple comparisons test (**P < 0.01). (C) COS-7 cells were cotransfected with FLAG-WT-PKCζ (WT) and HA-HOIL-1L; after 4 h of normoxia or 1.5% O2 exposure, plasma membrane proteins were isolated and used for coimmunoprecipitation using anti-FLAG antibody and immunoblotted for pPKCζ and HOIL-1L. FLAG was used as a loading control. β-Actin and CD29 are shown as loading controls for cytosolic and membrane fraction, respectively. Representative immunoblots from at least three independent experiments are shown. (D) PKCζ ubiquitination was evaluated in COS-7 cells transfected with FLAG-PKCζ-WT. After 4 h of hypoxia, an immunoprecipitation with anti-FLAG antibody was performed in isolated plasma membrane and immunoblotted for Lys-48 poliubiquitylation. Representative immunoblots from at least three independent experiments are shown.
Discussion
Adaptation to hypoxia requires coordination between energy demand and supply to promote cell homeostasis. The Na,K-ATPase is a major energy consumer, accounting for 20–70% of metabolic ATP depending of the tissue (38, 39). To conserve energy during acute hypoxia, the Na,K-ATPase α1 subunit is phosphorylated by PKCζ, which triggers its endocytosis, independently of HIF (1, 36). Here we describe that AEC exposure to prolonged hypoxia results in decreased Na,K-ATPase levels; however, the cells survive with the Na,K-ATPase level stabilized at a lower plateau (∼50% of normoxia levels) (Fig. 1 A and B) to conserve energy. This is achieved via HIF-induced up-regulation of the E3 ubiquitin ligase HOIL-1L and phosphorylation of PKCζ by AMPK. HOIL-1L then interacts with and ubiquitylates the phosphorylated PKCζ at the plasma membrane, triggering its degradation.
HOIL-1L is a member of LUBAC, which catalyzes the nondegradative linear ubiquitination of proteins. However, not only is the activity of HOIL-1L on PKCζ independent of the function of HOIP (the LUBAC catalytic component), but also HOIP competes with PKCζ for binding to HOIL-1L (20). We found that silencing of HOIP does not affect Na,K-ATPase levels at the plasma membrane during hypoxia (SI Appendix, Fig. S1E).
In RCC4 cells, which express constitutive levels of HIF1α, high levels of HOIL-1L, and low levels of PKCζ, hypoxia fails to induce Na,K-ATPase endocytosis, which is rescued by overexpression of PKCζ (Fig. 1D). In agreement with these data, silencing of HOIL-1L in hypoxia-exposed cells increased Na,K-ATPase down-regulation and cell death. The increase in cell death in the absence of HOIL-1L is rescued by expressing a phosphorylation-deficient Na,K-ATPase α1 subunit (Fig. 4A), indicating the specificity of the effect. As we have previously reported, the phosphorylation by PKCζ of the plasma membrane Na,K-ATPase-α1 subunits triggers the internalization of Na,K-ATPase molecules during hypoxia. It has been described that myristoylated alanine-rich C kinase substrate proteins (MARCKS) in the nonphosphorylated state sequester PIP2, preventing the formation of signaling complexes and thereby promoting the stability of plasma membrane proteins (40, 41). However, MARCKS is not a substrate for the atypical PKCs.
Concordant with the in vitro results, ATII cells isolated from WT mice exposed to 7% O2 for 7 d had increased HOIL-1L and decreased PKCζ levels (Fig. 2A). In contrast, PKCζ levels did not decrease in ATII cells from CreSPC/HOIL-1Lfl/fl mice exposed to hypoxia as they lacked HOIL-1L. We found significant α1-Na,K-ATPase down-regulation (Fig. 2E), more injury (Fig. 3), and increased apoptosis (Fig. 3) in lungs from CreSPC/HOIL-1Lfl/fl mice than in lungs from WT mice after low O2 treatment. We have previously reported that hypoxia moderately impairs the alveolar fluid clearance (42). However, this impairment did not result in evident alveolar edema, allowing the mice to adapt and survive hypoxic conditions. Interestingly, alveolar type I cells express lower levels of LUBAC compared with ATII cells (SI Appendix, Fig. S5). While ATII cells express only the Na,K-ATPase α1 isoform, ATI cells express both α1 and α2 Na,K-ATPase isoforms. The α2 isoform does not have a PKC consensus phosphorylation domain (Scansite 3 beta). Therefore, the alveolar fluid clearance could be conserved in ATI cells and protect against exaggerated lung injury.
The importance of HOIL-1L in the stabilization of α1-Na,K-ATPase during prolonged hypoxia was confirmed by the rescue of the epithelial function in CreSPC/HOIL-1Lfl/fl mice overexpressing a phosphorylation-resistant Na,K-ATPase-α1 subunit (Fig. 4). Low O2 exposure can be associated with increased immune cell recruitment and inflammatory response that has been linked with endothelial injury (43, 44). Interestingly, the increased lung injury observed in CreSPC/HOIL-1Lfl/fl mice during prolonged hypoxia does not appear to increase the hypoxia-induced inflammatory response in the lung but rather results from Na,K-ATPase down-regulation.
The activation of atypical PKCs is analogous to the activation of Akt, in which the loop phosphorylation is agonist-dependent and required for its translocation to the plasma membrane where the autoinhibition is removed, enabling the phosphorylation of substrates (45). In the current study, we provide evidence that the AMPK-induced phosphorylation at Thr-410 and activation are necessary for the ubiquitination and degradation of PKCζ. Translocation of PKCζ to the plasma membrane occurs within 10 min of hypoxia exposure, and it remains membrane-bound for at least 4 h (Fig. 5A). Similarly to PKCζ-WT, the degradation of PKCζ-T410E increased during hypoxia (Fig. 5C). These data suggest that both the hypoxia-induced phosphorylation of PKCζ and the HIF-dependent HOIL-1L up-regulation are required for PKCζ degradation to occur. Activation-dependent ubiquitylation and proteasomal degradation have been described for PKC isoforms (22, 46), suggesting that the ubiquitin proteasome system is critical for PKC regulation. Our data suggest that HOIL-1L interacts with and ubiquitylates the phosphorylated PKCζ at the plasma membrane to trigger PKCζ degradation (Fig. 6).
Accordingly, we provide evidence for an adaptive mechanism of cells to hypoxia, linking a HIF-regulated pathway to Na,K-ATPase stabilization at the plasma membrane (Fig. 7). During prolonged hypoxia, HIF up-regulates HOIL-1L, which targets PKCζ for degradation, thereby preventing it from further downregulating the Na,K-ATPase to conserve ATP and promote AEC survival. This mechanism represents a noncanonical pathway in which HOIL-1L acts as an E3 ligase for PKCζ to protect the alveolar epithelium against lung injury during severe hypoxia.
Fig. 7.
Cartoon depicting the proposed mechanism for Na,K-ATPase stabilization during chronic hypoxia. Hypoxia leads to stabilization of HIF, which regulates HOIL-1L, leading to PKCζ ubiquitylation to avoid an excessive depletion of Na,K-ATPase, and safeguarding the alveolar epithelial function as a mechanism of adaptation to hypoxic conditions.
Experimental Procedures
Generation of CreSPC/HOIL-1Lfl/fl Mice.
We generated a targeting vector containing LoxP sites flanking exon 8 in the HOIL-1 genomic allele, followed by a neomycin resistance gene (SI Appendix, Fig. S5). TT2 ES cells derived from C57BL/6 were transfected with the targeting vector, and neomycin-resistant colonies were screened for homologous recombination. Selected ES cells containing the appropriately targeted allele were injected into ICR mice blastocysts to generate chimeric mice, which then transmitted the targeted loxP-flanked HOIL-1L allele (HOIL-1Lfl) to their progeny and were backcrossed with C57BL/6 mice (47). The genotypes of offspring mice were determined by PCR analysis of DNA extracted from tail-biopsy specimens. The following primer set was used to distinguish the WT-HOIL-1L allele (1,100 bp) and floxed-HOIL-1L allele (273 bp): forward primer CAGCAGGAGGGGAACTACCTG and reverse primer TGACAGCACCTACTCATGCC. HOIL-1Lfl/fl mice were then crossed to mice expressing a Cre recombinase driven by the SP-C promoter (SP-C-Cre+), which were a gift from B. Hogan (Duke University, Durham, NC) and are described elsewhere (48). Expression of surfactant protein C promoter begins early in development in primary lung buds and continues throughout development (49). Deletion of HOIL-1L in CreSPC/HOIL-1Lfl/fl mice was verified by genotyping (Transnetyx) using specific probes designed for each gene, with the following set of primers: for the probe CRE, forward primer TTAATCCATATTGGCAGAACGAAAACG, reverse primer CAGGCTAAGTGCCTTCTCTACA, and reporter CCTGCGGTGCTAACC; for the probe Rbck1-1 EX, forward primer CAACCCCATACCTAGCAAGCTTAT, reverse primer CTTCCTCTTGCAAAACCACACT, and reporter CTCGAAGCTGACGTATAAC; for the probe Rbck1-1 FL, forward primer CAACCCCATACCTAGCAAGCTTAT, reverse primer TGGAGGAGTGGTTAGAGGTCAAG, and reporter CTAGATCTGCAGCCTCACTC; for the probe Rbck1-1 WT, forward primer TGCTCCACCTTGACCACAAC, reverse primer GTGCTGGAGGAGTGGTTAGAG, and reporter ACCTAGCAAGCTTTCTGC. C57BL/6 mice were use as WT mice.
Alveolar Epithelial Cell Isolation and Culture.
Mice and rats were provided with food and water ad libitum, maintained on a 14-h/10-h light–dark cycle, and handled according to National Institutes of Health guidelines and the experimental protocol approved by the Northwestern University Institutional Animal Care and Use Committee.
Rat ATII cells from the lungs of male Sprague–Dawley rats (weight: 200–225 g) (29) or mouse ATII cells from WT and CreSPC/HOIL-1Lfl/fl mice (age: 10–12 wk) (50) were isolated by the Pulmonary Division Cell Culture and Physiology Core from Northwestern University. The day of isolation and plating of rat ATII cells was designated day 0. All experiments were conducted on day 3.
The following cell lines were used: human adenocarcinoma A549 (ATCC CCL 185), African green monkey kidney COS-7 (ATCC CRL 1651), and pVHL-deficient human RCC4 (51). A549 cells stably expressing the rat Na,K-ATPase-α1 subunit WT or with a Ser-18 mutation have been described elsewhere (1). A549-GFP-α1 cells show similar enzymatic activity and respond to stimuli similarly to WT A549 cells (36). All cells were contamination-free and grown and maintained as described elsewhere (1). After treatment, cells were washed in ice-cold PBS and, when required, solubilized in 2× lysis buffer (Cell Signaling Technology). The lysates were cleared by centrifugation for 10 min at 14,000 × g.
Additional materials and methods can be found in SI Appendix, SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Hiam Abdala-Valencia for help with the construction of the libraries and sequencing and Mark Ciesielski for the isolation of rat and mouse ATII cells. This work was supported, in part, by National Institutes of Health Grants HL48129, HL-071643, and AG-049665. Research in the A.J.C. laboratory is supported by grants from the Dr. Miriam and Sheldon Adelson Medical Research Foundation (AMRF), the Israel Science Foundation (ISF), and the Israeli Centers of Research Excellence Program of the Planning and Budgeting Committee of the Council for High Education (CHE) and ISF Grant 1775/12. A.J.C. is an Israel Cancer Research Fund USA Professor. This work was also supported by the Northwestern University Flow Cytometry Core Facility, supported by National Cancer Institute (NCI) Cancer Center Support Grant CA060553. Flow cytometry cell sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH Grant 1S10OD011996-01. K.I. is supported by the Japan Society for the Promotion of Science KAKENHI Grants 24112002 and 17H06174I. Imaging work was performed at the Northwestern University Center for Advanced Microscopy, and histology services were provided by the Northwestern University Mouse Histology and Phenotyping Laboratory, both of which are supported by NCI Grant CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus database, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE102107 (accession no. GSE102107).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713563114/-/DCSupplemental.
References
- 1.Dada LA, et al. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-ζ. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 3.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer. 2016;16:663–673. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 8.Factor P, et al. Overexpression of the Na+,K+-ATPase alpha1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol. 1998;18:741–749. doi: 10.1165/ajrcmb.18.6.2918. [DOI] [PubMed] [Google Scholar]
- 9.Allemann Y, Scherrer U. High-altitude medicine: Important for trekkers and mountaineers, essential for progress in medicine. Prog Cardiovasc Dis. 2010;52:449–450. doi: 10.1016/j.pcad.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Mac Sweeney R, McAuley DF, Matthay MA. Acute lung failure. Semin Respir Crit Care Med. 2011;32:607–625. doi: 10.1055/s-0031-1287870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 12.Gille T, et al. Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway. Am J Respir Cell Mol Biol. 2014;50:526–537. doi: 10.1165/rcmb.2012-0518OC. [DOI] [PubMed] [Google Scholar]
- 13.Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: Reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol. 2001;25:554–561. doi: 10.1165/ajrcmb.25.5.4420. [DOI] [PubMed] [Google Scholar]
- 14.Vadász I, Sznajder JI. Gas exchange disturbances regulate alveolar fluid clearance during acute lung injury. Front Immunol. 2017;8:757. doi: 10.3389/fimmu.2017.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chibalin AV, et al. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- 16.Gusarova GA, et al. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gusarova GA, et al. Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton AC. Protein kinase C: Poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 20.Queisser MA, et al. HOIL-1L functions as the PKCζ ubiquitin ligase to promote lung tumor growth. Am J Respir Crit Care Med. 2014;190:688–698. doi: 10.1164/rccm.201403-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma L, et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell. 2013;152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobias IS, et al. Protein kinase Cζ exhibits constitutive phosphorylation and phosphatidylinositol-3,4,5-triphosphate-independent regulation. Biochem J. 2016;473:509–523. doi: 10.1042/BJ20151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pu Y, et al. Effects on ligand interaction and membrane translocation of the positively charged arginine residues situated along the C1 domain binding cleft in the atypical protein kinase C isoforms. J Biol Chem. 2006;281:33773–33788. doi: 10.1074/jbc.M606560200. [DOI] [PubMed] [Google Scholar]
- 24.Graybill C, Wee B, Atwood SX, Prehoda KE. Partitioning-defective protein 6 (Par-6) activates atypical protein kinase C (aPKC) by pseudosubstrate displacement. J Biol Chem. 2012;287:21003–21011. doi: 10.1074/jbc.M112.360495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Mallampalli RK. Decoding the growth advantage of hypoxia-sensitive lung cancer. Am J Respir Crit Care Med. 2014;190:603–605. doi: 10.1164/rccm.201408-1503ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dove KK, Klevit RE. RING-between-RINGs: Keeping the safety on loaded guns. EMBO J. 2012;31:3792–3794. doi: 10.1038/emboj.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brazee P, Dada LA, Sznajder JI. Role of linear ubiquitination in health and disease. Am J Respir Cell Mol Biol. 2016;54:761–768. doi: 10.1165/rcmb.2016-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu J, Shi Y, Iwai K, Wu ZH. LUBAC regulates NF-κB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 2011;30:3741–3753. doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridge KM, et al. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 30.Elton L, Carpentier I, Verhelst K, Staal J, Beyaert R. The multifaceted role of the E3 ubiquitin ligase HOIL-1: Beyond linear ubiquitination. Immunol Rev. 2015;266:208–221. doi: 10.1111/imr.12307. [DOI] [PubMed] [Google Scholar]
- 31.Zhou G, et al. Hypoxia-mediated Na-K-ATPase degradation requires von Hippel Lindau protein. FASEB J. 2008;22:1335–1342. doi: 10.1096/fj.07-8369com. [DOI] [PubMed] [Google Scholar]
- 32.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 33.Lecuona E, et al. Na,K-ATPase {alpha}1-subunit dephosphorylation by protein phosphatase 2A is necessary for its recruitment to the plasma membrane. FASEB J. 2006;20:2618–2620. doi: 10.1096/fj.06-6503fje. [DOI] [PubMed] [Google Scholar]
- 34.Bertorello AM, et al. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell. 2003;14:1149–1157. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fröhlich S, Boylan J, McLoughlin P. Hypoxia-induced inflammation in the lung: A potential therapeutic target in acute lung injury? Am J Respir Cell Mol Biol. 2013;48:271–279. doi: 10.1165/rcmb.2012-0137TR. [DOI] [PubMed] [Google Scholar]
- 36.Dada LA, et al. Phosphorylation and ubiquitination are necessary for Na,K-ATPase endocytosis during hypoxia. Cell Signal. 2007;19:1893–1898. doi: 10.1016/j.cellsig.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 38.Milligan LP, McBride BW. Energy costs of ion pumping by animal tissues. J Nutr. 1985;115:1374–1382. doi: 10.1093/jn/115.10.1374. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: Functional sites and their interactions. Annu Rev Physiol. 2003;65:817–849. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- 40.Sheats MK, Sung EJ, Adler KB, Jones SL. In vitro neutrophil migration requires protein kinase C-delta (δ-PKC)-mediated myristoylated alanine-rich C-kinase substrate (MARCKS) phosphorylation. Inflammation. 2015;38:1126–1141. doi: 10.1007/s10753-014-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer M, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 42.Litvan J, et al. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem. 2006;281:19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- 43.Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: The prominent role of p42/ p44 and PI3 kinase pathways. FASEB J. 2004;18:1090–1092. doi: 10.1096/fj.03-0991fje. [DOI] [PubMed] [Google Scholar]
- 44.Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Tokunaga F, Sakata S, Iwai K. Mutual regulation of conventional protein kinase C and a ubiquitin ligase complex. Biochem Biophys Res Commun. 2006;351:340–347. doi: 10.1016/j.bbrc.2006.09.163. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki Y, et al. Defective immune responses in mice lacking LUBAC-mediated linear ubiquitination in B cells. EMBO J. 2013;32:2463–2476. doi: 10.1038/emboj.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 49.Flodby P, et al. Knockout mice reveal a major role for alveolar epithelial type I cells in alveolar fluid clearance. Am J Respir Cell Mol Biol. 2016;55:395–406. doi: 10.1165/rcmb.2016-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheresh P, et al. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am J Respir Cell Mol Biol. 2015;52:25–36. doi: 10.1165/rcmb.2014-0038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cockman ME, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.