ABSTRACT
This monocenter, descriptive, prospective, non-interventional study evaluated the long-term immune responses following routine vaccination with one or 2 doses of a licensed inactivated hepatitis A (HA) vaccine (Avaxim® 80U Pediatric) at age 11–23 months in a cohort of children from Mendoza, Argentina. Antibodies to hepatitis A virus (anti-HAV) were quantified annually up to Y5, and at Y7. Children whose titer decreased to below the seroprotection threshold (defined as an anti-HAV antibody concentration of ≥ 10 mIU/mL in a microparticle enzyme immunoassay up to Y5, or ≥ 3 mIU/mL in an electrochemiluminescence immunoassay at Y7) received a routine booster dose of the same HA vaccine. This report summarizes the data at 7 year after the first vaccination. Of 546 participants initially included, 264 participants remained at Y7 and provided blood samples. Of these, 204 having received one HA primary dose as a toddler were still seroprotected at Y7; titers for a further 7 also having received one HA dose as a toddler fell to below the seroprotection threshold and they therefore received a booster; all 53 having received 2 HA doses as a toddler and still present at Y7 remained seroprotected at Y7. One or 2 primary doses of this HA vaccine in toddlers result in very good persistence of anti-HAV up to 7 year post-first vaccination.
KEYWORDS: antibody persistence, Argentina, hepatitis A vaccine, single dose, follow-up, Argentina
Introduction
Hepatitis A (HA) is a vaccine-preventable disease, caused by the HA virus (HAV) and strongly correlated with socio-economic factors such as access to good quality drinking water and food supply as well as appropriate sanitation. In early childhood symptoms of HA are not always apparent, but severity generally increases after 5 year of age and becomes more symptomatic, including fever, malaise, nausea, diarrhea, joint pain, abdominal pain, or vomiting.1 In Argentina HA has always been endemic but during 2003–2004 there was a country wide outbreak with the burden of acute liver failure due to HA infection being mainly in children – one study in Argentina showed that in 210 children (median age 5.33 years) with acute liver failure, 61% had HA infection2; in adults, acute liver failure is associated more often with hepatitis B infection.3
Argentina has diverse environmental and socio-economic conditions and associated diverse rates of HAV endemicity. To combat these regional disparities, universal single dose HA vaccination for toddlers at 12 months of age was introduced in the regions of Mendoza in 2004, and throughout Argentina in 2005 with significant projected benefits to both public health and expenditure.4 Vaccine coverage (Avaxim™ 80U Pediatric [Sanofi Pasteur], Havrix™ [GlaxoSmithKline], Vaqta™ [MSD], or Virohep-A Junior™ [Novartis]) has been high (≥ 90% since 2006)5 and this program has been very successful in reducing the national incidence of HA, as shown by the incidence of HA notified to the National Epidemiological Surveillance System in children under 1 year of age falling from 1087 cases between 2000 and 2005 to 156 cases between 2006 to 2013.6 This reduction in HA incidence children under 1 year of age illustrates a strong herd effect, with a similar effect observed in all age groups of young children.6 A higher proportion of cases was observed in individuals aged over 14 years, but this represented a small number of cases none of which had received the HA vaccine.7
In middle-income countries and regions with transitional HAV prevalence, such as Mendoza where circulation of wild HAV is still observed, a single-dose vaccination strategy may be effective, but should be validated versus a complete vaccination course (one primary vaccine dose followed by one booster vaccination). In this long-term study we evaluate the immunogenicity of one and 2 HAV vaccine doses given at 11 to 23 months of age in a cohort in Mendoza. Such strategies have been evaluated in adults8,9 but not in children.
The aims of this study, therefore, were to better characterize the effect of a single-dose regimen in toddlers in terms of long-term immunogenicity, compared with alternative vaccination schedules. The incidence of HAV infection throughout the study and the socio-economic environment were also assessed. Data at 3 and 5 years post-vaccination have been published elsewhere,10,11 and showed good seropositivity and geometric mean concentrations (GMCs) following a single dose and in those who received a booster vaccination; at 5 year post-vaccination the highest GMCs occurred in those who received 2 vaccinations and these results also supported a flexible time window for the booster vaccination. Here we present the follow-up data up to Year 7 in the same cohort in Mendoza, and place these data in the context of those previously presented for Years 1 to 5.
Results
Disposition of participants
Of the 546 participants originally included in the study at Visit 1 (1 year post-vaccination) 277 participants remained in the study at Visit 6 (6 year post-vaccination), which represents the withdrawal of 134 participants since Visit 5 (5 year post-vaccination) mainly due to voluntary withdrawal. At Visit 7, 266 returned, of whom 264 provided a blood sample. Of these 264, 204 participants were in Group 1, 53 participants in Group 2, and 7 participants in Group 3. Study disposition for Years 1 to 6 is presented in Figure 1.
Figure 1.
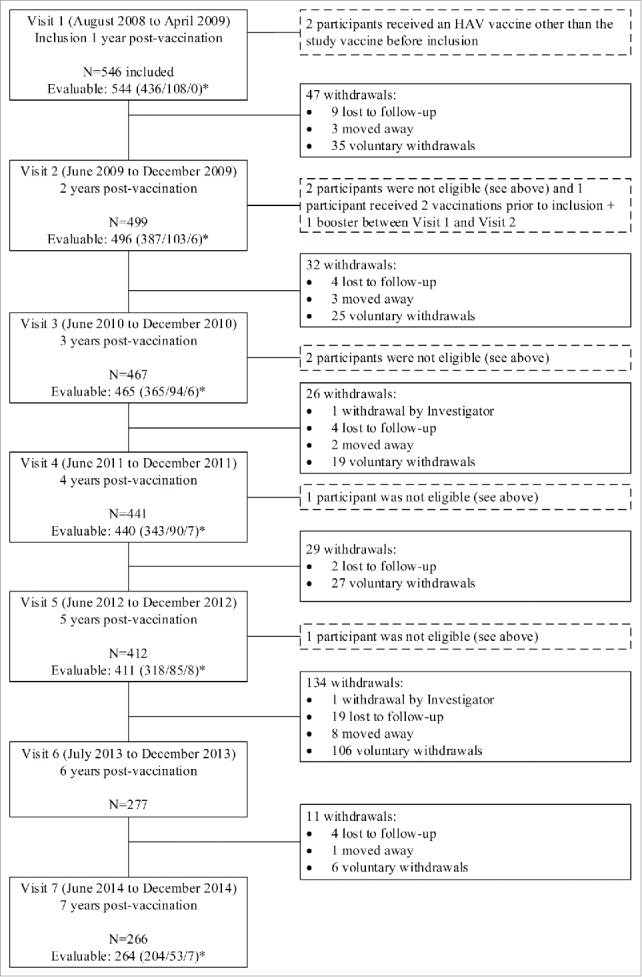
Disposition of study participants. *Group 1/Group 2/Group 3.
At baseline (inclusion) there was a similar number of males (49.6%) and females (50.4%), with mean ± standard deviation (SD) age of 28.5 ± 2.44 months. At Year 7, 50.5% of participants were male and 49.5% were female. From Visit 1 to Visit 7, mean weight and mean height ± SD increased from 13.42 ± 1.82 kg to 30.26 ± 7.61 kg and from 90.5 ± 3.9 cm to 128.9 ± 5.8 cm. Mean ± SD body mass index increased from 16.34 ± 1.52 kg/m2 at Visit 1 to 18 ± 3.27 kg/m2 at Visit 7.
Persistence of immunity
At Year 7, the seroprotection rate (percentage of participants with anti-HAV concentration ≥ 3 mIU/mL by electrochemiluminescence immunoassay [ECLIA]) was 100% in each group, demonstrating maintenance of the high seroprotection rates observed in Years 1 to 5 (using the threshold of 10 mIU/mL by microparticle enzyme immunoassay [MEIA]. At Year 7, of the 211 participants who received only one dose before inclusion, and for whom blood samples were available (Groups 1 and 3) 204 participants (96.7%) remained seroprotected without a booster dose (Group 1) (Table 1). The remaining 7 participants at Year 7 (Group 3) had received a booster when low anti-HAV antibodies were detected, as described in Table 1, to achieve seroprotection. The Year 7 GMCs were 125.6, 712.5, and 257.2 mIU/mL for Groups 1, 2 and 3, although as these were derived using ECLIA they should not be directly compared in numerical terms to the GMCs over Years 1 to 5, which were derived using MEIA. However, as for Years 1 to 5, the highest GMC was observed in Group 2 (2 doses of the HAV vaccine before inclusion and no booster during the study). Due to the different assays used for Years 1 and 7, GMCR data (i.e. the ratio of Year 7/Year 1) are not applicable for Year 7.
Table 1.
Persistence of anti-HAV antibodies for Years 1 to 5 (MEIA) and Year 7 (ECLIA) after first vaccination.
| Year 1 (95% CI) | Year 2 (95% CI) | Year 3 (95% CI) | Year 4 (95% CI) | Year 5 (95% CI) | Year 7 (95% CI) | |
|---|---|---|---|---|---|---|
| Group 1: one dose of Avaxim™ 80U Pediatric prior to inclusion | ||||||
| M | 435a | 387 | 365 | 343 | 318 | 204 |
| ≥10 mIU/mL (n [%]) | 429 (98.6) | 387 (100) | 364 (99.7) | 342 (99.7) | 317 (99.7) | — |
| ≥3 mIU/mL (n [%]) | — | — | — | — | — | 204 (100) |
| GMC (95% CI) | 209.7 (190.6;230.6) | 216.0 (198.0;235.7) | 170.2 (155.7;186.0) | 150.3 (137.4;164.4) | 122.5 (111.2;135.0) | 125.6 (118.8;141.1) |
| GMCR (95% CI) | — | 0.95 (0.90;1.00) | 0.74 (0.70;0.79) | 0.66 (0.61;0.70) | 0.53 (0.49;0.57) | – |
| Group 2: two doses of Avaxim™ 80U Pediatric prior to inclusion | ||||||
| M | 108 | 103 | 94 | 90 | 85 | 53 |
| ≥10 mIU/mL (n [%]) | 107 (99.1) | 103 (100) | 94 (100) | 90 (100) | 85 (100) | — |
| ≥3 mIU/mL (n [%]) | — | — | — | — | — | 53 (100) |
| GMC (95% CI) | 1433.9 (1108.4;1855.1) | 1353.8 (1116.2;1641.9) | 872.9 (710.2;1073.0) | 814.6 (667.4;994.1) | 591.7 (479.9;729.4) | 712.5 (526.4;964.5) |
| GMCR (95% CI) | — | 0.88 (0.72;1.07) | 0.57 (0.47;0.70) | 0.54 (0.44;0.66) | 0.40 (0.33;0.50) | – |
| Group 3: one dose of Avaxim™ 80U Pediatric prior to inclusion and one booster doseb | ||||||
| M | 0 | 6 | 6 | 7 | 8 | 7 |
| ≥10 mIU/mL (n [%]) | — | 6 (100) | 6 (100) | 7 (100) | 8 (100) | — |
| ≥3 mIU/mL(n [%]) | — | — | — | — | — | 7 (100) |
| GMC (95% CI) | — | — | — | — | — | 257.2 (81.3;813.6) |
| Booster performed after visit | ||||||
| M | 6 | 0 | 1 | 1 | 1 | 0 |
| Pre-booster GMC (95% CI)c | 5 (3.7;6.7) | — | 7 (NC) | 9 (NC) | 9 (NC) | — |
| Post-booster GMC ([95% CI)d | 551.3 (130.3;2332.2) | — | 3000 (NC) | 153.0 (NC) | 2622.0 (NC) | — |
| Post-/pre- booster GMCR (95% CI) | 110.1 (27.5;440.6) | — | 428.57 (NC) | 17.0 (NC) | 291.33 (NC) | — |
Immunogenicity data missing for one participant.
No participant had a booster at Visit 1. Following Visit 1, 6 participants had low anti-HAV concentrations and received a booster dose – these participants were included in the 1 dose/booster set at Visit 2. Following Visit 3, 1 participant had low anti-HAV concentration and received a booster dose – this participant was included in the 1 dose/booster set at Visit 4. Following Visit 4, 1 participant had low anti-HAV concentration and received a booster dose – this participant was included in the 1 dose/booster set at Visit 5.
Sample taken at the indicated visit.
Sample taken after the booster regardless of the time window between booster and blood sampling.
n, number of subjects; M, number of participants with available data; CI, confidence interval; GMC, geometric mean concentration (mIU/mL); GMCR, geometric mean concentration ratio against Year 1 (not applicable for Year 7 due to different assays used for Years 1 to 5 [MEIA] and Year 7 [ECLIA]) or post-/pre-booster for Group 3; NC, not calculated (as only 1 participant).
There were no suspected cases of hepatitis A in family members at the Year 6 or Year 7 visits, and so it is not possible to fully assess the possibility of a natural boosting effect on anti-HAV immunity of the study participants.
Socio-economic factors
The participants' socio-economic conditions were similar in Years 6 and 7 as in Years 1 to 5. For Years 6 and 7 respectively, the population as a whole remained mainly urban (80.5% and 82.3%) or suburban (18.1% and 15.8%) with only 1.4% and 1.9% being rural. All participants' had a toilet at home, with 97.5% and 97.7% of these being indoors; 98.9% and 99.2% had access to potable water; and 92.8% and 92.1% had access to a sewage network.
The proportion of participants cared for outside the home (in day care centers) in Year 6 (99.6%) and Year 7 (100%) was similar to Year 5 (99.6%) which had shown an increase from 25.3% at inclusion. Also similar to Year 5, most children were cared for together with a group of ≥ 20 others in Year 6 (82.7%) and Year 7 (86.7%) (in Year 5 this was 80.3%); this was only 28.0% in Year 3 and 50.3% in Year 4. For 99.6% (Year 6) and 100% (Year 7) of participants, this childcare was for 5 d per week.
In Years 6 and 7, respectively, 97.0% and 96.9% of fathers and 55.7% and 54.6% of mothers had working activity. This reflected the pattern reported for Years 1 to 5.
Discussion
In the absence of long-term immunogenicity data following 1- or 2-dose inactivated HAV vaccine regimens in children, the data provided in this ongoing follow-up study are important to understand the optimal HAV vaccination strategy in this pediatric population. Previously, immunogenicity data as well HA incidence and socio-economic factors at 1 and 5 years of age in 3 groups according to the vaccination regimen have been described (10, 11). Here we provide these data to 7 year of follow-up.
This study was originally planned for a 5-year post-vaccination follow-up period, which was subsequently extended to 10 year. As such, it was necessary to obtain informed assent from each participant who remained in the study after that time, i.e., those aged 7 year at Visit 6, and also to obtain new informed consent from these participants' parent(s) or legally acceptable representative at this time (in addition to the informed consent that had previously been obtained from each participant's parent[s] or legally acceptable representative). This resulted in a total of 134 participants who withdrew voluntarily from the study between Visit 5 and Visit 6.
The assay used to measure anti-HAV IgG at Year 7 (ECLIA) differed to the one used previously to assess the response for Years 1 to 5 (MEIA) due to the discontinuation of the MEIA kit by its manufacturer. As quantification of anti-HAV IgG depends on the assay used (e.g. the ECLIA assay is more sensitive than the MEIA assay), and since no universally accepted level for seroprotection has been identified,12-17 the anti-HAV IgG concentration data from Years 1 to 5 and from Year 7 cannot be directly compared. However, although equivalence cannot be demonstrated for seroprotection between the 2 assays, comparisons in terms of seroprotection based on the threshold for each assay are valid (10 mIU/mL for MEIA and 3 mIU/mL for ECLIA, even though there is no absolute definition of a seroprotection level for anti-HAV antibodies [as described above]).
In Years 6 and 7 after vaccination there were no symptomatic cases of HA in participants' family members, which correlates with the declining incidence of HA in Argentina since the introduction of universal single dose vaccination in 2005.6 Furthermore, in Year 7 the seroprotection was 100% in each group, indicating that no participant dropped below the seroprotection threshold between Year 5 and Year 6, and demonstrating a very good persistence of protection afforded by each vaccination regimen. In terms of anti-HA IgG concentrations, the highest GMCs were observed in Group 2, i.e., 2 doses of HAV vaccine before inclusion and no booster during the study, although for those who received only 1 dose before inclusion (Groups 1 and 3) 96.7% did not require a booster dose to remain seroprotected (Group 1), and the 7 participants who did require a booster achieved seroprotection (Group 3). These data confirm the continued antibody persistence that has been described for Years 1 to 5 for each of the 3 vaccination regimens and support the single-dose vaccination approach in this population. There were no changes in socio-economic factors in the study population.
Limitations of the study include the reduced number of participants at Years 6 and 7 due to the high number of withdrawals between Years 5 and 6 (as described above), and also the change in assay used to assess anti-HA IgG after Year 5. However, the number of remaining participants at Year 7 is still considered sufficient for the descriptive analyses, and despite the change in assay – which precludes a comparison of GMCs between Year 7 and Years 1 to 5 - the seroprotection rate remains an effective comparator.
These data show very positive results for 7 year after the first vaccination with the HAV vaccine with regard to persistence of anti-HA IgG antibodies.
In conclusion, good antibody persistence has been demonstrated for 7 year following one or 2 vaccinations of Avaxim™ 80U Pediatric in a large pediatric population in Mendoza, Argentina, with very high seroprotection at Year 7.
Materials and methods
This was a monocenter, descriptive, prospective, non-interventional study conducted in Argentina. The study evaluated only immunogenicity; no safety data were collected.
Participants and vaccine administered
Healthy participants who had received at least one dose of inactivated HAV vaccine (Avaxim™ 80U Pediatric) between June and December 2007 (when aged 11 to 23 months) were eligible for inclusion in this study, which started in August 2008. The main exclusion criterion was any obvious or previously documented health condition that could interfere with the participant's immune response to the vaccine. Avaxim™ 80U Pediatric is part of the national immunization schedule in Argentina. Each 0.5 mL dose of Avaxim™ 80U Pediatric vaccine contains 80 U inactivated HA virus, 0.15 mg aluminum hydroxide, 2.5 µL 2-phenoxyethanol, 12.5 µg formaldehyde, and ≤ 0.5 mL water for injection.
Ethics
The study was consistent with the ethical standards established by the Declaration of Helsinki and complied with the International Conference on Harmonization guidelines for Good Clinical Practice, as well as with all local and national regulations and directives. Informed consent was obtained initially from each study participant's parent(s) or legally acceptable representative; additionally, an assent form was also signed at Year 6 by children aged 7 year and older and the informed consent was re-signed by the parent(s) or legally acceptable representative of these participants at this time.
Study design and assays
All participants were followed up on a yearly basis. For the evaluation of the persistence of the anti-HAV immune response, a first blood sample (Year 1) was taken between August 2008 and April 2009, and for each participant further blood samples were taken at Year 2, Year 3, Year 4, Year 5, and Year 7. The intervals accorded to the date of the first vaccination that had been received before entry into the study.
To measure the anti-HAV antibody concentration up to and including Year 5 a MEIA (seropositivity defined as anti-HAV antibody concentration ≥ 10 mIU/mL) was used; thereafter an ECLIA (seropositivity defined as anti-HAV antibody concentration ≥ 3 mIU/mL) was used (due to the discontinuation of the MEIA kit by its manufacturer). All assays were performed by the Bio Analytical Research Corporation (BARC) laboratory (Ghent, Belgium) using a commercial ELISA assay kit (HAV 2.0 quantitative AXSYM HAVAB microparticle ELISA, Abbott Laboratories, Abbott Park, IL, USA) for MEIA and a commercial laboratory test (Roche Elecsys Anti-HAV, Roche Diagnostics, IN, USA) for ECLIA. For MEIA and ECLIA, quantitative anti-HAV levels are expressed as GMCs (mIU/mL) as determined by comparison to a serial dilution of a WHO reference serum. Due to inherent differences, these 2 commercial serological assays cannot be compared with respect to their sensitivities and specificities. The lower and upper limits of quantification (LLOQ and ULOQ), respectively, are 5 and 20,000 mIU/mL for MEIA and 3 and 12,800 mIU/mL for ECLIA.
Participants who had received one dose of the HAV vaccine and who had an anti-HAV IgG titer <10 mIU/mL (MEIA) at any scheduled visit up to and including Year 5 or <3 mIU/mL thereafter (ECLIA) were offered a booster vaccination outside the scope of this study. For these participants, an additional blood sample was taken ideally at 10 d after the booster vaccination to measure anti-HAV antibody concentration; if the Day 10 sample was missing, the next scheduled sample was used to evaluate the immune response to the booster.
Participants who had received 2 doses of the HAV vaccine (either 2 doses before the study, or one dose before the study and a subsequent booster as described above) and who had an anti-HAV IgG titer <10 mIU/mL (MEIA) at any scheduled visit up to and including Year 5 or <3 mIU/mL thereafter (ECLIA) were not offered any further vaccination and were withdrawn from the study.
Statistical methods
The long-term immunogenicity was analyzed according to the number of doses received (one or 2 doses) either before inclusion or before inclusion and during the study, as described above. Participants were therefore analyzed based on whether they received 1 dose before inclusion and no booster during the study (Group 1); 2 doses before inclusion and no booster during the study (Group 2); or 1 dose before inclusion and 1 booster (Group 3).
For the primary objective, seroprotection rates (percentage of participants with anti-HAV anti-Ig concentration ≥ 10 mIU/mL [using MEIA, from Year 1 to Year 5, inclusive] or ≥ 3 mIU/mL [using ECLIA, Year 7]) were calculated with their 95% confidence intervals (CIs) using the Clopper-Pearson exact binomial method, as quoted by Newcombe.18 The relationship between suspected cases of HA (if any) in family members and the participant's antibody levels (to evaluate the possibility of a natural boosting effect) was to be assessed using the chi-square test (or Fisher's exact test when the conditions for using the chi-square test were not met). Socio-economic data were analyzed descriptively. The analysis population comprised all included participants.
No statistical hypothesis was used for the calculation of sample size. Assuming an annual dropout rate of 8%, it was estimated that 600 participants enrolled would result in 429 participants at Year 5 and 283 participants at Year 10. Assuming that 10–15% of participants would have anti-HAV antibody levels below the minimum threshold for seroprotection at Year 5 (i.e., 10 mIU/mL by MEIA), a sample size of 429 participants at Year 5 would be sufficient to obtain a precision of 3.5% for the 95% CI of the proportion of participants achieving the minimum concentration. No sample size calculation was done for the period after 5 year of follow-up.
The statistical analysis was done under the responsibility of the study sponsor using Statistical Analysis Software (SAS®), Version 9.1 (SAS Institute, Cary, NC, USA).
Disclosure of potential conflicts of interest
CE, LB, ML, HC, ILC did not receive any direct payment from Sanofi Pasteur with regard to their contributions to this manuscript, but could receive expenses for conference attendance for the presentation of data from this study.
YT and AR are employees of Sanofi Pasteur.
Acknowledgments
The authors would like to thank the children who took part in the study and their parents/legal guardian(s). They also thank Victor Ortiz, Maria del Carmen Ortiz, and Adriana Chirino for their support in conducting the annual visits, as well as Valérie Bosch-Castells (Sanofi Pasteur) for her critical review of the manuscript.
This manuscript was prepared with the assistance of a professional medical writer, Dr Andrew Lane (Lane Medical Writing), in accordance with the European Medical Writers Association guidelines and Good Publication Practice and funded by Sanofi Pasteur.
Funding
This study was supported by Sanofi Pasteur, Lyon, France.
Authors' contributions
All authors contributed to the conception, design, or conduct of the study, or the analysis and interpretation of study data, and all approved the final version of this article. The publication was managed by Jean-Sébastien Persico (Sanofi Pasteur) with the full involvement of the authors.
References
- [1].WHO Position paper on hepatitis A vaccines. Wkly Epidemiol Rec. 2012;87(28/29):261-76. PMID:22905367 [PubMed] [Google Scholar]
- [2].Ciocca M, Ramonet M, Cuarterolo M, Lopez S, Speranza A, Imventarza O, Alvarez F.. Acute liver failure in children: experience with 210 patients. J Pediatr Gastroenterol Nutr. 2004;39:S31. doi: 10.1097/00005176-200406001-00064 [DOI] [Google Scholar]
- [3].Bernal W, Wendon J. Acute liver failure. New Eng J Med. 2013;369(26):2525-34. doi: 10.1056/NEJMra1208937. PMID:24369077 [DOI] [PubMed] [Google Scholar]
- [4].Lopez E, Debbag R, Coudeville L, Baron-Papillon F, Armoni J. The cost-effectiveness of universal vaccination of children against hepatitis A in Argentina: results of a dynamic health-economic analysis. J Gastroenterol. 2007;42(2):152-60. doi: 10.1007/s00535-006-1984-x. PMID:17351805 [DOI] [PubMed] [Google Scholar]
- [5].Gentile A, Ramonet MD, Ciocca M. [Hepatitis A immunisation in the Argentinean mandatory schedule]. Arch Argent Pediatr. 2013;111(2):155-61. doi: 10.5546/aap.2013.155. PMID:23568072 [DOI] [PubMed] [Google Scholar]
- [6].Vizzotti C, Gonzalez J, Rearte A, Uruena A, Perez Carrega M, Calli R, Gentile A, Uboldi A, Ramonet M, Cañero-Velasco M, et al.. Single-Dose Universal Hepatitis A Immunization in Argentina: Low Viral Circulation and High Persistence of Protective Antibodies Up to 4 Years. J Pediatric Infect Dis Soc. 2015;4(4):e62-7. doi: 10.1093/jpids/piu068. PMID:26582885 [DOI] [PubMed] [Google Scholar]
- [7].Vizzotti C, Gonzalez J, Gentile A, Rearte A, Ramonet M, Canero-Velasco MC, Pérez Carrega ME, Urueña A, Diosque M. Impact of the single-dose immunization strategy against hepatitis A in Argentina. Pediatr Inf Dis J. 2014;33(1):84-8. doi: 10.1097/INF.0000000000000042 [DOI] [PubMed] [Google Scholar]
- [8].Iwarson S, Lindh M, Widerstrom L. Excellent booster response 4-6 y after a single primary dose of an inactivated hepatitis A vaccine. Scand J Infect Dis. 2002;34(2):110-1. doi: 10.1080/00365540110077362. PMID:11928839 [DOI] [PubMed] [Google Scholar]
- [9].Van Herck K Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol. 2001;63(1):1-7. doi: 10.1002/1096-9071(200101)63:1%3c1::AID-JMV1000%3e3.0.CO;2-U. PMID:11130881 [DOI] [PubMed] [Google Scholar]
- [10].Espul C, Benedetti L, Cuello H, Houillon G, Rasuli A. Persistence of immunity from 1 year of age after one or two doses of hepatitis A vaccine given to children in Argentina. Hepat Med. 2012;4:53-60. doi: 10.2147/HMER.S33847. PMID:24367232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Espul C, Benedetti L, Linares M, Cuello H, Rasuli A. Five-year follow-up of immune response after one or two doses of inactivated hepatitis A vaccine given at 1 year of age in the Mendoza Province of Argentina. J Viral Hepat. 2015;22(4):453-8. doi: 10.1111/jvh.12317. PMID:25262590 [DOI] [PubMed] [Google Scholar]
- [12].Berger R, Just M, Althaus B. Time course of hepatitis A antibody production after active, passive and active/passive immunisation: the results are highly dependent on the antibody test system used. J Virol Methods. 1993;43(3):287-97. doi: 10.1016/0166-0934(93)90147-J. PMID:8408443 [DOI] [PubMed] [Google Scholar]
- [13].Miller WJ, Clark W, Hurni W, Kuter B, Schofield T, Nalin D. Sensitive assays for hepatitis A antibodies. J Med Virol. 1993;41(3):201-4. doi: 10.1002/jmv.1890410306. PMID:8263501 [DOI] [PubMed] [Google Scholar]
- [14].Wiedmann M, Boehm S, Schumacher W, Swysen C, Zauke M. Evaluation of three commercial assays for the detection of hepatitis a virus. Eur J Clin Microbiol Infect Dis. 2003;22(2):129-30. PMID:12627291 [DOI] [PubMed] [Google Scholar]
- [15].Advisory Committee on Immunization Practices, Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortality Wkly Rep. 2006;55(RR-7):1-23 [PubMed] [Google Scholar]
- [16].Lemon SM, Binn LN. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis. 1983;148(6):1033-9. doi: 10.1093/infdis/148.6.1033. PMID:6317766 [DOI] [PubMed] [Google Scholar]
- [17].Van Damme P Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K Shouval D, Bonanni P, Connor B, Cooksley G, et al.. Hepatitis A booster vaccination: is there a need? Lancet. 2003;362(9389):1065-71 [DOI] [PubMed] [Google Scholar]
- [18].Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998;17(8):857-72. doi: 10.1002/(SICI)1097-0258(19980430)17:8%3c857::AID-SIM777%3e3.0.CO;2-E. PMID:9595616 [DOI] [PubMed] [Google Scholar]


