Trypanosoma brucei, the pathogen that causes sleeping sickness, has a clever and insidious trick to help it navigate its hosts’ innards: It changes shape depending on its surroundings. In bodily fluids, the bacterium assumes a long and narrow shape to propel itself forward, whipping its tail-like flagellum and spinning like a corkscrew. In other settings and life stages, when it doesn't need motility, it shortens into a stumpy blob.
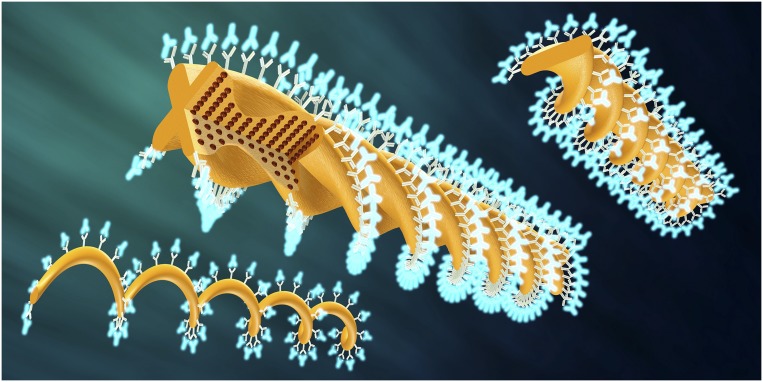
In 2014, researchers at ETH Zurich devised microrobots that propel themselves by twisting, something like an artificial flagellum. Researchers direct the microrobots with external magnetic fields. Reproduced with permission from ref. 12.
To Bradley Nelson, an engineer at ETH Zurich, the T. brucei’s potentially deadly shape-shifting ability was both revelation and inspiration. “Parasites have evolved these interesting strategies to survive and move,” he says. “As an engineer, I’m not sure I’d ever have sat down and described something like that from scratch.” His laboratory focuses on developing microrobots that can navigate the human body to perform tasks, such as delivering drugs to a tumor or mechanically clearing blocked blood vessels.
In July 2016, Nelson and his team introduced a T. brucei-inspired “origami robot,” a self-folding micromachine, made from a hydrogel, that optimizes its shape according to the viscosity and temperature of its environment (1). It propels itself forward—in experiments, through a viscous sugar solution—by whipping a flagellum-like tail. Scientists steer it by manipulating external magnetic fields.
Nelson is among a cadre of scientists who are designing ever-smaller robots that have the potential to improve the precision of medicine. Broadly, the field of “medical microrobots” includes small devices from a millimeter down to a few microns. All aim to safely invade the body and improve health—by diagnosing or monitoring a disease, such as Alzheimer’s, in real time, measuring glucose levels in a person with diabetes, sending a swarm to deliver targeted therapies directly to tumors, or performing delicate surgery perhaps in the eye or even in the brain.
Researchers have been chasing microrobots for decades, but the task is more difficult than miniaturizing current approaches to human-scale robots. “The physics changes when we go to the microscale or nanoscale,” says Shuhei Miyashita at the University of York, in the United Kingdom. Says Nelson, “You have to rethink your intuition at these levels.”
For tiny devices attempting to navigate this terrain, surface area becomes more important than volume, which means attraction and contact forces, such as adhesion, have a greater influence on movement than does gravity, which is almost negligible because of the minuscule mass. As a result, building microrobots often requires counter intuitive approaches to physics and material design, and it’s only in the last few years that researchers have made proof-of-concept advances that could lead to useful devices.
Nelson predicts we’ll see the first clinical applications in 5–10 years. Researchers are addressing the challenges of small-scale engineering both by building machines inspired by nature—such as Nelson’s—and by actually coopting living microswimmers, such as bacteria, heart cells, or sperm.
The Locomotion Challenge
Microrobots have been a long time coming. At Cal Tech in 1959, physicist Richard Feynman mused on the benefits of controlling devices at small scales. He speculated about controlling atoms and building computing devices inspired by biological ideas. Feynman shared a wild idea from his friend, the
“You can't control the robot because you cannot see the road,” he says. “We have to get better at moving them around.”
—Sylvain Martel
mathematician Albert Hibbs: “it would be interesting in surgery if you could swallow the surgeon” (2). In an article published in PNAS in 1981, Massachusetts Institute of Technology (MIT) futurist and engineer Kim Eric Drexler described an approach to molecular machinery that used protein molecules to build atomic-scale robots (3).
Such proposals inspire science-fiction visions, but there are important caveats. In the 1966 film Fantastic Voyage, scientists shrink a submarine to smaller than a red blood cell and drive it through blood vessels en route to the brain in an attempt to remove a life-threatening clot. The fantastical notion of shrinking people to a few microns aside, the narrative was physics challenged in other ways. At such a small scale and in a fluid as thick as blood, the shrunken submarine's teeny propellers wouldn't be able to move the ship, for example.
Keeping such challenges in mind, researchers have developed a variety of ways to move and steer microrobots to their destinations. Nelson’s device mimics the motion of a bacterium’s flagellum and can be guided by magnetic fields. At a conference in May 2016 (4), researchers in Daniela Rus’s laboratory at MIT, in Cambridge, introduced an origami-like robot that's made of a magnetic sliver attached to a film made from dried pig intestines. It folds up in a pill to be ingested and unfolds inside the body. Fully extended, it’s about a centimeter long. Guided by external magnetic fields, it moves by sticking to a surface by friction and then redistributing its weight to slip off.
The robot could be tweaked for specific tasks, such as performing minor surgeries in the body, says Miyashita, who worked on the device as a postdoctoral researcher in Rus's laboratory. It might be designed to patch small wounds or rescue dangerous swallowed objects, such as batteries or glass, from a person's stomach. The researchers tested its capabilities via a simulated esophagus and stomach, which had been 3D printed from silicone to match the structure and viscosity of the real thing. Using magnetic fields, the researchers navigated the unfolded robot into the stomach, where it attached to and removed a battery embedded in the lining. In other experiments, Miyashita and his collaborators showed how to navigate the surgery-performing robot to patch a small stomach wound.
Miyashita says he’s continuing to work on making the robot smaller. He also wants to give it the ability to navigate independently. Magnetic fields offer high precision for navigation, and they're optimal for low-cost, high-precision microrobotic medicine.
“Ideally,” says Miyashita, “we’d let the robot decide its reaction to its environment.” Instead of needing to be steered, the device could find its own way to its destination by following chemical cues in the bloodstream, for example, and complete its mission.
Natural Leanings
Many microrobot researchers aren’t just looking to nature for inspiration; they’re harnessing the existing tools in nature’s smallest denizens. In 2013, a group at the Institute for Integrative Nanosciences in Dresden, Germany, debuted a device that traps bull sperm in magnetic nanotubes and uses the cells for propulsion (5); magnetic fields are used to steer. Last year, the same group (6) unveiled a remote-controlled “spermbot,” which can fit on a slow sperm and escort it to an egg as a possible treatment for infertility; in a preprint published on the arXiv in April 2017, they described a similar nanotube sperm helmet that may carry cancer-treating drugs (7). In 2014, researchers from the University of Illinois at Urbana–Champaign built a device that propels itself through viscous fluids, such as blood, using constantly contracting heart cells (8).
Other recent devices deliver on Drexler's 1981 predictions. In 2012, Shawn Douglas at Harvard University (now at the University of California, San Francisco) led the design of a device made from DNA, designed to autonomously respond to its environment without external steering (9). He says that 2012 work was a proof-of-concept model. In those laboratory experiments, Douglas and his team attached proteins to folded DNA in random configurations and showed how the molecular robot could deliver drugs to target cells. But to be effective, the proteins and DNA have to be oriented in particular directions—and getting precise orientations has turned out to be a significant technical challenge, Douglas says. Now, he and his team are testing different protein–DNA orientations that may perform useful functions in the body.
In March 2017, robotics researchers at Tohoku University, in Japan, introduced an “amoeba-like” molecular robot made of natural molecules—including DNA, lipids, and proteins—that changes its shape in response to chemical and light cues (10).
Sylvain Martel, at the Polytechnique Montréal in Canada, says that autonomy is critical to making medical biorobots useful for applications like cancer therapy. External magnetic fields have a natural limit. While internal imaging approaches can achieve a resolution of about 100 microns, the leaky blood vessels that would offer inroads for drug delivery robots are only a few microns in diameter. “You can’t control the robot because you cannot see the road,” he says. “We have to get better at moving them around.”
A better strategy, Martel says, is to take advantage of navigation mechanisms that already exist. In his laboratory, he’s been investigating magnetotactic bacteria, which travel along magnetic field lines and gravitate, generally, to low-oxygen areas. Such microbes won’t be useful for every task, he says, but cancer therapies present some interesting possibilities.
Cancerous tumors are riddled with hypoxic areas where cells reproduce quickly, resulting in low oxygen. Martel's idea is to send harmless magnetotactic bacteria into the tumors, loaded with trace amounts of encapsulated targeted drugs. In August 2016, he and his team reported that when they injected drug-bearing bacteria near the cancer site in mice, more than half of the microbes migrated into the heart of the tumors (11), autonomously seeking hypoxic areas after being steered the right region with magnetic fields.
Martel's work combines the built-in natural navigational abilities of microorganisms with knowledge about disease and targeted drugs. He hypothesizes that hybrids like these, which combine the functionality of genetically modified microorganisms with state-of-the-art technology, have the best chance of becoming part of medical treatments in the future.
“Nature creates the right specifications,” Martel says. “If we can exploit those, we’ll have a device that behaves exactly like a nanorobot of the future.”
Supplementary Material
References
- 1.Huang HW, Sakar MS, Petruska AJ, Pané S, Nelson BJ. Soft micromachines with programmable motility and morphology. Nat Commun. 2016;7:12263. doi: 10.1038/ncomms12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feynman RP. There’s plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- 3.Drexler KE. Molecular engineering: An approach to the development of general capabilities for molecular manipulation. Proc Natl Acad Sci USA. 1981;78:5275–5278. doi: 10.1073/pnas.78.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita S, et al. 2016 IEEE International Conference on Robotics and Automation (ICRA) IEEE; New York: 2016. Ingestible, controllable, and degradable origami robot for patching stomach wounds; pp. 909–916. [Google Scholar]
- 5.Magdanz V, Sanchez S, Schmidt OG. Development of a sperm-flagella driven micro-bio-robot. Adv Mater. 2013;25:6581–6588. doi: 10.1002/adma.201302544. [DOI] [PubMed] [Google Scholar]
- 6.Medina-Sánchez M, Schwarz L, Meyer AK, Hebenstreit F, Schmidt OG. Cellular cargo delivery: Toward assisted fertilization by sperm-carrying micromotors. Nano Lett. 2016;16:555–561. doi: 10.1021/acs.nanolett.5b04221. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, et al. 2017 Sperm-hybrid micromotor for drug delivery in the female reproductive tract. arXiv:170308510 [physics, q-bio]. Available at: http://arxiv.org/abs/1703.08510. Accessed August 14, 2017.
- 8.Williams BJ, Anand SV, Rajagopalan J, Saif MTA. A self-propelled biohybrid swimmer at low Reynolds number. Nat Commun. 2014;5:3081. doi: 10.1038/ncomms4081. [DOI] [PubMed] [Google Scholar]
- 9.Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335:831–834. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Hiratsuka Y, Kawamata I, Murata S, Nomura SM. Micrometer-sized molecular robot changes its shape in response to signal molecules. Sci Robot. 2017;2:eaal3735. doi: 10.1126/scirobotics.aal3735. [DOI] [PubMed] [Google Scholar]
- 11.Felfoul O, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11:941–947. doi: 10.1038/nnano.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters C, et al. Superparamagnetic twist-type actuators with shape-independent magnetic properties and surface functionalization for advanced biomedical applications. Adv Funct Mater. 2014;24:5269–5276. [Google Scholar]