ABSTRACT
Medical scientific societies have the core mission of producing, pooling and disseminating solid and updated scientific information. We report the successful experience of the partnership of four national Medical Scientific Societies active in Italy in producing scientific advice on vaccines and vaccination. In particular, i) the Italian Society of Hygiene, Preventive Medicine and Public Health; SitI, ii) the Italian Society of Paediatrics; SIP, iii) the “Italian Federation of General Practitioners”; FIMP, and iv) the Italian Federation of General Medicine FIMMG) have worked together since 2012 to produce shared evidence-based recommendations on vaccination schedules, namely the “Lifetime Immunization Schedule” which introduced for the first time in Italy a life-course approach to vaccination. The 2014 edition of the “Lifetime Immunization Schedule” was used as a basis to develop the 2017–2019 Italian National Prevention Plan, approved by The Italian Ministry of Health in February 2017. In this report, we present the structure, content and supporting evidence of the new 2016 “Lifetime Immunization Schedule” and we expand on the influential role of medical scientific societies in researching and advocating for effective and safe vaccination programmes’ implementation at the national level.
KEYWORDS: vaccines, immunization, life course vaccination, adults' vaccination, scientific societies
The role and mission of medical scientific societies in promoting population health
Medical scientific societies have the core mission of producing, pooling and disseminating solid and updated scientific information. As recently pointed out by Vercellini et al. in BMJ Open, medical scientific societies should “foster research, (..) promote medical education and develop guidelines”.1 But how can these activities serve society in reaching the ultimate aim of improving population health? What is the role of medical scientific societies as key stakeholders in the health arena? “Authoritative medical associations, Vercellini et al. continue, are [those] influential in modulating practice, counselling administrators, advising politicians regarding public healthcare programmes”.1
We report the successful experience of the partnership of four national Medical Scientific Societies active in Italy in producing scientific advice on vaccines and vaccination and, of relevance, we report their key contribution in supporting the process that led to the recent approval of the new 2017–2019 Italian National Immunization Prevention Plan.2 In recent times, although national and international health authorities have renewed their commitments to reduce the burden and related costs of vaccine preventable diseases (VPDs) through effective vaccination programmes,3,4 vaccines have lost public confidence.5 In this context, the role of medical scientific societies can be crucial to both i) inform the planning and implementation of effective and evidence-based national-level vaccination policies, and ii) restore the culture of vaccination as one of the most efficient primary prevention tools for promoting individual and public health.6,7
Immunization polices in Italy
In Italy, immunization programs are managed in the context of the National Health Service (Servizio Sanitario Nazionale or SSN). SSN provides universal health coverage: the national level sets the health systems' fundamental principles and goals, defines the core benefit package of health services to be guaranteed to all citizens (‘Livelli Essenziali di Assistenza’ or LEA) and allocates national funds to the Regions. Regions are responsible for planning, financing, and implementing healthcare services.8 In the field of vaccination, this structure translates into each Region adopting its own regional immunization plan and schedule. Italy has currently no National Immunization Technical Advisory Group (NITAG) to provide advice for National immunization programs.9 The Ministry of Health periodically issues the National Immunization Prevention Plan (PNPV), a guidance document for immunization polices intended to be of technical support to Regions and which defines vaccines that have to be actively offered free-of-charge to target populations throughout the country. The PNPV is composed in close consultation with the High Health Council (Consiglio Superiore di Sanità or CSS), experts from the National Institute of Health (Istituto Superiore di Sanità or ISS), from the Directorate General for Prevention of the Ministry of Health and with input and support from scientific societies.
A strong multidisciplinary partnership to advocate for immunization polices
In Italy, four medical scientific societies active in the field of public health, preventive medicine and primary care for both children and adults came together for the first time in 2012 to jointly work on evidence-based immunization recommendations. The four scientific societies namely are: the Italian Society of Hygiene, Preventive Medicine and Public Health (SitI), the Italian Society of Paediatrics (SIP), the Italian Federation of Family Paediatricians (FIMP) and the Italian Federation of General Medicine (FIMMG). The outcomes of this joint collaboration have been considered one of the most important innovations promoting Italian vaccination policies in recent years,7,10,11 because: i) it introduced a multidisciplinary approach to vaccination combining public health and primary prevention competencies with primary care ones, ii) it allowed to play a strong advocacy role for vaccination at the institutional level as representatives from all four scientific societies were consulted by the Ministry of Health during the approval process of the PNPV, iii) it allowed to produce strong evidence-based recommendations for immunization polices in Italy, including the introduction of a life-course approach to vaccination
In fact, in 2012, SItI, SIP, FIMP and FIMMG worked together to produce, for the first time in Italy, shared recommendations on vaccines' schedules, the “Lifetime Immunization Schedule” which introduced in the country the life-course approach to immunization. The 2012 edition of the “Lifetime Immunization Schedule” was then updated in 2014 on the basis of the new accumulated scientific evidence; it was published 10 and presented at national and international conferences. Importantly, it was shared with decision-makers at the Italian national and regional level with the aim of advocating for evidence-based, effective and safe vaccines schedules and supporting their implementation in different Italian settings. On several circumstances, during this period, the four medical societies SItI, SIP, FIMP and FIMMG consulted for public heath bodies and authorities, were called to present their scientific thesis, and to interpret doubts and scientific questions on vaccines and immunizations raised by consumers, civil society, other stakeholders and the media.11-13
The joint SItI, SIP, FIMP and FIMMG scientific and advocacy action has aimed at supporting with scientific evidence the need for expanding vaccination offer to additional target populations, including children, adolescents, adults and the elderly and to new efficacious, safe and cost-effective vaccines; this leaving to central and regional health authorities the role of planning and implementing immunization programmes in terms of: vaccine procurement, health services organization and human and economic resources allocation.
The 2017–2019 edition of the PNPV, issued by the Italian Ministry of Health has been very recently published in the Italian Official Gazette (Februrary 18th 2017) 2 as a binding document for all Regional health systems. The final approval of the 2017–2019 PNPV comes after two-year of scientific and political debate where case of Italy was raised on international journals in 2015, including the British Medical Journal.14,15 Nonetheless, the new Italian Plan, internationally recognized to be one of the world's most advanced immunization schedules where vaccines are actively offered free of charge to the general population as part of LEAs, was drafted in close consultation with SItI, SIP, FIMP and FIMMG representatives. The 2014 “Lifetime Immunization Schedule” constituted a solid basis for discussion in the building of the 2017–2019 PNPV,11 every scientific society identified selected representative experts and a coordinator to participate to the consultation meetings organized by the Ministry of Health.
As stated in the Plan, the Italian Ministry of Health's formally supports the introduction of new vaccines or schedules, provided they are sustained by strong efficacy, safety and cost-effectiveness data. In this context, since the SItI, SIP, FIMP and FIMMG partnership commitment is to periodically update its immunization recommendations, pooling new accumulated scientific evidence in subsequent editions of the “Lifetime Immunization Schedule”, a new 2016 edition of the “Lifetime Immunization Schedule” is now available.
The way to a new 2016 “Lifetime Immunization Schedule”
The new SItI, SIP, FIMP and FIMMG 2016 “Lifetime Immunization Schedule” “Lifetime Immunization Schedule” was drafted taking into consideration the need of: i) on one side, recommending the most complete and advanced immunization schedules which could offer individual-level protection as well as population-level VPDs' burden drecrease, ii) recommending schedules manageable by health services and iii) acceptable by the general population.6,16 The proposal for the 2016 “Lifetime Immunization Schedule” was formulated by integrating and updating the 2014 edition,10 complemented by an extensive literature review aimed at selecting the most recent scientific evidence concerning the efficacy and safety of vaccination policies.17-21 Top Italian experts in the field of hygiene and public health, pediatrics and family medicine and primary care were involved in the drafting process, engaged in an enriching and multidisciplinary scientific debate which included a careful revision of vaccination schedules and immunization recommendations issued by the most important national and international scientific boards (i.e. Advisory Committee on Immunization Practices-ACIP, European Center for Disease Prevention and Control ECDC, etc.).22,23
An innovative “Lifetime Immunization Schedule”
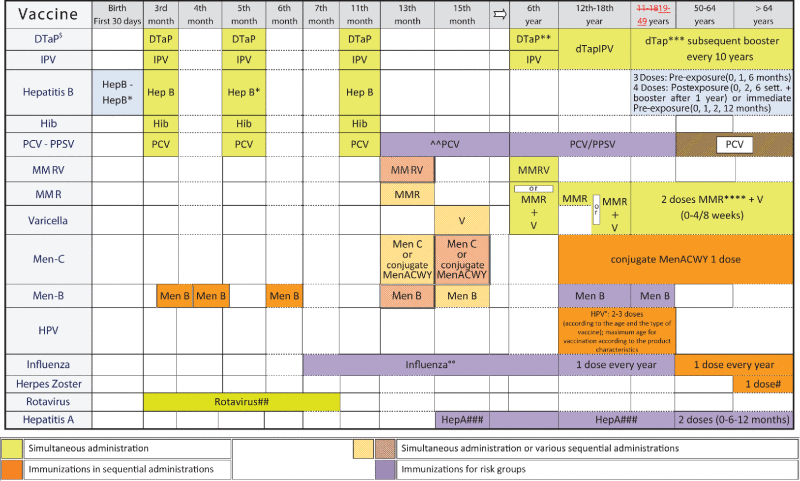
The complete 2016 “Lifetime Immunization Schedule” is presented in Table 1, where the recommended immunization programmes are listed by specific vaccine, age, target and risk categories. The 2016 edition builds on the 2014 one and extends immunization offer to include new vaccines as well as new target populations. Recommended schedules comprise the following vaccines; for the first year of life: hexavalent, pneumococcus, meningococcus B and rotavirus vaccines; for the second year of life: meningococcus C vaccine, vaccines against measles, mumps and rubella (MMR), together with the varicella vaccine. For adolescent populations, the recommended schedule includes the HPV vaccine for both genders and a quadrivalent meningococcal vaccine dose for both the unvaccinated subjects and those already immunized when toddlers. Influenza vaccine is recommended for children as well as for subjects aged >50 years, and at-risk groups, pneumococcal and zoster vaccines are recommended in the elderly populations (at least one age cohort with free of charge active offer). In addition, booster doses and vaccines schedules for at- risk categories are listed in Table 1 footnotes.
Table 1.
(Continued).
Hexavalent combination: DTaP = Diphteria, Tetanus and Acellular Pertussis vaccine – paediatric formulation, IPV = Inactivated Polio vaccine, Hep B = Hepatitis B vaccine, Hib = Haemophilus influenzae type b vaccine
MenB = Meningoccal B vaccine, Rotavirus = Rotavirus vaccine
dTap = Diphteria, Tetanus and Acellular Pertussis vaccine – adult/adolescent formulation
dTap-IPV = Diphteria, Tetanus , Acellular Pertussis and Inactivated Polio – adult/adolescent formulation
MMR = Measles, Mumps and Rubella vaccine, MMRV = Measles, Mumps, Rubella and Varicella vaccine, V = Varicella vaccine
PCV = Pneumococcal Conjugate vaccine, PPSV = Pneumococcal polysaccharide vaccine
MenC = Meningococcal C Conjugate vaccine, Men ACWY = Meningococcal ACWY conjugate vaccine
HPV = Human papilloma virus vaccine
Influenza = Influenza vaccine
Hep A = Hepatitis A vaccine
Notes:
*For children born from HBsAg positive mothers four-dose schedule: 1st dose within the first 12-24 hours of life concurrently with specific Hepatitis B immunoglobulins; 2nd dose after 4 weeks, 3rd dose following the lifetime immunization schedule, after the 60th day of (hexavalent vaccine).
Although the final decision should be taken by Regional health administrations according to the local organization of vaccination services, a possible recommended sequence of immunization is the following (the days are suggestive and not mandatory):
Hexavalent vaccine + Pneumococcal vaccine (PCV) at the start of the 3rd month of life (61st day of life)
Meningococcal B vaccine after 15 days (76th day)
Meningococcal B vaccine after 1 month (106th day)
Hexavalent vaccine + Pneumococcal vaccine (PCV) after 15 days, at the start of the 5th month of life (121st day of life)
Meningococcal B vaccine after 1 month, at the start of the 6th month of life (151st day of life)
Hexavalent vaccine + Pneumococcal vaccine (PCV) at the start of the 12th month of life
Meningococcal B vaccine starting from the 13th month of life
Meningococcal C vaccine, always the 1st year of life
Possible co-administration of MMR or MMRV vaccine with Meningococcal C or B vaccine, according to the different regional schedules (see possible combination in the scheme)
In case of co-administration of Meningococcal B vaccine with MMR or MMRV vaccine a counseling service for parents is advisable, with the aim of explaining possible fever occurrence in the hours following vaccination and/or 7-10 days after immunization.
**The third dose must be administered at least 6 months after the second dose. The fourth dose, the last of the first course, must be administered during the 5th–6th year. Use of the adult formulation (dTap) is possible since the 5th year of life if coverage with the 5th dose at adolescence is granted.
***Subsequent booster every 10 years.
*** In response to the outbreaks occurred in the past years, catch-up of the susceptible individuals and an active search of unvaccinated individuals (mop-up) is strongly recommended.
^Individuals without history of varicella: Administration of two doses of vaccine. Second dose at least 4 weeks after the first.
^^Two doses for children who start immunization during the second year of life. One dose only if immunizartion starts during the third year of life.
§One dose. Meningococcal C or Conjugate Men ACWY vaccine is administered at 13-15 months of life. A dose of Men ACWY is recommended at 12-14 years of life both to previously unvaccinated subjects, and to those already immunized as toddlers or children with the MenC or the Men ACWWY conjugate vaccine. Meningococcal C vaccine can be administered to at-risk individuals since the third month of life following a three dose schedule: the third dose should be given T after the 1st year of life.
°Two doses at 0 and 6 months (bivalent and nonovalent vaccine for individuals aged 9-14 years; quadrivalent vaccine for individuals aged 9-13 years), three doses at 0, 1 and 6 months (bivalent) or 0, 2 and 6 months (quadrivalent and nonovalent) for older individuals. A multi-cohort strategy including the cohort of 12-year old males, older female adolescents and/or young women is recommended in order to accelarate HPV diseases prevention. Co-payment at social price of HPV vaccine should be ensured for all those who do not have access to free-of-charge immunization.
°°Immunization with the seasonal vaccine for individuals considered at risk by the Italian Minister of Health and for children who attend kindergartens or other communities. It's recommended to extend Universal offer of free-of-charge vaccination should be extended to all subjects aged ≥50 years.
#Administration is recommended on the basis of age for at least one cohort of individuals ≥60 years and for subjects at risk.
##Universal free of charge offer. Rotavirus vaccine can be co-administred with all other vaccines used in the first months of life.
###Recommendations for highly endemic areas (2 cohorts, at 15/18 months and 12 years). Universal vaccination free of charge for children (0-14 years) who travel abroad.
Table 1.
 |
|---|
Hexavalent combination: DTaP = Diphteria, Tetanus and Acellular Pertussis vaccine – paediatric formulation, IPV = Inactivated Polio vaccine, Hep B = Hepatitis B vaccine, Hib = Haemophilus influenzae type b vaccine
MenB = Meningoccal B vaccine, Rotavirus = Rotavirus vaccine
dTap = Diphteria, Tetanus and Acellular Pertussis vaccine – adult/adolescent formulation
dTap-IPV = Diphteria, Tetanus , Acellular Pertussis and Inactivated Polio – adult/adolescent formulation
MMR = Measles, Mumps and Rubella vaccine, MMRV = Measles, Mumps, Rubella and Varicella vaccine, V = Varicella vaccine
PCV = Pneumococcal Conjugate vaccine, PPSV = Pneumococcal polysaccharide vaccine
MenC = Meningococcal C Conjugate vaccine, Men ACWY = Meningococcal ACWY conjugate vaccine
HPV = Human papilloma virus vaccine
Influenza = Influenza vaccine
Hep A = Hepatitis A vaccine
Notes:
*For children born from HBsAg positive mothers four-dose schedule: 1st dose within the first 12-24 hours of life concurrently with specific Hepatitis B immunoglobulins; 2nd dose after 4 weeks, 3rd dose following the lifetime immunization schedule, after the 60th day of life, (hexavalent vaccine).
*Although the final decision should be taken by Regional health administrations according to the local organization of vaccination services, a possible recommended sequence of immunization is the following (the days are suggestive and not mandatory):
Hexavalent vaccine + Pneumococcal vaccine (PCV) at the start of the 3rd month of life (61st day of life)
Meningococcal B vaccine after 15 days (76th day)
Meningococcal B vaccine after 1 month (106th day)
Hexavalent vaccine + Pneumococcal vaccine (PCV) after 15 days, at the start of the 5th month of life (121st day of life)
Meningococcal B vaccine after 1 month, at the start of the 6th month of life (151st day of life)
Hexavalent vaccine + Pneumococcal vaccine (PCV) at the start of the 12th month of life
Meningococcal B vaccine starting from the 13th month of life
Meningococcal C vaccine, always the 1st year of life
Possible co-administration of MMR or MMRV vaccine with Meningococcal C or B vaccine, according to the different regional schedules (see possible combination in the scheme)
In case of co-administration of Meningococcal B vaccine with MMR or MMRV vaccine a counseling service for parents is advisable, with the aim of explaining possible fever occurrence in the hours following vaccination and/or 7-10 days after immunization.
**The third dose must be administered at least 6 months after the second dose. The fourth dose, the last of the first course, must be administered during the 5th–6th year. Use of the adult formulation (dTap) is possible since the 5th year of life if coverage with the 5th dose at adolescence is granted.
***Subsequent booster every 10 years.
*** In response to the outbreaks occurred in the past years, catch-up of the susceptible individuals and an active search of unvaccinated individuals (mop-up) is strongly recommended.
^Individuals without history of varicella: Administration of two doses of vaccine. Second dose at least 4 weeks after the first.
^^Two doses for children who start immunization during the second year of life. One dose only if immunizartion starts during the third year of life.
§One dose. Meningococcal C or Conjugate Men ACWY vaccine is administered at 13-15 months of life. A dose of Men ACWY is recommended at 12-14 years of life both to previously unvaccinated subjects, and to those already immunized as toddlers or children with the MenC or the Men ACWWY conjugate vaccine. Meningococcal C vaccine can be administered to at-risk individuals since the third month of life following a three dose schedule: the third dose should be given after the 1st year of life.
°Two doses at 0 and 6 months (bivalent and nonovalent vaccine for individuals aged 9-14 years; quadrivalent vaccine for individuals aged 9-13 years), three doses at 0, 1 and 6 months (bivalent) or 0, 2 and 6 months (quadrivalent and nonovalent) for older individuals. A multi-cohort strategy including the cohort of 12-year old males, older female adolescents and/or young women is recommended in order to accelarate HPV diseases prevention. Co-payment at social price of HPV vaccine should be ensured for all those who do not have access to free-of-charge immunization.
°°Immunization with the seasonal vaccine for individuals considered at risk by the Italian Minister of Health and for children who attend kindergartens or other communities. It's recommended to extend Universal offer of free-of-charge vaccination should be extended to all subjects aged ≥50 years.
#Administration is recommended on the basis of age for at least one cohort of individuals ≥60 years and for subjects at risk.
##Universal free of charge offer. Rotavirus vaccine can be co-administred with all other vaccines used in the first months of life.
###Recommendations for highly endemic areas (2 cohorts, at 15/18 months and 12 years). Universal vaccination free of charge for children (0-14 years) who travel abroad.
The 2016 Lifetime Immunization Schedule's novel aspects, as compared to the 2014 edition are:
-
▪
a strong recommendation for extending influenza vaccination to pre-school age healthy children, in line with what has been recommended for years in other countries;24-26
-
▪
rotavirus vaccination is recommended at six weeks of age, earlier than previously recommended, for two reasons: on one hand, to obtain the strongest vaccine effectiveness against a disease whose severity is inversely proportional to age; and, on the other hand, to obtain maximum safety levels. In fact, although cases of intussusception following the first vaccine dose are extremely rare, their probability is directly related to age 27,28
-
▪
meningococcal B immunization, already recommended for the first year of life, is foreseen in the coming years for adolescents, due to their role in the transmission of infection 29-31 (please note Men B vaccine schedule recommendation is based on available evidence on both Men-B disease burden and Men B vaccine efficacy, 19)
-
▪
the introduction of influenza vaccine recommendation to pregnant women during the second or third quarter in order to avoid the risk of serious disease complications for both the mother and the fetus. In addition, a booster immunization against diphtheria, tetanus and pertussis (dTpa) is also recommended to pregnant women between week 27 and 36 at every pregnancy.32-37
The way forward
Immunization programs are key preventive interventions and have largely contributed – over the last century – to reduce the burden of infectious diseases and to decrease the related morbidity, mortality and healthcare costs.13,38-40 International and national health authorities have recently renewed their commitment to promote vaccine preventable diseases prevention and strengthening immunization programs. As a milestone in the European political agenda for public health, the EU has recently adopted the Council Conclusions on “Vaccinations as an effective tool in public health”.3 Along the same line, the WHO European Region Vaccine Action Plan 2015–2020 (EVAP) – defining immunization priority action areas and targets – calls on countries to implement effective immunization policies and programs.4
As medicine and technology progress, new vaccines become available and new evidence accumulates in the field of vaccinology and immunization polices, it is of fundamental importance for health authorities to have updated and evidence-based recommendations. In this context, medical scientific societies can play a crucial role in informing and supporting this process, producing, pooling and disseminating solid and updated scientific information.
We present the successful case of Italy where, in the last five years, a multidisciplinary partnership of medical scientific societies representing public health, primary care and paediatrics have consolidated a fruitful collaboration in a joint effort to produce three consecutive editions of the “Lifetime Immunization Schedule”. Its scientific solidity and comprehensiveness have grown over the years. Their output and activity at large is now nationally and internationally recognized, not only within the scientific community, but also by institutions, health authorities, healthcare managers and, last but not least, by the general population, thus enabling a strong advocacy action to promote immunization in the country.
It is estimated that, on average, the time lag between the marketing authorization of a new vaccine and its introduction in national immunization schedules and routine administration is six years.41 Documents such as the “Lifetime Immunization Schedule”, incorporating new available evidence in updated recommendations can help to reduce such time lag. The “Lifetime Immunization Schedule” was built on the basis of available efficacy, safety as well as effectiveness data, pooled in a complete and comprehensive immunization schedule for children, adolescents, adults and elder populations.
We firmly believe primary prevention of VPDs through immunization, and prevention in general, are among the pillars of well-functioning health systems.42-45 In times of changing socio-demographic and welfare patterns, where the sustainability of health systems is threatened by the ongoing economic crises, it is our strong commitment to advocate to preserve a priceless heritage for our country: the National Health Service, that still provides universal health coverage. As members of the scientific community, we devote our knowledge, skills, efforts and commitment to support national and regional health authorities in planning, implementing and evaluating safe and effective immunization programmes and policies with the ultimate aim of reducing the clinical and economic burden of vaccine preventable diseases in Italy.
Disclosure of potential conflicts of interest
In the last two years – Paolo Bonanni reports personal fees from Sanofi Pasteur MSD, MSD, GSK, Pfizer and Seqiros to take part to Scientific Advisory Boards or participate in CME events as speaker/moderator. He has also acted as member of the Data Safety Monitoring Board (DSMB) for an experimental vaccine against bacterial diarrhoea developed by the GSK Vaccine Institute for Global Health (GVGH) intended to be used in endemic countries with limited resources – Giampietro Chiamenti reports the participation in Advisory Boards organized by Abbvie, Sanofi-MSD and Gap- Tommasa Maio reports the participation in Advisory Boards organized by Sanofi Pasteur MSD and Glaxosmithkline – Silvestro Scotti reports the participation in two Advisory Boards organized by Sanofi Pasteur MSD -Alberto Villani reports the participation in Advisory Boards organized by Sanofi Pasteur MSD, GSK, Pfizer e Abbvie – Carlo Signorelli reports the participation in one Advisory Board organized by Sanofi Pasteur MSD – The other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1].Vercellini P, Vigano P, Frattaruolo MP, Somigliana E. Proliferation of gynaecological scientific societies and their financial transparency: an Italian survey. BMJ Open. 2016;6(1):e008370. PMID:26769777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Italian Ministry of Health National Immnunization Prevention Plan 2017–2019. IPublished on the italian official gazette, Februrary 18th 2017. Available at http://www.gazzettaufficiale.it/eli/id/2017/02/18/17A01195/sg [accessed: 18/03/2017].
- [3].Council of The European Union Council conclusions on vaccinations as an effective tool in public health, 2014. http://www.consilium.europa.eu/uedocs/cms_data/docs/pressdata/en/lsa/145973.pdf [Accessed 26.12.2014].
- [4].The World Health Organization Regional office for europe. European region vaccine action plan 2015–2020. 2014. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020-2014 [Accessed 31.12.2014].
- [5].Odone A, Signorelli C. When vaccine hesitancy makes headlines. Vaccine. 2017;35(9):1209-10. PMID:26657186 [DOI] [PubMed] [Google Scholar]
- [6].Odone A, Fara GM, Giammaco G, Blangiardi F, Signorelli C. The future of immunization policies in Italy and in the European Union: The Declaration of Erice. Hum Vaccin Immunother. 2015;11(5):1268-71. PMID:25806425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Signorelli C, Odone A. Advocacy communication, vaccines and the role of scientific societies. Ann Ig. 2015;27(5):737-47. PMID:26661915 [DOI] [PubMed] [Google Scholar]
- [8].Ferrè F, de Belvis AG, Valerio L, Longhi S, Lazzari A, Fattore G, Ricciardi W, Maresso A. Italy: Health system review. Health Syst Transit. 2014;16(4):1-168. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/263253/HiT-Italy.pdf?ua = 1. PMID:25471543 [PubMed] [Google Scholar]
- [9].Ricciardi GW, Toumi M, Weil-Olivier C, Ruitenberg EJ, Danko D, Duru G, Picazo J, Zöllner Y, Poland G, Drummond M. Comparison of NITAG policies and working processes in selected developed countries. Vaccine. 2015;33(1):3-11. PMID:25258100 [DOI] [PubMed] [Google Scholar]
- [10].Bonanni P, Azzari C, Castiglia P, Chiamenti G, Conforti G, Conversano M, Corsello G, Ferrera G, Ferro A, Icardi G, et al.. [The 2014 Lifetime Immunization Schedule approved by the Italian scientific societies. Italian Society of Hygiene, Preventive Medicine, and Public Health. Italian Society of Pediatrics. Italian Federation of Pediatric Physicians. Italian Federation of General Medical Physicians]. Epidemiol Prev. 2014;38(6 Suppl 2):131-46. PMID:25759359 [PubMed] [Google Scholar]
- [11].Bonanni P, Ferro A, Guerra R, Iannazzo S, Odone A, Pompa MG, Rizzuto E, Signorelli C. Vaccine coverage in Italy and assessment of the 2012–2014 national immunization prevention plan. Epidemiol Prev. 2015;39(4 Suppl 1):146-58. PMID:26499433 [PubMed] [Google Scholar]
- [12].Signorelli C, Odone A, Bonanni P, Russo F. New Italian immunisation plan is built on scientific evidence: Carlo Signorelli and colleagues reply to news article by Michael Day. BMJ. 2015;351:h6775. PMID:26667823 [DOI] [PubMed] [Google Scholar]
- [13].Signorelli C. Vaccines: building on scientific excellence and dispelling false myths. Epidemiol Prev. 2015;39(3):198-201. PMID:26522283 [PubMed] [Google Scholar]
- [14].Signorelli C, Guerra R, Siliquini R, Ricciardi W. Italy's response to vaccine hesitancy: An innovative and cost effective National Immunization Plan based on scientific evidence. Vaccine. 2017;35(33):4057-9. doi: 10.1016/j.vaccine.2017.06.011. [DOI] [PubMed] [Google Scholar]
- [15].Day M. Italian expert questions need for expanded vaccination schedule. BMJ. 2015;351:h6181. https://doi.org/ 10.1136/bmj.h6181 PMID:26573246 [DOI] [PubMed] [Google Scholar]
- [16].Biasio LR, Corsello G, Costantino C, Fara GM, Giammanco G, Signorelli C, Vecchio D, Vitale F. Communication about vaccination: A shared responsibility. Hum Vaccin Immunother. 2016;12(11):2984-7. https://doi.org/ 10.1080/21645515.2016.1198456 PMID:27458874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonanni P, Signorelli C. Anti-rotavirus: no evidence to discontinue the universal vaccination policy. Ig San Pub. 2015;LXXI.5.2015:559-567. [PubMed] [Google Scholar]
- [18].Signorelli C, Chiesa V, Odone A. Meningococcal serogroup B vaccine in Italy: state-of-art, organizational aspects and perspectives. J Prev Med Hyg. 2015;56(3):E125-32. PMID:26788733 [PMC free article] [PubMed] [Google Scholar]
- [19].Crosignani P, De Stefani A, Fara GM, Isidori AM, Lenzi A, Liverani CA, Lombardi A, Mennini FS, Palu' G, Pecorelli S, et al.. Towards the eradication of HPV infection through universal specific vaccination. BMC Public Health. 2013;13:642. https://doi.org/ 10.1186/1471-2458-13-642 PMID:23845195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Audisio RA, Icardi G, Isidori AM, Liverani CA, Lombardi A, Mariani L, Mennini FS, Mitchell DA, Peracino A, Pecorelli S, et al.. Public health value of universal HPV vaccination. Crit Rev Oncol Hematol. 2016;97:157-67. PMID:26346895 [DOI] [PubMed] [Google Scholar]
- [21].Blasi F, Aliberti S, Bonanni P, Mantero M, Odone A, Signorelli C. [Pneumococcal vaccination in adults: recommendations from the Italian Society of Respiratory Medicine (SIMeR) and the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI)]. Epidemiol Prev. 2014;38(6 Suppl 2):147-51. PMID:25759360 [PubMed] [Google Scholar]
- [22].Advisory Committee on Immunization Practices-ACIP. Available at http://www.cdc.gov/vaccines/acip/index.html [accessed: 17/03/2017].
- [23].European Center for Disease Prevention and Control.Vaccine Schedule. Available at: http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx. [accessed: 17/03/2017].
- [24].Rose MA, Damm O, Greiner W, Knuf M, Wutzler P, Liese JG, Krüger H, Wahn U, Schaberg T, Schwehm M, et al.. The epidemiological impact of childhood influenza vaccination using live-attenuated influenza vaccine (LAIV) in Germany: predictions of a simulation study. BMC Infect Dis. 2014;14:40. https://doi.org/ 10.1186/1471-2334-14-40 PMID:24450996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. https://doi.org/ 10.1371/journal.pmed.1001527 PMID:24115913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Department of Health The flu immunisation programme 2013/14 – extension to children, 2013. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/225360/Children_s_flu_letter_2013.pdf [accessed: 07.04.2017].
- [27].Yen C, Healy K, Tate JE, Parashar UD, Bines J, Neuzil K, Santosham M, Steele AD. Rotavirus vaccination and intussusception – Science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low and middle income countries. Hum Vaccin Immunother. 2016;12(10):2580-9. https://doi.org/ 10.1080/21645515.2016.1197452 PMID:27322835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vesikari T, Van Damme P, Giaquinto C, Dagan R, Guarino A, Szajewska H, Usonis V. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: Update 2014. Pediatr Infect Dis J. 2015;34(6):635-43. https://doi.org/ 10.1097/INF.0000000000000683 PMID:25860532 [DOI] [PubMed] [Google Scholar]
- [29].Vetter V, Baxter R, Denizer G, Safadi MA, Silfverdal SA, Vyse A, Borrow R. Routinely vaccinating adolescents against meningococcus: Targeting transmission & disease. Expert Rev Vaccines. 2016;15(5):641-58. https://doi.org/ 10.1586/14760584.2016.1130628 PMID:26651380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health: Official publication of the Society for Adolescent Medicine. 2016;59(2 Suppl):S3-s11. https://doi.org/ 10.1016/j.jadohealth.2016.04.012 [DOI] [PubMed] [Google Scholar]
- [31].AAP Committee on Infectious Diseases. Recommendations for Serogroup B Meningococcal Vaccine for Persons 10 Years and Older. Pediatrics. 2016;138(3):e20161890. [DOI] [PubMed] [Google Scholar]
- [32].Nunes MC, Aqil AR, Omer SB, Madhi SA. The effects of influenza vaccination during pregnancy on birth outcomes: A systematic review and meta-analysis. Am J Perinatol. 2016;33(11):1104-14. https://doi.org/ 10.1055/s-0036-1586101 PMID:27603545 [DOI] [PubMed] [Google Scholar]
- [33].Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: A systematic review and meta-analysis. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America. 2015;60(5):e11-9. https://doi.org/ 10.1093/cid/ciu915 PMID:25409473 [DOI] [PubMed] [Google Scholar]
- [34].Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555-64. https://doi.org/ 10.1056/NEJMoa0708630 PMID:18799552 [DOI] [PubMed] [Google Scholar]
- [35].Forsyth K, Plotkin S, Tan T, Wirsing von Konig CH. Strategies to decrease pertussis transmission to infants. Pediatrics. 2015;135(6):e1475-82. https://doi.org/ 10.1542/peds.2014-3925 PMID:25963002 [DOI] [PubMed] [Google Scholar]
- [36].Atkins KE, Fitzpatrick MC, Galvani AP, Townsend JP. Cost-effectiveness of pertussis vaccination during pregnancy in the United States. Am J Epidemiol. 2016;183(12):1159-70. https://doi.org/ 10.1093/aje/kwv347 PMID:27188951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Regan AK. The safety of maternal immunization. Hum Vaccin Immunother. 2016;12(12):3132-6. https://doi.org/ 10.1080/21645515.2016.1222341 PMID:27541370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ten great public health achievements–United States, 2001–2010{, 2011 #26}. MMWR Morb Mortal Wkly Rep. 2011;60(19):619-23. PMID:21597455 [PubMed] [Google Scholar]
- [39].Hinman AR, Orenstein WA, Schuchat A. Vaccine-preventable diseases, immunizations, and MMWR–1961-2011. MMWR Surveill Summ. 2011;60 Suppl 4:49-57. [PubMed] [Google Scholar]
- [40].Roush SW, Murphy TV. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298(18):2155-63. https://doi.org/ 10.1001/jama.298.18.2155 PMID:18000199 [DOI] [PubMed] [Google Scholar]
- [41].Blank PR, Schwenkglenks M, Sardos CS, Patris J, Szucs TD. Population access to new vaccines in European countries. Vaccine. 2013;31(27):2862-7. https://doi.org/ 10.1016/j.vaccine.2013.04.039 PMID:23632307 [DOI] [PubMed] [Google Scholar]
- [42].Signorelli C, Odone A, Bianco D, Di Vivo N, Bevere F. [Health expenditure for prevention in Italy (2006-2013): Descriptive analysis, regional trends and international comparisons]. Epidemiol Prev. 2016;40(5):374-80. PMID:27764919 [DOI] [PubMed] [Google Scholar]
- [43].U.S. Department of Health & Human Services A Review and Analysis of Economic Models of Prevention Benefits, 2013. https://aspe.hhs.gov/basic-report/review-and-analysis-economic-models-prevention-benefits [accessed 8.6.2017].
- [44].Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Diabetes care. 2008;31(8):1686-96. https://doi.org/ 10.2337/dc08-9022 PMID:18663233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sassi F, Cecchini M, Lauer J, Chisholm D. Improving Lifestyles, Tackling Obesity: The Health and Economic Impact of Prevention Strategies, OECD Health Working Papers, No. 48, OECD Publishing, Paris. 2009, DOI: 10.1787/220087432153. [DOI] [Google Scholar]


