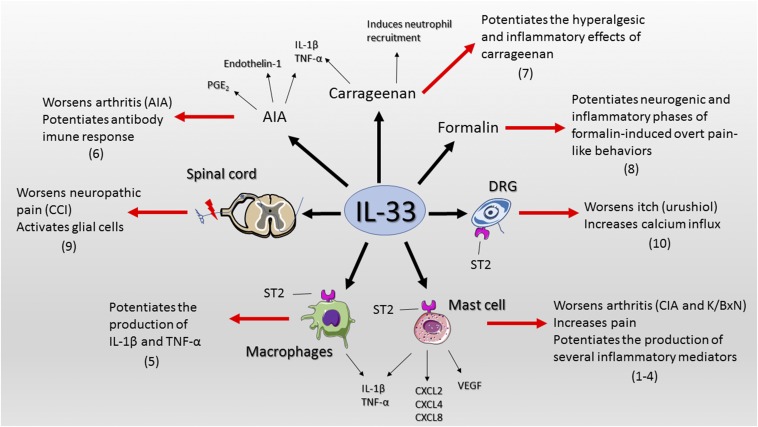
We read with interest the work of Taracanova et al. (1) on the synergic effect of substance P (SP) and IL-33 over TNF-α production. Notably, IL-33 potentiates SP-induced TNF-α production by more than 100-fold in mast cells. Both IL-33 and SP increase the expression of each other receptor, and more interestingly, ST2 coimmunoprecipitates with neurokinin-1 (NK-1, SP receptor), demonstrating a formation of an ST2–NK-1 complex. The Taracanova et al. study expands the importance of mast cells in inflammation and the understanding that IL-33 potentiates the effects of other peptides to enhance inflammation. IL-33 also enhances by 50% SP-induced vascular endothelial growth factor release by human mast cells (2). At the single-cell level, IL-33 augments the frequency and magnitude of FcεRI (using suboptimal concentrations) -induced mast cell degranulation and production of chemokines (3). In a model of collagen-induced arthritis, intraperitoneal injection of IL-33 and adoptive transfer of wild-type mast cells to mice lacking ST2 worsens clinical index. Thus, IL-33 exacerbates arthritis (4) and potentiates mast cell activation. IL-33 also potentiates the response of other immune cells and dorsal root ganglion neurons. IL-33 potentiates TNF-α and IL-1β production by LPS-primed and ATP-stimulated macrophages (5). Targeting IL-33/ST2 signaling reduces hyperalgesia in antigen-induced arthritis and IL-33 induces hyperalgesia in immunized mice (6). IL-33 synergizes with carrageenan (using suboptimal doses of both compounds) to induce mechanical hyperalgesia and the production of TNF-α and IL-1β (7). IL-33 also enhances formalin-induced overt pain-like behavior (8). Furthermore, spinal cord oligodendrocyte-derived IL-33 mediates mechanical hyperalgesia in a chronic constrict injury model of neuropathic pain by activating other glial cells (9). The intrathecal injection of IL-33 into chronic constrict injury mice augments the mechanical hyperalgesia (9). Regarding itch, IL-33 increases the calcium influx in dorsal root ganglion neurons of urushiol-challenged mice (10). Taken together, these data demonstrate that IL-33 worsens diseases by potentiating inflammatory and nociceptive stimuli even at low concentrations (Fig. 1). Conversely, the inflammatory mediators triggered by these noxious stimuli induce IL-33 production. For example, coincubation of TNF-α and IL-1β increases IL-33 production in human fibroblast from patients with rheumatoid arthritis (4). Therefore, preclinical data demonstrate that targeting IL-33/ST2 signaling ameliorates varied diseases and thereby may represent a potential target for new drugs. Given that IL-33 potentiates the production of several mediators in a wide range of conditions (Fig. 1), a better clinical profile would be achieved using IL-33/ST2-targeting drugs alongside with current treatments.
Fig. 1.
IL-33 boosts inflammation and pain in diseases. References in parenthesis are placed next to the observed outcome. AIA, antigen-induced arthritis; CIA, collagen-induced arthritis; CCI, chronic constrict injury; DRG, dorsal root ganglion; VEGF, vascular endothelial growth factor.
Supplementary Material
Acknowledgments
The authors received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Footnotes
The authors declare no conflict of interest.
References
- 1.Taracanova A, et al. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci USA. 2017;114:E4002–E4009. doi: 10.1073/pnas.1524845114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theoharides TC, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joulia R, L'Faqihi FE, Valitutti S, Espinosa E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J Allergy Clin Immunol. 2017;140:497–509.e10. doi: 10.1016/j.jaci.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinassous Q, et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- 6.Verri WA, Jr, et al. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci USA. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarpelon AC, et al. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: Role of cytokines, endothelin-1 and prostaglandin E2. Br J Pharmacol. 2013;169:90–101. doi: 10.1111/bph.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han P, et al. Interleukin-33 mediates formalin-induced inflammatory pain in mice. Neuroscience. 2013;241:59–66. doi: 10.1016/j.neuroscience.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Zarpelon AC, et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016;30:54–65. doi: 10.1096/fj.14-267146. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci USA. 2016;113:E7572–E7579. doi: 10.1073/pnas.1606608113. [DOI] [PMC free article] [PubMed] [Google Scholar]